Particle growth mechanism of nanocrystalline zirconia powder during high temperature heat treatment
来源期刊:中国有色金属学报(英文版)2007年第5期
论文作者:刘纯波 于连生 蒋显亮
文章页码:1022 - 1022
Key words:nanocrystalline zirconia; thermal barrier coating; spray drying; agglomeration; nano-particle growth
Abstract: Agglomerated nanocrystalline ZrO2-8%Y2O3 powder prepared by spray drying was heat-treated in air at temperatures from 1 200 ℃ to 1 400 ℃ for 2 h. Scanning electron microscopy was used to examine the changes of particle size and morphology, and X-ray diffraction was used to analyze the change of constituent phases before and after the high temperature heat treatment. Nano-particle growth behavior was also investigated. The results show that the major constituent phase of the agglomerated nanocrystalline powder is tetragonal, and non-uniform growth of the nano-particles occurs while the heat treatment temperature reaches 1 300 ℃. This non-uniform growth phenomenon is related with the inhomogeneous distribution of Y2O3 in ZrO2. Nano-particles grow into micron particles through the mechanisms of gradual merging of nano-particles in some areas and sudden merging of nano-particles in other areas.

LIU Chun-bo(刘纯波), YU Lian-sheng(于连生), JIANG Xian-liang(蒋显亮)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 31 October 2006; accepted 12 January 2007
Abstract: Agglomerated nanocrystalline ZrO2-8%Y2O3 powder prepared by spray drying was heat-treated in air at temperatures from 1 200 ℃ to 1 400 ℃ for 2 h. Scanning electron microscopy was used to examine the changes of particle size and morphology, and X-ray diffraction was used to analyze the change of constituent phases before and after the high temperature heat treatment. Nano-particle growth behavior was also investigated. The results show that the major constituent phase of the agglomerated nanocrystalline powder is tetragonal, and non-uniform growth of the nano-particles occurs while the heat treatment temperature reaches 1 300 ℃. This non-uniform growth phenomenon is related with the inhomogeneous distribution of Y2O3 in ZrO2. Nano-particles grow into micron particles through the mechanisms of gradual merging of nano-particles in some areas and sudden merging of nano-particles in other areas.
Key words: nanocrystalline zirconia; thermal barrier coating; spray drying; agglomeration; nano-particle growth
1 Introduction
In order to reduce fuel consumption and increase thrust-to-mass ratio and life expectation of future gas turbine engine, many advanced materials with low density, good toughness at room temperature and high temperature strength have been used extensively. Heat insulation, anti-wear and corrosion-resistant coatings have been applied to engine components. However, traditional micron coatings with laminar layers are brittle, easily cracking and spalling, which results in the decrease of the service life of the coatings. They are difficult to meet the increasing demand for a variety of applications such as aerospace industry. To solve these problems, nanostructured ceramic coatings are developed. Nanostructured coatings can be deposited by plasma spraying and applied on metals to enhance coating performance[1-3]. When ceramic coatings consist of nanostructure, nano-particles in the coatings can slide each other under stress so that its brittleness is reduced and toughness is improved[4-5]. Plasma spray forming of nano-oxide ceramic coatings has become a hot research field in recent years[6-13]. However, during the process of deposition and use of nanostructured ceramic coatings at high temperature, nano-particles in the nano-coatings will grow, which leads to the decrease of its relevant properties[14]. Therefore, the temperature limit of nano-coating applications is the key issue that must be resolved.
It is reported that the grain growth occurs in nanostructured zirconia-based thermal barrier coating when heat treatment or annealing temperature reaches beyond some points[15-16]. In addition to grain or particle growth, phase transformation also happens in thermal barrier coating. The transformation from tetragonal phase to monoclinic phase strongly depends on grain size. The phase transformation will happen only when grain size in the coating exceeds a minimum size[17]. In this study, nano-particle growth behavior and growth mechanism of the agglomerated nanocrystalline yttria-stablized zirconia(YSZ) powder prepared by spray drying and with subsequent heat treatment under the temperatures from 1 200 ℃ to 1 400 ℃ were studied. The experimental results and corresponding findings will provide important reference to the development and application of the nanostructured zirconia ceramic coating.
2 Experimental
ZrO2-8%Y2O3 (mass fraction, the same below) powder agglomerated by the method of spray drying was used for the experiment of high temperature heat treatment. Raw nanocrystalline ZrO2-8%Y2O3 powder was purchased from Shijiazhuang Yisite Patent Development Corp., Hebei Province, China. Agglomeration of the nanocrystalline powder by the method of spray drying was conducted in Hunan Bestful Materials Corp. Ltd, Hunan Province, China.
