铝酸钠溶液中硫和铁组元间的相互作用
来源期刊:中国有色金属学报(英文版)2015年第2期
论文作者:李小斌 李重洋 彭志宏 刘桂华 周秋生 齐天贵
文章页码:608 - 614
关键词:高硫铝土矿;铝酸钠;铁化合物;脱硫;机理
Key words:high-sulfur bauxite; sodium aluminate; iron compounds; desulfurization; mechanism
摘 要:对铝酸钠溶液中铁化合物与含硫组元间的反应行为进行研究。结果表明:铝酸钠溶液中铁化合物能显著脱除溶液中的S2-,对S2O32-、SO32-和SO42-等硫化合物则没有脱除效果。当Fe(Ⅲ)和Fe(Ⅱ)化合物加入量达到铁硫摩尔比为2:1时,100 °C下S2-的脱除率分别达到86.10%和92.70%。在Fe(Ⅲ)和Fe(Ⅱ)化合物的除硫过程中,均有水合硫代铁酸钠、赤铁矿、无定型硫化亚铁、聚合硫铁化合物和硫酸铁等物质生成,主要区别在于Fe(Ⅱ)化合物反应初期生成的水合硫代铁酸钠会继续转化为不含钠的二硫化亚铁。
Abstract: Reaction behaviors of sulfur and iron compounds in sodium aluminate solutions were investigated. The results show that iron compounds can remarkably remove the S2- but cannot get rid of and in sodium aluminate solutions. The removal efficiency of S2- using ferrous compound and ferric compound can reach 86.10% and 92.70% respectively when the iron compounds were added with a molar ratio of 2:1 compared with the sulfur in liquors at 100 °C. Moreover, several same compounds are formed in those two desulfurization processes with ferrous or ferric compounds, including erdite, hematite, amorphous ferrous sulfide, polymerized sulfur-iron compounds and ferric sulfate. The major difference between these two processes is that the erdite generated from ferrous compounds at the initial reaction stage will convert to a sodium-free product FeS2 at the subsequent stage.
Trans. Nonferrous Met. Soc. China 25(2015) 608-614
Xiao-bin LI, Chong-yang LI, Zhi-hong PENG, Gui-hua LIU, Qiu-sheng ZHOU, Tian-gui QI
National Engineering Laboratory for Efficient Utilization of Refractory Nonferrous Metals Resources,
Central South University, Changsha 410083, China
Received 29 April 2014; accepted 13 July 2014
Abstract: Reaction behaviors of sulfur and iron compounds in sodium aluminate solutions were investigated. The results show that iron compounds can remarkably remove the S2- but cannot get rid of 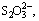
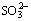 and
and 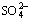 in sodium aluminate solutions. The removal efficiency of S2- using ferrous compound and ferric compound can reach 86.10% and 92.70% respectively when the iron compounds were added with a molar ratio of 2:1 compared with the sulfur in liquors at 100 °C. Moreover, several same compounds are formed in those two desulfurization processes with ferrous or ferric compounds, including erdite, hematite, amorphous ferrous sulfide, polymerized sulfur-iron compounds and ferric sulfate. The major difference between these two processes is that the erdite generated from ferrous compounds at the initial reaction stage will convert to a sodium-free product FeS2 at the subsequent stage.
in sodium aluminate solutions. The removal efficiency of S2- using ferrous compound and ferric compound can reach 86.10% and 92.70% respectively when the iron compounds were added with a molar ratio of 2:1 compared with the sulfur in liquors at 100 °C. Moreover, several same compounds are formed in those two desulfurization processes with ferrous or ferric compounds, including erdite, hematite, amorphous ferrous sulfide, polymerized sulfur-iron compounds and ferric sulfate. The major difference between these two processes is that the erdite generated from ferrous compounds at the initial reaction stage will convert to a sodium-free product FeS2 at the subsequent stage.
Key words: high-sulfur bauxite; sodium aluminate; iron compounds; desulfurization; mechanism
1 Introduction
The constraint on sustainable development of alumina industry in China by bauxite resources has become increasingly obvious because of the large industrial production capacity of alumina, the exhausted high-grade quality bauxite resources in China and the restrictive export policies of foreign bauxite [1]. The reserve of high sulfur-containing diasporic bauxite has reached 560 million tons in China and is expected to rise greatly to 2 billion tons in the future, which is mainly located in Guizhou Province and Chongqing Municipality. In addition, more than 60% of the high sulfur-containing diasporic bauxite in Guizhou Province is high-grade quality bauxite and it is suitable for the Bayer process [2,3]. During the high temperature digestion of Bayer process, the sulfur minerals can completely or partially react with the aluminate solutions, as a result, the sulfur enters into and accumulates in the Bayer liquor mainly in the form of S2-[4], which will obviously increase the caustic soda consumption and iron content in the alumina product and seriously corrode the steel equipment. Unfortunately, the current methods for sulfur removal could not treat high sulfur-containing diasporic bauxite effectively and economically, which results in the rarely using of high sulfur bauxite in alumina production [5,6]. However, it is worth noting that the disadvantages of high sulfur bauxite mentioned above involve the reaction behaviors of sulfur species and iron compounds in sodium aluminate solutions, and it would be of great significance to investigate the behaviors of sulfur compounds and iron compounds in sodium aluminate solutions.
