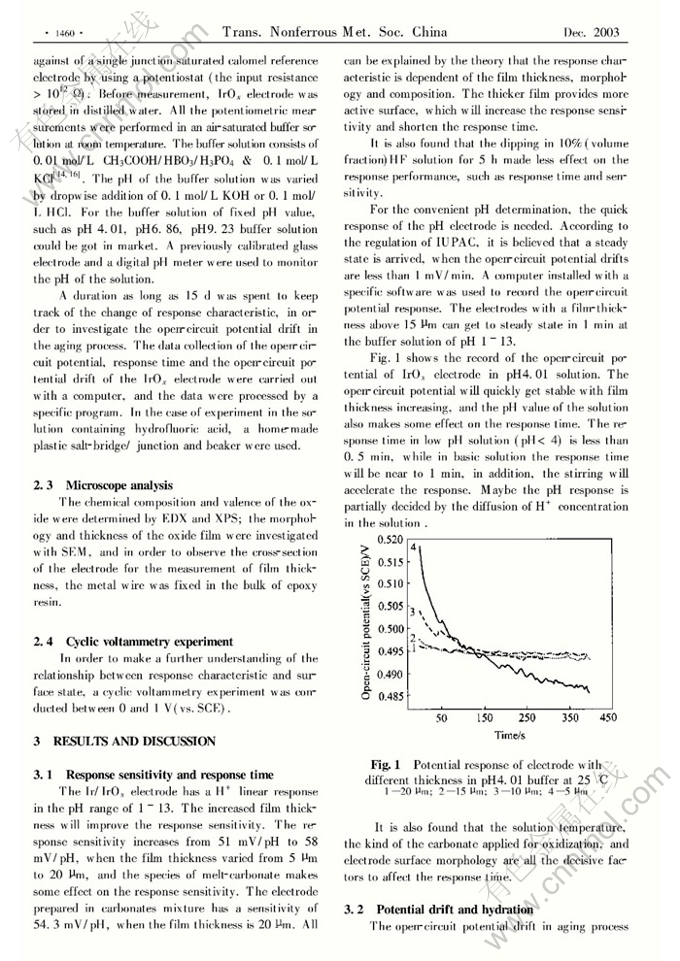
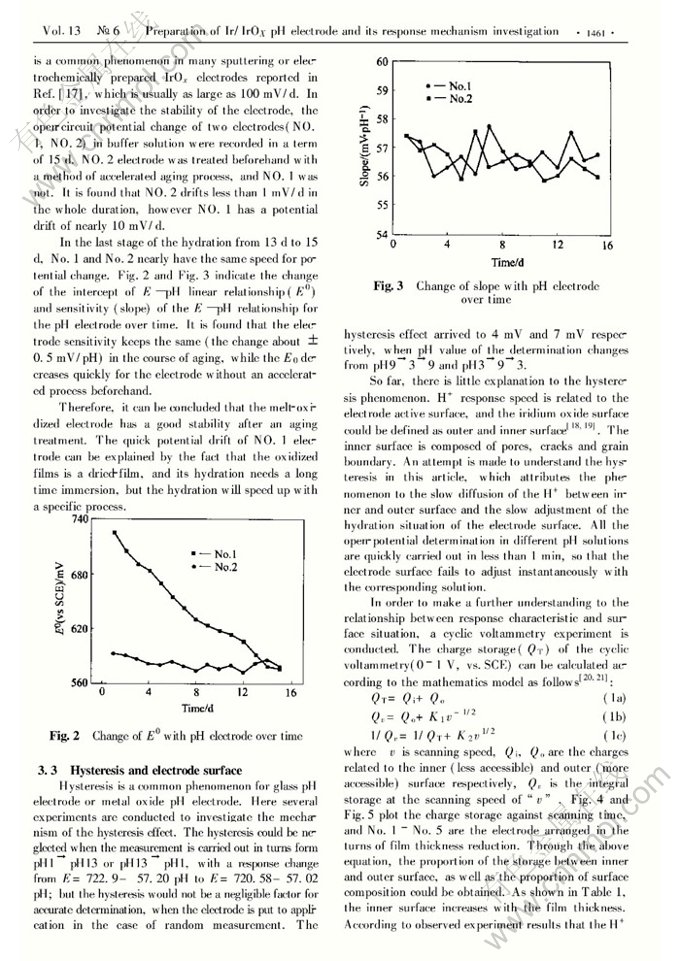
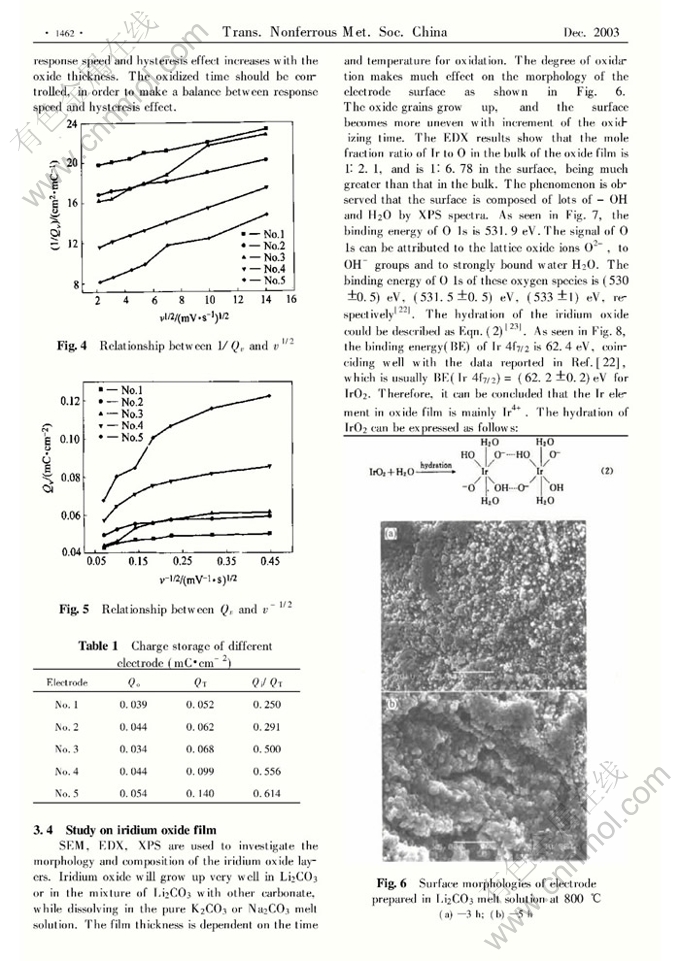
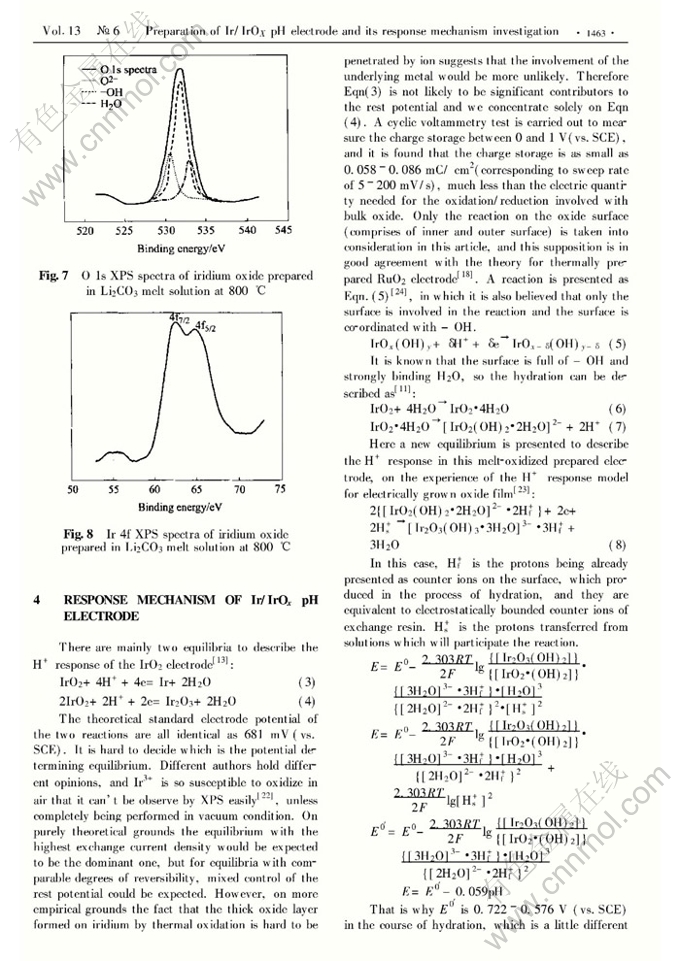
Preparation of Ir/IrOx pH electrode based on melt-oxidation and its response mechanism investigation
来源期刊:中国有色金属学报(英文版)2003年第6期
论文作者:陈东初 郑家燊 付朝阳
文章页码:1459 - 1464
Key words:pH; electrode; iridium; oxide; mechanism
Abstract: A method of melt-oxidation at high temperature is adopted to prepare Ir/IrOx pH electrode, and different carbonates such as Li2CO3, K2CO3, Na2CO3 and their mixture were used in this process. It is shown that the electrode prepared in carbonate melt has good pH response characteristics, such as stability, sensitivity, response time, and anti-corrosion ability in the solution containing F-, etc. The hysteresis mechanism was also investigated based on a storage model with a cyclic voltammetry experiment. It is found that the valence of Ir in the oxide film is +4, and the iridium oxide on the surface of the film is co-ordinate with -OH and H2O from the result of XPS spectra. The mole fraction ratio of Ir to O is 1∶2.1 in the bulk of the oxide, but x(Ir)∶x(O) is 1∶6.78 on the surface of the oxide film by EDX technology. The hydration effect of the thermally prepared iridium oxide was investigated. Therefore, a H+ response mechanism is suggested, being much different from the reported theory for thermally prepared iridium oxide electrode, which previously does not take the hydration of oxide into consideration.