Heat treatment was conducted in air in SX-12-16 box furnace using MoSi2 heating elements. The aims of the high temperature heat treatment are to remove the organic binder of polyvinyl alcohol(PVA) from the agglomerated particles, allowing nano-particles sintering together, and to find an application temperature limit. As a result, the nano-particles of the agglomerated powder did not need to separate again during subsequent processing and the thermal barrier coating deposited from the powder can be safely used. During high temperature heat treatment of the agglomerated powder, appropriate powder samples were put into five zirconia ceramic crucibles. Heat treatment temperatures were 1 200, 1 250, 1 300, 1 350 and 1 400 ℃, respectively and the heat treatment time was 2 h. After each heat treatment, the powder sample was taken out from the furnace and cooled in air. Particle morphology and size of the powders before and after the high temperature heat treatment were examined by KYKY2800 scanning electron microscope. Phase constituent analysis was made on D/MAX2500 X-ray diffractometer using Cu Kα.
For the purpose of comparison, pure nanocrystalline Al2O3 powder was also heat-treated under identical experimental conditions.
3 Results and discussion
3.1 Morphology of raw nanocrystalline ZrO2- 8%Y2O3 powder
Particle morphology of the raw ZrO2-8%Y2O3 nano- powder prepared by chemistry precipitation is shown in Fig.1. It can be seen from Fig.1(a) that the raw powder is non-uniform and nano-particle aggregates are present in the raw powder. Some of these aggregates are loose (soft agglomeration) and can be dispersed in the subsequent processing, however the others are tight (hard agglomeration) and cannot be dispersed in the subsequent processing. Due to the poor flowability of the raw powder, the powder is easily blocked in powder feed line and the deposition efficiency is low during plasma spraying. Therefore, the raw nano-powder has to be agglomerated into the powder with large particle size and spherical particle shape by the method of spray drying to meet the requirement of plasma spraying. It can be seen from Fig.1(b) at high magnification that the sizes of nano-particles are in the range of 70-300 nm, mostly in the range of 100-200 nm.
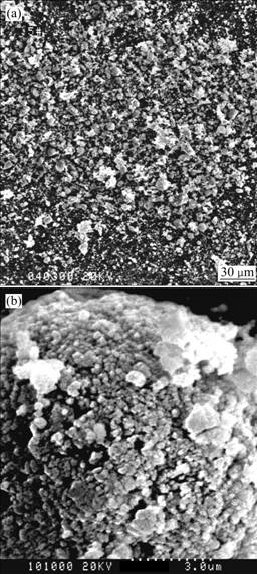
Fig.1 SEM micrographs of raw nanocrystalline ZrO2-8%Y2O3 powder
3.2 Morphology of agglomerated ZrO2-8%Y2O3 powder before heat treatment
The agglomerated nanocrystalline powder before heat treatment is shown in Fig.2. It can be seen from Fig.2(a) that the particle size of the agglomerated powder is in the range of 10-60 μm and the particle morphology is nearly spherical or donut-shape. The powders consisting of the particles with large size and spherical shape have good flowability and are suitable for plasma spray. Fig.2(b) shows the surface morphology of an agglomerated particle with the size of about 30 μm that consists of many small nano-particles combined by the organic binder of PVA.
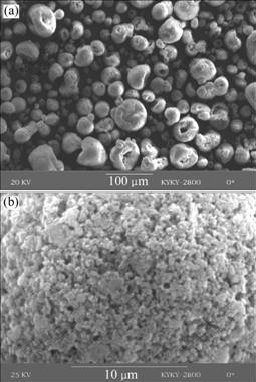
Fig.2 SEM micrographs of YSZ powder with different sizes without high temperature heat treatment: (a) 10-60 μm; (b) 30 μm
3.3 Morphology of agglomerated ZrO2-8%Y2O3 powder after heat treatment
Particle morphology of the yttria-stabilized zirconia powder after heat treatment at 1 200 ℃ for 2 h is shown in Fig.3. It can be seen from Fig.3(a) that the particle size and the particle morphology of the agglomerated powder are similar to those of the powder before high temperature heat treatment, i.e. 10-60 μm of particle size, near-spherical and donut shape. Some large agglomerated particles sinter together. The sintering of the large agglomerated particles is very weak and the connection of the large agglomerated particles can be easily broken up through sifting.