Up to now, considerable studies have been proceeding, such as decomposition of sulfur or iron minerals in bauxite, increase of iron content in alumina product resulting from the more soluble sulfur-iron compounds, and corrosion of steel equipment. Pyrite in high sulfur bauxite can easily react with alkali liquors during the Bayer process [7]. The iron-hydroxyl complexes are generated firstly by the reaction of Fe2+ on the pyrite surface with OH- in sodium aluminate solution, then the complexes detach from the surface of pyrite, and the sulfur enters into the solution mainly in the form of S2- with a small quantity of 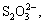
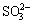 and
and 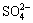 [8]. At the same time, the iron content in aluminate solutions increases sharply with the accumulation of S2-. KUZNETSOV et al [9] and HE [10] held that iron would combine with oxygen or hydroxyl to generate coordination complexes in aluminate solutions at high temperature, and then the S2- in solutions would substitute the oxygen or hydroxyl and form a more soluble Na2FeS2(OH)2·2H2O, which results in the increase of iron content in solutions and subsequent contamination of alumina product. Additionally, the formation of Na2FeS2(OH)2·2H2O would destroy the passive film on the surface of steel equipments and accelerate the corrosion of low alloy steel [11]. Recently, LI et al [12] have found that the sulfur could be removed by the addition of specific iron compounds, thus the problem of caustic soda consumption and alumina product contamination could be solved simultaneously. But the further research has not been reported. In this work, the reaction behavior of iron complexes and sulfur compounds in aluminate solutions were investigated, a new idea and an important fundamental basis of removing sulfur from aluminate solutions were provided and an effective protocol to utilize high sulfur bauxite was developed.
[8]. At the same time, the iron content in aluminate solutions increases sharply with the accumulation of S2-. KUZNETSOV et al [9] and HE [10] held that iron would combine with oxygen or hydroxyl to generate coordination complexes in aluminate solutions at high temperature, and then the S2- in solutions would substitute the oxygen or hydroxyl and form a more soluble Na2FeS2(OH)2·2H2O, which results in the increase of iron content in solutions and subsequent contamination of alumina product. Additionally, the formation of Na2FeS2(OH)2·2H2O would destroy the passive film on the surface of steel equipments and accelerate the corrosion of low alloy steel [11]. Recently, LI et al [12] have found that the sulfur could be removed by the addition of specific iron compounds, thus the problem of caustic soda consumption and alumina product contamination could be solved simultaneously. But the further research has not been reported. In this work, the reaction behavior of iron complexes and sulfur compounds in aluminate solutions were investigated, a new idea and an important fundamental basis of removing sulfur from aluminate solutions were provided and an effective protocol to utilize high sulfur bauxite was developed.
2 Experimental
2.1 Materials
Sodium aluminate solutions were prepared by dissolving industrial aluminum trihydroxide in hot solutions of industrial sodium hydroxide. The ferric compound and ferrous compound were respectively synthesized by the sedimentation of ferric chloride solution and ferrous chloride solution with the sodium hydroxide solution at 25 °C. The sulfur compounds were dissolved in sodium aluminate solutions to prepare sodium aluminate solutions to a certain sulfur concentration as element S. Sodium thiosulfate (AR purity) and sodium sulfite (AR purity) and sodium sulfate (AR purity) were purchased from Kermel Chemical Reagent Corporation of Tianjin, China. Sodium sulfide nonahydrate which contains small amounts of high valence state sulfur compounds was purchased from Xilong Chemical Corporation Limited (Guangdong, China).
2.2 Experimental procedure
The experiments were carried out in the DY-8 low pressure reaction kettles (Central South University Machinery Factory) in which stainless bombs (150 mL) sealed were immersed and rotated in glycerol with the temperature precision of 1 °C. The sulfur-containing aluminate solutions and ferric compound or ferrous compound as well as several stainless balls for agitation were added to the bomb and remained in the low pressure reaction kettle for 1 h at 100 °C. Then the bombs were cooled rapidly in cold water to room temperature, and the slurry was subsequently filtered and washed with hot water. The residue was dried at 50 °C for more than 24 h. The concentrations of S2-, 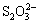 and
and 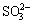 in the filtrate were analyzed by titration method [13] and the concentration of
in the filtrate were analyzed by titration method [13] and the concentration of 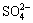 in the filtrate was determined by ion chromatography (Dionex China Limited). The concentration of iron impurities (labeled as Fe2O3) in solutions was determined by atomic absorption spectrometry. The phases of residues were further characterized by XRD analyzer D/MAX2500X (Rigaku Corporation, Japan), X-ray photoelectron spectrometer ESCALAB MK-II (Vaccum Generator Corporation, UK) and FT-IR 6700 spectrometer (Nicolet Corporation, USA), respectively.
in the filtrate was determined by ion chromatography (Dionex China Limited). The concentration of iron impurities (labeled as Fe2O3) in solutions was determined by atomic absorption spectrometry. The phases of residues were further characterized by XRD analyzer D/MAX2500X (Rigaku Corporation, Japan), X-ray photoelectron spectrometer ESCALAB MK-II (Vaccum Generator Corporation, UK) and FT-IR 6700 spectrometer (Nicolet Corporation, USA), respectively.
3 Results and discussion
3.1 Interaction of sulfur and iron compounds in sodium aluminate solutions
The sulfur enters the sodium aluminate solutions and exists as S2-, 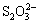 ,
, 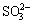 and
and 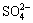 due to the redox reaction, and the amounts of sulfur species vary greatly with different production processes during the alumina production. The reactions of ferrous compound and ferric compound (the molar ratio of Fe to S represents the dosage of iron compound, n(Fe)/n(S)) with aluminate solutions with a sulfur concentration of 5 g/L (as element S) were firstly proceeded in order to examine the desulfurization efficiencies of iron compounds. The results are given in Tables 1 and 2.
due to the redox reaction, and the amounts of sulfur species vary greatly with different production processes during the alumina production. The reactions of ferrous compound and ferric compound (the molar ratio of Fe to S represents the dosage of iron compound, n(Fe)/n(S)) with aluminate solutions with a sulfur concentration of 5 g/L (as element S) were firstly proceeded in order to examine the desulfurization efficiencies of iron compounds. The results are given in Tables 1 and 2.
Table 1 Removal efficiency of sulfur compounds using ferrous compound
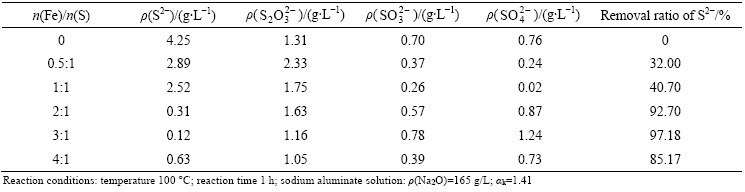
Table 2 Removal efficiency of sulfur compounds using ferric compound
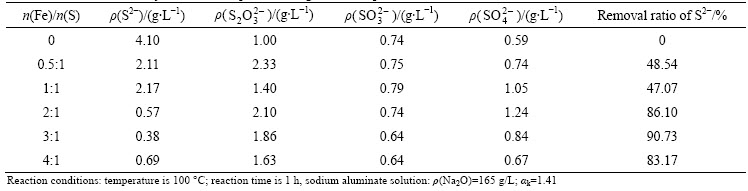
It can be seen from Table 1 that the S2- concentration decreases obviously with the increase of n(Fe)/n(S), while the concentrations of S2O32-, SO32- and SO42- change a little. For instance, the removal ratio of S2- reaches 92.70% as the n(Fe)/n(S) increases to 2:1, and then remains stable with more addition of ferrous compounds. However, the variation of sulfur concentration in aluminate solutions reacted with ferric compound exhibits differently (Table 2). With increasing the amount of ferric compounds, the S2- concentration decreases distinctly as well, but the 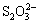 and
and 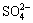 concentrations increase in some degree and the
concentrations increase in some degree and the 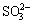 concentration reduces a little. It can be concluded that the iron compounds can remarkably remove the S2- but hardly eliminate
concentration reduces a little. It can be concluded that the iron compounds can remarkably remove the S2- but hardly eliminate 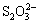 ,
, 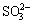 and
and 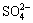 in sodium aluminate solutions. Furthermore, the S2- and
in sodium aluminate solutions. Furthermore, the S2- and 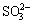 are easily oxidized by ferric compound to relatively stable
are easily oxidized by ferric compound to relatively stable 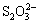 and
and 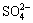 in alkaline solution, which leads to the enrichment of
in alkaline solution, which leads to the enrichment of 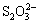 and
and 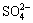 in aluminate solutions. At the same time, the iron concentration in the aluminate solutions was analyzed when the n(Fe)/n(S) varied from 0.5:1 to 2:1. The iron contents in the sulfur containing aluminate solutions reacted with ferric compound and ferrous compounds decreased from above 100 mg/L to 32.09 mg/L and 7.36 mg/L, respectively. The above results imply that the iron compounds cannot only remove S2- effectively but also decrease the iron content to a reasonable range in aluminate solutions.
in aluminate solutions. At the same time, the iron concentration in the aluminate solutions was analyzed when the n(Fe)/n(S) varied from 0.5:1 to 2:1. The iron contents in the sulfur containing aluminate solutions reacted with ferric compound and ferrous compounds decreased from above 100 mg/L to 32.09 mg/L and 7.36 mg/L, respectively. The above results imply that the iron compounds cannot only remove S2- effectively but also decrease the iron content to a reasonable range in aluminate solutions.