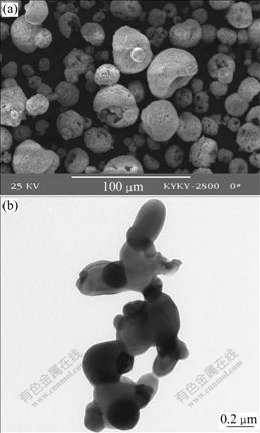
Fig.3 Micrographs of YSZ powder with different sizes after heat treatment at 1 200 ℃ for 2 h: (a) 10-60 μm; (b) 100-300 nm
Fig.3(b) illustrates the nano-particle morphology at high magnification. The nano-particle size is about 100-300 nm. The nano-particles connect together, indicating that sintering of the nano-particles occurs after the organic binder of PVA is burnt.
Fig.4 shows the nano-particle growth process after the agglomerated yttria-stabilized zirconia powder is heat-treated at the temperatures from 1 250 ℃ to 1 350℃. It can be seen in Fig.4(a) that no single nano-particle is isolated and all of the nano-particles sinter together after the powder is heat-treated at 1 250 ℃. However, the growth of the nano-particles is insignificant at this temperature. The nano-particle size is 300-400 nm. The nano-particle morphology of the powder after heat treatment at 1 300 ℃ for 2 h is shown in Fig.4(b). The nano-particles remarkably grow up. Some of the nano-particles grow into 600-700 nm. Fig.4(c) shows the result of significant nano-particle growth after the powder is heat-treated at 1 350 ℃ for 2 h. Some of the nano-particles grow into 800-900 nm.
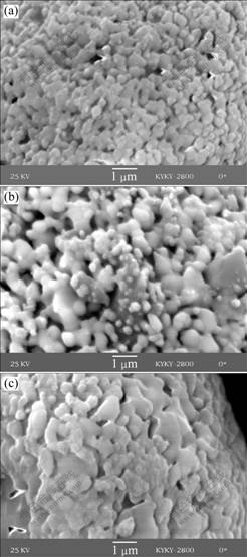
Fig.4 SEM micrographs of YSZ powders after heat treatment at different temperatures for 2 h: (a) 1 250 ℃; (b) 1 300 ℃; (c) 1 350 ℃
Non-uniform growth phenomenon appears when heat treatment temperature is above 1 300 ℃. One kind of growth is the gradual merging of the nano-particles with the increase of heat treatment temperature, finally becoming micron particle, and another kind of growth is the sudden merging of many small nano-particles when the heat treatment temperature is sufficiently high.
Morphology of the agglomerated yttria-stabilized zirconia powder after heat treatment at 1 400 ℃ for 2 h is shown in Fig.5. Non-uniform growth is obvious. It can be seen in Fig.5(a) that some of the nano-particles grow into micron size while others are in nano-size or sub-micron size. These small particles are polygonal and meet each other at 120?. Almost all of these small particles sinter together, making the large agglomerated particles integral. Fig.5(b) illustrates the formed nano- particle block after the high temperature sintering.
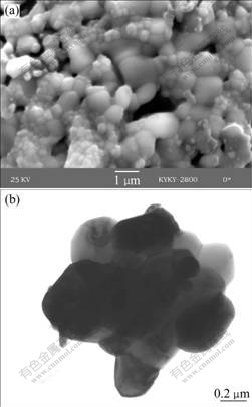
Fig.5 Micrographs of YSZ powder after heat treatment at 1 400 ℃ for 2 h: (a) Micron size particles; (b) Nano-particle block
3.4 Phase analysis of powders before and after heat treatment
X-ray diffraction pattern of the ZrO2-8%Y2O3 agglomerated powder before heat treatment is shown in Fig.6(a). It can be seen in Fig.6(a) that the powder is composed of tetragonal phase and monoclinic phase. The major constituent phase is tetragonal. No impurity is found. When heat treatment is conducted at a temperature higher than 400 ℃, the organic binder of PVA in the agglomerated powder is completely burnt. X-ray diffraction results on the powder samples heat-treated from 1 200 ℃ to 1 400 ℃ show that the content of tetragonal phase is increased and the content of the monoclinic phase is decreased when the heat treatment temperature is increased. When the heat treatment temperature reaches 1 400 ℃, the monoclinic phase almost vanishes and the predominant phase is tetragonal, as shown in Fig.6(b). The tetragonal phase is desired because the phase transformation from tetragonal to monoclinic accompanies 3%-5% volume change and causes cracking when zirconia coatings are used repeatedly from low temperature to high temperature.