3.2 Analyses of desulfurization residues from reaction of iron compounds with sulfide
X-ray diffraction patterns of desulfurization residues from the reaction of ferrous compound and ferric compound with sulfide are displayed in Fig. 1 and Fig. 2 respectively in order to study the morphology of the residues and the reaction mechanism of iron compounds with sulfide. The ambiguous diffractograms in Fig. 1 imply that the crystal form of residues from ferrous compound for different retentions is imperfect. The residue with duration of 1 h exhibits peaks associating with the presence of maghemite and a broad background at 2θ=16.5° attributing to the poor crystallinity erdite (NaFeS2·2H2O). Accordingly, the XRD pattern of residue with duration of 10 h clearly shows well-defined peaks associated with maghemite, and the FeS2 (010) crystal face was identified according to the most intensity peak at 2θ=16.5°. Furthermore, there is no big difference between the peaks of FeS2 over a reaction time of 10 h and 20 h, but the maghemite phases disappear and the hematite could be identified with longer duration time. Therefore, it can be deduced that the desulfurization residues from the reaction of ferrous compounds with sulfide initially consist of erdite and maghemite, then the erdite and maghemite would transform to FeS2 and hematite, respectively.

Fig. 1 XRD patterns of residues from desulfurization by ferrous compound under different durations
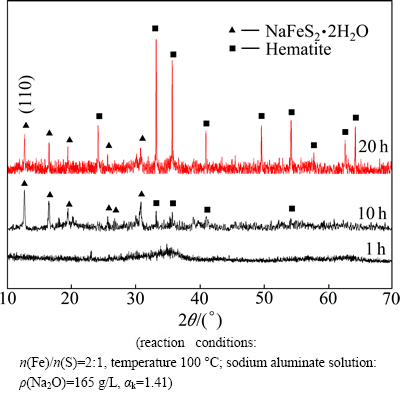
Fig. 2 XRD patterns of residues from desulfurization by ferric compound under different durations
In Fig. 2, the XRD patterns show that the desulfurization residue from the reaction of ferric compound with sulfide is amorphous after 1 h treatment. Over a period of 10 h, the hematite and erdite are identified as the main constituents. However, those low-intensity and broadening peaks indicate that the crystallization of residues is poor. The XRD patterns show that the more intense and well-defined peaks after 20 h treatment are attributed to the erdite and hematite because of the grain growth with the reaction time increasing. The above results demonstrate that the main phases of the residues from the reaction of ferric compound with sulfide are erdite and hematite.
The ambiguous diffractograms for the desulfurization residues suggest that other amorphous precipitates may exist except the above phases. The identification of the solid products over a reaction time of 20 h is complemented by X-ray photoelectron spectrometer (XPS) and the results are described in Figs. 3-6. The XPS spectra of S 2p and Fe 2p for the desulfurization residue from ferrous compound are shown in Figs. 3 and 4, respectively. The decomposition of the S 2p spectrum shows five major peaks after the data fitting, which indicates that various sulfur compounds may be presented. The peaks at 161.37, 163.28, 163.59, 168.35 and 169.59 eV are attributed to FeS, FeS2, Sn, 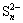 and
and 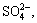 respectively [14]. However, the binding energies associated to FeS and FeS2 are greater than the values in reference because of the specific chemical environment. The XPS spectrum of Fe 2p is more complicated than the spectrum of S2p due to the peaks of Fe 2p1/2 and Fe 2p3/2. The binding energy value of Fe 2p3/2 is usually about 13 eV greater than that of Fe 2p1/2 and the studies often focus on the Fe 2p3/2 peaks. The values for the Fe 2p3/2 peak reported in this literature are 710.29, 710.81, 713.29, 717.04 and 720.17 eV. The peak at 710.29 eV is unequivocally attributed to Fe(Ⅱ). The peak at 710.81 eV could be attributed to Fe2O3 or Fe(OH)3. The peak at 713.29 eV corresponds to Fe2(SO4)3. The peaks at 717.04 and 720.17 eV may be associated with satellite peaks for an electron transition of the iron atom [15,16]. These results clearly show that an evidence of amorphous-type FeS, polymerized sulfur-iron compounds and Fe2(SO4)3 in the residue can be distinguished.
respectively [14]. However, the binding energies associated to FeS and FeS2 are greater than the values in reference because of the specific chemical environment. The XPS spectrum of Fe 2p is more complicated than the spectrum of S2p due to the peaks of Fe 2p1/2 and Fe 2p3/2. The binding energy value of Fe 2p3/2 is usually about 13 eV greater than that of Fe 2p1/2 and the studies often focus on the Fe 2p3/2 peaks. The values for the Fe 2p3/2 peak reported in this literature are 710.29, 710.81, 713.29, 717.04 and 720.17 eV. The peak at 710.29 eV is unequivocally attributed to Fe(Ⅱ). The peak at 710.81 eV could be attributed to Fe2O3 or Fe(OH)3. The peak at 713.29 eV corresponds to Fe2(SO4)3. The peaks at 717.04 and 720.17 eV may be associated with satellite peaks for an electron transition of the iron atom [15,16]. These results clearly show that an evidence of amorphous-type FeS, polymerized sulfur-iron compounds and Fe2(SO4)3 in the residue can be distinguished.
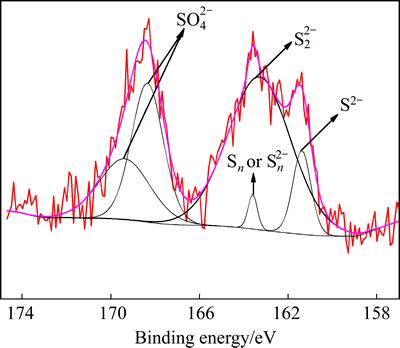
Fig. 3 XPS spectra of S 2p from desulfurization residue by ferrous compound
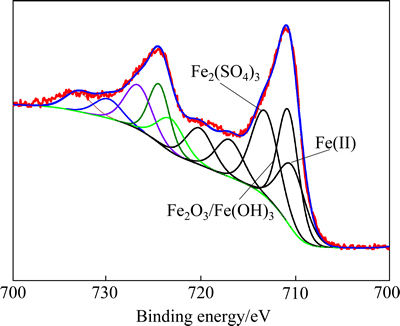
Fig. 4 XPS spectra of Fe 2p from desulfurization residue by ferrous compound
The XPS spectra of S 2p and Fe 2p for the residue from ferric compound are shown in Figs. 5 and 6, respectively. The binding energies of S 2p are 160.80, 161.97, 163.29 and 168.34 eV. Compared with Fig. 3, they are attributed to FeS, NaFeS2·2H2O, 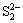 or
or 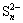 ,
, 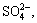 respectively. The values for the Fe 2p3/2 peaks in Fig. 6 are 710.03, 710.89, 713.86, 717.49 and 720.34 eV. Accordingly, the peak at 710.03 eV corresponds to Fe(Ⅱ), the peak at 710.89 eV is attributed to Fe2O3 or Fe(OH)3, the peak at 713.86 eV corresponds to Fe2(SO4)3, and the peaks at 717.49 eV and 720.34 eV are associated with satellite peaks for an electron transition of the iron atom. From the results obtained in Fig. 5 and Fig. 6, the existence of FeS, Fe(OH)3 and Fe2(SO4)3 can be verified. Nevertheless, the sedimentation of FeS2 or polymerized sulfur-iron compounds is uncertain.
respectively. The values for the Fe 2p3/2 peaks in Fig. 6 are 710.03, 710.89, 713.86, 717.49 and 720.34 eV. Accordingly, the peak at 710.03 eV corresponds to Fe(Ⅱ), the peak at 710.89 eV is attributed to Fe2O3 or Fe(OH)3, the peak at 713.86 eV corresponds to Fe2(SO4)3, and the peaks at 717.49 eV and 720.34 eV are associated with satellite peaks for an electron transition of the iron atom. From the results obtained in Fig. 5 and Fig. 6, the existence of FeS, Fe(OH)3 and Fe2(SO4)3 can be verified. Nevertheless, the sedimentation of FeS2 or polymerized sulfur-iron compounds is uncertain.

Fig. 5 XPS spectra of S2p from desulfurization residue by ferric compound
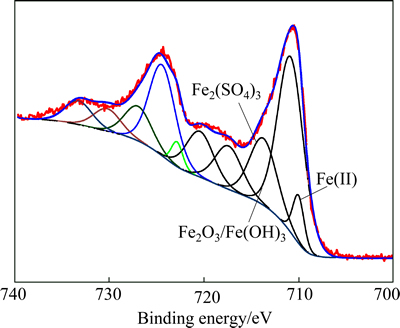
Fig. 6 XPS spectra of Fe 2p from desulfurization residue by ferric compound
In an attempt to further understand the constituents of the desulfurization residues, the samples were subjected to FTIR analyses, the results are shown in Fig. 7. For residue (Curve a) from reaction of ferrous compound with sulfide, characteristic bands of the hydroxy in water (3200-3550 cm-1, 1627.57 cm-1), 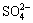 (1119.86 cm-1) and Fe-O2 (566.13 cm-1) can be identified. Furthermore, a number of absorption bands observed in the near infrared region (360-490 cm-1) at 453.46 cm-1 and 419.21 cm-1 would correspond to the elemental sulfur and
(1119.86 cm-1) and Fe-O2 (566.13 cm-1) can be identified. Furthermore, a number of absorption bands observed in the near infrared region (360-490 cm-1) at 453.46 cm-1 and 419.21 cm-1 would correspond to the elemental sulfur and 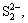 respectively. And the band at 378.58 cm-1 is assigned to S—Fe symmetric stretching vibration in spite of blue shifts. Therefore, the above results by XRD, XPS and FTIR indicate that there are FeS2, Fe2O3, amorphous-type phases FeS, element sulfur, polymerized sulfur-iron compounds and Fe2(SO4)3 in the solid product from the reaction of ferrous compound with sulfide.