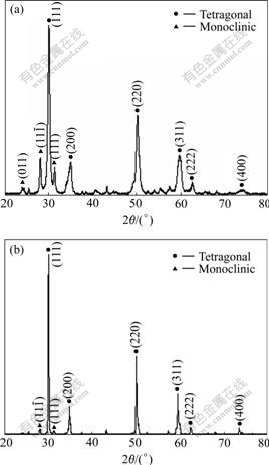
Fig.6 XRD patterns of ZrO2-8%Y2O3 agglomerated powder before heat treatment (a) and after heat treatment at 1 400 ℃ for 2 h (b)
3.5 Yttria-stablized zirconia nano-particle growth mechanism
In order to stabilize the tetragonal phase of zirconia, yttria is often added into zirconia. The distribution of Y2O3 in ZrO2 has two states: one state is the yttria present in the lattice of ZrO2, forming solid solution; the other state is the yttria accumulated on the surface or boundary of ZrO2. Because only 8% Y2O3 is added into ZrO2, the solid solution formed is not saturated with Y2O3 and no Y2O3 precipitates on ZrO2 grain boundary or ZrO2 nano-particle surface. Because Y3+ ion radius and Zr4+ ion radius are somehow different, lattice distortion will be created and micro-stress will be generated when Y3+ ions enter the lattice of ZrO2 and replace Zr4+. This kind of micro-stress plays a role in inhibiting the phase transformation from high temperature tetragonal phase to low temperature monoclinic phase.
SEM observation on the heat-treated powder reveals that the yttria-stablized zirconia nano-particles grow slowly and uniformly when heat treatment temperature is below 1 300 ℃ and obvious nano-particles growth occurs only when heat treatment temperature is above 1 300 ℃. With the further increase of heat treatment temperature, the nano-particles grow from nano-size to sub-micron or micron-size. The nano-particles growth is not uniform. Energy dispersive analysis of X-ray(EDAX) of different small particles reveals that there is a difference of yttria content in zirconia in different areas of the large agglomerated particle, as shown in Fig.7.

Fig.7 SEM micrograph and EDAX result of YSZ powder after heat treatment at 1 400 ℃ for 2 h
In order to reveal the growth mechanism of YSZ nano-particles during the high temperature heat treatment,the growth behavior of Al2O3 nano-particles was studied for the purpose of comparison. Raw alumina nano- particles have the average size of about 100 nm. Dispersion of the raw alumina powder is rather poor. Some nano-particles are aggregated. The aggregation phenomenon of the nanocrystalline powder is due to the high surface energy and high moisture adsorption capability of nano-particles. The aggregates having soft agglomeration can be dispersed by milling/ultrasonic processing; while the aggregates having hard agglomeration can not be dispersed, which will be remained in the large particles agglomerated by spray- drying. Fig.8(a) shows nano-particle size, shape and state on the surface of an agglomerated nanocrystalline Al2O3 particle at a high magnification. This agglomerated powder is not heat-treated.

Fig.8 SEM micrographs of agglomerated nanocrystalline Al2O3 powder: (a) Without heat treatment; (b) Heat treated at 1 400 ℃ for 2 h
Heat treatment of agglomerated Al2O3 powder was conducted under identical conditions as used for heat treatment of agglomerated ZrO2-8%Y2O3 powder. The surface morphology of agglomerated Al2O3 powder heat-treated at 1 400 ℃ for 2 h is shown in Fig.8(b). From this high-magnification SEM micrograph, it can be seen that Al2O3 nano-particles grow into 300-400 nm, but the growth rate is much lower than that of the ZrO2-8%Y2O3 nano-particles. Unlike yttria stabilized zirconia nano-particles, all Al2O3 nano-particles grow gradually and uniformly with the increase of heat treatment temperature. The uniform growth of all Al2O3 nano-particles implies that the pre-existed aggregates are not the sites for priority growth and will not affect the growth of nano-particles.
Through the comparative analysis of the growth behaviors of Al2O3 nano-particles and ZrO2-8%Y2O3 nano-particles after the high temperature treatment, it can be inferred that the non-uniform growth of ZrO2- 8%Y2O3 nano-particles is related with the inhomogeneous distribution of Y2O3 in ZrO2.
4 Conclusions
1) Nano-particles of agglomerated ZrO2-8%Y2O3 powder grow obviously when heat treatment temperature reaches 1 300 ℃, which limits the application temperature of nanocrystalline ZrO2-8%Y2O3 material.