respectively. And the band at 378.58 cm-1 is assigned to S—Fe symmetric stretching vibration in spite of blue shifts. Therefore, the above results by XRD, XPS and FTIR indicate that there are FeS2, Fe2O3, amorphous-type phases FeS, element sulfur, polymerized sulfur-iron compounds and Fe2(SO4)3 in the solid product from the reaction of ferrous compound with sulfide.
The FTIR spectra of desulfurization residue from the reaction of ferric compound with sulfide (Curve b) contain peaks from water (3200-3550 cm-1, 1627.57 cm-1),  (1587.21 cm-1), amorphous ferric hydroxide Fe-OH (1344.42 cm-1), Fe2O3 (542.24 cm-1and 469.14 cm-1) and
(1587.21 cm-1), amorphous ferric hydroxide Fe-OH (1344.42 cm-1), Fe2O3 (542.24 cm-1and 469.14 cm-1) and 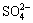 (1118.69 cm-1 and 997.85 cm-1) [17]. Beyond those, the band resulting from S—Fe symmetric stretching vibration is also found. But the characteristic bands of the elemental sulfur and S22- are absent. It is possible that the band of elemental sulfur may be over layered by the bands of Fe2O3. In conclusion, the residues from the reaction of ferric compound with sulfide consist of erdite, hematite, amorphous FeS and ferric hydroxide, polymerized sulfur-iron compounds and Fe2(SO4)3 without FeS2.
(1118.69 cm-1 and 997.85 cm-1) [17]. Beyond those, the band resulting from S—Fe symmetric stretching vibration is also found. But the characteristic bands of the elemental sulfur and S22- are absent. It is possible that the band of elemental sulfur may be over layered by the bands of Fe2O3. In conclusion, the residues from the reaction of ferric compound with sulfide consist of erdite, hematite, amorphous FeS and ferric hydroxide, polymerized sulfur-iron compounds and Fe2(SO4)3 without FeS2.
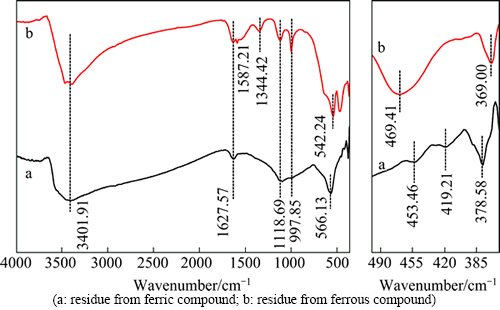
Fig. 7 FTIR spectra of residues from desulfurization
3.3 Reaction process of iron compounds with sulfide in sodium aluminate solutions
Based on the above discussion, it is concluded that the components of residues from the reaction of iron compounds with sulfide are roughly the same, and the major distinction is that the erdite from ferrous compounds at the reaction front subsequently converts to FeS2. Furthermore, the thermodynamic analysis of the reactions of iron compounds with sulfide in aluminate solutions was carried out. Iron compounds in aluminate solutions mainly exist in the form of Fe(OH)3, 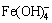 , Fe(OH)2 and
, Fe(OH)2 and 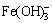 according to the φ-pH diagram for the Fe-H2O system at 100 °C [18]. Simultaneously, considering that the compounds of iron with
according to the φ-pH diagram for the Fe-H2O system at 100 °C [18]. Simultaneously, considering that the compounds of iron with 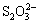 or
or 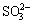 are unstable, we mainly studied the thermodynamics of the reactions of iron with sulfide and sulfate with the aid of the methods and data in Ref. [19], and the results are listed as
are unstable, we mainly studied the thermodynamics of the reactions of iron with sulfide and sulfate with the aid of the methods and data in Ref. [19], and the results are listed as
Fe(OH)3+(3/2)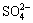 =(1/2)Fe2(SO4)3+3OH- (1)
=(1/2)Fe2(SO4)3+3OH- (1)
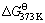 = 211.94 kJ/mol
= 211.94 kJ/mol
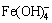 +(3/2)
+(3/2)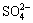 =(1/2)Fe2(SO4)3+4OH- (2)
=(1/2)Fe2(SO4)3+4OH- (2)
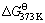 =178.42 kJ/mol
=178.42 kJ/mol
Fe(OH)2+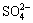 =FeSO4+2OH- (3)
=FeSO4+2OH- (3)
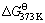 =95.62 kJ/mol
=95.62 kJ/mol
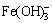 +
+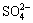 =FeSO4+3OH- (4)
=FeSO4+3OH- (4)
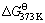 = 61.39 kJ/mol
= 61.39 kJ/mol
Fe(OH)3+2S2-+Na++2H2O=NaFeS2·2H2O+3OH- (5)
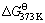 = 16.20 kJ/mol
= 16.20 kJ/mol
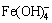 +2S2-+Na++2H2O=NaFeS2·2H2O+4OH- (6)
+2S2-+Na++2H2O=NaFeS2·2H2O+4OH- (6)
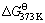 =-19.58 kJ/mol
=-19.58 kJ/mol
Fe(OH)2+S2-=FeS+2OH- (7)
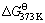 =-4.46 kJ/mol
=-4.46 kJ/mol
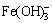 +S2-=FeS+3OH- (8)
+S2-=FeS+3OH- (8)
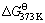 = -37.44 kJ/mol
= -37.44 kJ/mol
The standard Gibbs free energy changes of Reactions (1)-(4) at 100 °C are all positive. It is evident that ferric sulfate and ferrous sulfate are difficult to precipitate during the desulfurization. In contrast, the standard Gibbs free energy changes of reactions of iron compounds with sulfide are negative except Reaction (5). This suggests that iron compounds can remove sulfide to produce precipitates in aluminate solutions. Actually, the ferric compound can be reduced by sulfide to ferrous compound, and then the ferrous compound reacts with sulfide to form FeS, resulting in the amorphous FeS in the residues from ferric compound.
As for the ferrous compound, it either reacts with sulfide to form FeS or transforms to ferric compound due to its instability. So the erdite is also discovered in the earlier reaction stage. Nevertheless, the erdite is an intermediate product in pyrite syntheses and abundant ferrous compounds provide strong reduction environments [20]. It is anticipated that the erdite transforms into pyrite as the reaction time passes. The reactions in this process may be described as
Fe(OH)2+2NaFeS2·2H2O=FeS2+2FeS+2Na++2OH-+4H2O (9)
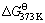 =-86.05 kJ/mol
=-86.05 kJ/mol
Fe(OH)3-+NaFeS2·2H2O=FeS2+2FeS+2Na++3OH-+4H2O (10)
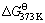 =-119.01 kJ/mol
=-119.01 kJ/mol
Furthermore, the unreacted iron compounds in liquors would transform to iron oxide and iron hydroxide and remain in the residues. Meanwhile, the element sulfur, polymerized sulfur-iron compounds and ferric sulfate are also found in the desulfurization residues. This may be attributed to the equilibrium of the sulfur species and the combination of iron and those sulfur compounds. Another relevant factor to be considered is that the element sulfur and polymerized sulfur-iron compounds are formed during the transformation of residues after desulfurization. In contrast, the ferric sulfate is hard to precipitate from the aluminate solutions. Therefore, it may be formed after the desulfurization because of the transformation or oxidization of residues.
4 Conclusions
1) Iron compounds can remarkably remove the S2- but cannot remove 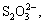
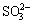 and
and 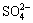 in sodium aluminate solutions. The ferric compound can oxidize sulfide to produce high valence state sulfur compounds which are difficult to eliminate from liquors.
in sodium aluminate solutions. The ferric compound can oxidize sulfide to produce high valence state sulfur compounds which are difficult to eliminate from liquors.
2) Initially, the main constituents of residues are erdite, hematite, amorphous ferrous sulfide and ferric hydroxide during the desulfurization, and then the erdite from ferrous compound sequentially converts to a sodium-free product FeS2. Finally, the element sulfur, polymerized sulfur-iron compounds and ferric sulfate are formed as the reaction is progressing.
References
[1] International Aluminum Institute. The aluminum industry’s sustainable development report [R]. London: The Swallow House Group of Companies, 2002.
[2] YIN Jian-guo, XIA Wen-tang, HAN Ming-rong. Resource utilization of high-sulfur bauxite of low-median grade in chongqing china [C]// Light Metals. Chongqing, 2011: 19-22.
[3] ZHANG Nian-bing, LI Zhi-ying, GUO Pei-li. Desulfurization mechanism and application of high sulfur bauxite [J]. Metalurgia International, 2013, 18(3): 29-32.
[4] ABIKENOVA G K,KOVAZLENKO V A, AMBARNIKOVA G A, IBRAGIMOV A T. Investigation of the effect and behavior of sulfur compounds on the technological cycle of alumina production [J]. Metallurgy of Non-ferrous Metals, 2008, 49(2): 91-96.