2) The nano-particles show non-uniform growth. This non-uniform growth phenomenon is related with the inhomogeneous distribution of Y2O3 in ZrO2. Some nano-particles grow into a micron particle by the way of gradual merging of nano-particles with the increase of heat treatment temperature, while other nano-particles become micron particles by the way of sudden merging of many nano-particles when heat treatment temperature is higher than 1 300 ℃.
References
[1] SHAW L L, GOBERMAN D, REN R, GELL M, JIANG S, WANG Y, XIAO D, STRUTT P. The dependency of microstructure and properties of nanostructured coatings on plasma spray conditions [J]. Surface and Coatings Technology, 2000, 130(1): 1-8.
[2] LIMA R S, KUCUK A, SENTURK U, BERNDT C C. Properties and microstructures of nanostructured partially stabilized zirconia coatings [J]. Journal of Thermal Spray Technology, 2001, 10(1): 150-152.
[3] CHEN H, DING C X. Nanostructured zirconia coating prepared by atmospheric plasma spraying [J]. Surface and Coatings Technology, 2002, 150(1): 31-36.
[4] JIANG X L, JORDAN E, SHAW L, GELL M. Deformation behavior of nanostructured ceramic coatings deposited by thermal plasma spray [J]. Journal of Materials Science and Technology, 2004, 20(4): 479-480.
[5] JIANG X L, JORDAN E, SHAW L, GELL M. Plasma spray forming of nanostructured composite coatings [J]. Journal of Materials Science and Technology, 2002, 18(3): 287-288.
[6] LIMA R S, KUCUK A, BERNDT C C. Integrity of nanostructured partially stabilized zirconia after plasma spray processing [J]. Materials Science and Engineering A, 2001, 313(1/2): 75-82.
[7] CHEN H, ZHANG Y F, DING C X. Tribological properties of nanostructured zirconia coatings deposited by plasma spraying [J]. Wear, 2002, 253(7/8): 885-893.
[8] CHEN H, DING C X, LEE S. Phase composition and microstructure of vacuum plasma sprayed nanostructured zirconia coating [J]. Materials Science and Engineering A, 2003, 361(1/2): 58-66.
[9] ZHOU C G, WANG N, WANG Z B, GONG S K, XU H B. Thermal cycling life and thermal diffusivity of a plasma-sprayed nanostructured thermal barrier coating [J]. Scripta Materialia, 2004, 51(9): 945-948.
[10] LIANG B, DING C X. Phase composition of nanostructured zirconia coatings deposited by air plasma spraying [J]. Surface and Coatings Technology, 2005, 191(2/3): 267-273.
[11] LIANG B, DING C X. Thermal shock resistances of nanostructured and conventional zirconia coatings deposited by atmospheric plasma spraying [J]. Surface and Coatings Technology, 2005, 197(2/3): 185-192.
[12] LIN Feng, YU Yue-guang, JIANG Xian-liang, ZENG Ke-li, REN Xian-jing, LI Zhen-duo. Microstructure and properties of nanostructured TBCs fabricated by plasma spraying [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(3): 482-487. (in Chinese)
[13] ZHOU B, WANG Q S, LIU Y B, MA Z. Grain size and thermal conductivity of nano-zirconia thermal barrier coating [J]. Journal of Materials Protection, 2006, 39(3): 1-3.
[14] WANG Z B, ZHOU C G, XU H B, GONG S K. Effect of thermal treatment on the grain growth of nanostructured YSZ thermal barrier coating prepared by air plasma spraying [J]. Chinese Journal of Aeronautics, 2004, 17(2): 119-123. (in Chinese)
[15] CHOI S R, ZHU D M, MILLER R A. Effect of sintering on mechanical properties of plasma-sprayed zirconia-based thermal barrier coatings [J]. Journal of American Ceramic Society, 2005, 88(10): 2859-2867.
[16] MOON J, CHOI H, KIM H, LEE C. The effect of heat treatment on the phase transformation behavior of plasma-sprayed stabilized ZrO2 coatings [J]. Surface and Coatings Technology, 2002, 155(1): 1-10.
[17] ZENG F, HUANG L H, JIANG X L. Research on agglomerated Al2O3/TiO2/SiO2 nanocrystalline powder for thermal spray [J]. China Powder Science and Technology, 2005, 11(2): 24-28.
Foundation item: Project supported by the Priority Development Program of the Human Resources Ministry of China for Oversea Students
Corresponding author: JIANG Xian-liang; Tel: +86-731-8876307; Fax: +86-731-8876692; E-mail: xljiang@mail.csu.edu.cn