[5] LV Zhi-guo, ZHANG Yan-an, BAO Li, DOU Zhi-he, ZHAO Ai-chun, QU Hai-cui, NI Pei-yuan. Roasting pretreatment of high-sulfur bauxite and digestion performance of roasted ore [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(9): 1684-1689. ( in Chinese)
[6] HU Xiao-lian, CHEN Wen-mi. Desulfurization from sodium aluminate solution by wet oxidation [J]. Journal of Central South University: Science and Technology, 2011, 42(10): 2911-2916. (in Chinese)
[7] SONG Chao, PENG Zhi-hong, WEI Xin-xin, QI Tian-gui. The reaction behavior of pyrite in process of bayer digestion [J]. Nonferrous Metals Science and Engieering, 2011, 2(5): 1-5. (in Chinese)
[8] LI Xiao-bin, LI Chong-yang, QI Tian-gui, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Reaction behavior of pyrite during Bayer digestion at high temperature [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(3): 830-835. (in Chinese)
[9] KUZNETSOV S I, GRACHEV V V, TYURIN N G. Interaction of iron and sulfur in alkaline aluminate solutions [J]. Zh Prikl Khim, 1975, 48(4): 748-750.
[10] HE Qiang. Research of iron content of sulfur-rich bauxite in Bayer process after heat-exchange and filtration of green and pregnant liquors [J]. Light Metals, 2010(8): 12-16. (in Chinese)
[11] XIE Qiao-ling, CHEN Wen-mi. Effect of S2- on corrosion behavior of low alloy steel in sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3462-3469. (in Chinese)
[12] LI Xiao-bin, LI Chong-yang, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng, QI Tian-gui, TAN Jie. A method for the removal of sulfur and iron from sodium aluminate solutions: CN, 102976381A [P]. 2013-03-20. (in Chinese)
[13] CHEN Wen-mi, HU Qin. Research on the analysis of low-valence sulphion in sodium aluminate solution [J]. Light Metals, 2012(10): 17-20. (in Chinese)
[14] TONIAZZO V, MUSTIN C, PORTAL J M, HUMBERT B, BENOIT R, ERRE R. Elemental sulfur at the pyrite surfaces: Speciation and quantification [J]. Applied Surface Science, 1999, 143(1): 229-237.
[15] YAMASHITA T, HAYES P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials [J]. Applied Surface Science, 2008, 254(8): 2441-2449.
[16] DESCOSTES M, MERCIER F, THROMAT N, BEAUCAIRE C, GAUTIER S M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: Constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium [J]. Applied Surface Science, 2000, 165(10): 288-302.
[17] CALDEIRA C L, CIMINELLI V S T, DIAS A, OSSEO-ASARE K. Pyrite oxidation in alkaline solutions: nature of the product layer [J]. International Journal of Mineral Processing, 2003, 72(1): 373-386.
[18] LE H H, GHALI E. Interpretation of the E-pH diagrams of Fe-H2O system connected with the caustic embrittlement of steels [J]. Journal of Applied Electrochemistry, 1993, 23(1): 72-77.
[19] LIU Gui-hua, LI Xiao-bin, LI Yong-fang, PENG Zhi-hong. The linear relationship between complex inorganic compounds and thermodynamic data and its preliminary application [J]. Chinese Science Bulletin, 2000, 45(13): 1386-1392. (in Chinese)
[20] HONMA H, NAKATA M, KOBAYASHI K. Formation Condition of Erdite in System FeCO3-NaHS Solution at 150 °C [J]. Bulletin of Tokyo Gakugei University, 2003, 55(4): 39-44.
李小斌,李重洋,彭志宏,刘桂华,周秋生,齐天贵
中南大学 难冶有色金属资源高效利用国家工程实验室,长沙 410083
摘 要:对铝酸钠溶液中铁化合物与含硫组元间的反应行为进行研究。结果表明:铝酸钠溶液中铁化合物能显著脱除溶液中的S2-,对S2O32-、SO32-和SO42-等硫化合物则没有脱除效果。当Fe(Ⅲ)和Fe(Ⅱ)化合物加入量达到铁硫摩尔比为2:1时,100 °C下S2-的脱除率分别达到86.10%和92.70%。在Fe(Ⅲ)和Fe(Ⅱ)化合物的除硫过程中,均有水合硫代铁酸钠、赤铁矿、无定型硫化亚铁、聚合硫铁化合物和硫酸铁等物质生成,主要区别在于Fe(Ⅱ)化合物反应初期生成的水合硫代铁酸钠会继续转化为不含钠的二硫化亚铁。
关键词:高硫铝土矿;铝酸钠;铁化合物;脱硫;机理
(Edited by Yun-bin HE)
Foundation item: Project (51374239) supported by the National Natural Science Foundation of China
Corresponding author: Chong-yang LI; Tel/Fax: +86-731-88830453; E-mail: lichongyang@csu.edu.cn
DOI: 10.1016/S1003-6326(15)63643-3

