
Effect of mineral processing wastewater on flotation of sulfide minerals
CHEN Jian-ming(陈建明)1, 2, LIU Run-qing(刘润清)1, SUN Wei(孙 伟)1, QIU Guan-zhou(邱冠周)1
1. School of Resource Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Liuzhou China Tin Group Company, Limited, Liuzhou 545000, China
Received 4 May 2008; accepted 20 September 2008
Abstract: The effects of mineral processing wastewater on sulfide minerals were investigated by flotation, infrared spectrometry and electrochemistry test. The results show that lead-concentrate water can improve the flotation of galena, while the sulfur-concentrate water has negative effect on flotation of galena compared with distilled water. The flotation behavior of pyrite is contrary to that of galena in three kinds of water. Infrared spectra indicate that the residual collector in the lead-concentrate water is beneficial to the formation of lead xanthate on the surface of galena. Electrochemistry results indicate that electrochemistry reaction on galena surface has apparent change. The anode polarization is improved and cathode polarization is depressed.
Key words: mineral processing; sulfide mineral; recycled wastewater; flotation
1 Introduction
Water resource problem has become more and more important in the world because it is closely related to human life and environment. A lot of water has been consumed and wasted in mining to obtain metal resources. Wastewater discharged from mines takes 1/10 of the total amount of the industrial wastewater discharged in China[1]. The wastewater produced in mineral processing contains a lot of suspended solid particles, heavy metal ions, flotation reagents, organics and other pollutants etc, resulting in waste of water resource and environmental pollution[2-6]. Therefore, wastewater treatment and reuse have much academic and practical significance.
There are many reports about wastewater treatment and reuse in the past[7-13]. It was shown that pellucid wastewater after physical and chemical purification could be recycled successfully. The studies of XIE et al [14] and YUAN et al[15] indicated that purified and treated wastewater by activated carbon could be reused. Furthermore, the quality of Pb flotation could be improved by controlling activated carbon consumption. A lot of researches have been done in wastewater treatment and utilization of mineral processing, and
much achievement has been made. However, they only focused on the technology development and the study on mineral flotation mechanism was rarely carried out. Up to now, there are few reports about the mineral processing wastewater of different systems and sites. Wastewater is classified and disposed selectively, and then returns to the different floatation operations according to the effect of wastewater on mineral surface reaction. This method could enhance the efficiency, utilize the chemical component of wastewater, and decrease greatly the cost. It has been innovatively proposed to use the recycled water according to its characteristics.
In this work, the effects of mineral processing wastewater on flotation of galena and pyrite are investigated and the reaction mechanism between reagents and mineral is discussed.
2 Experimental
2.1 Materials
Galena and pyrite sample used in this study was from Pb/Zn mine of Fankou. These mineral samples were crushed to below 3 mm and removed to porcelain mill, then fraction of 0.147-0.045 mm was obtained by screening as flotation samples.
The wastewater sample was taken from production site directly. The wastewater was settled for some time firstly in order to remove precipitated solids and then upper liquor was taken as flotation experimental water sample based on the experiment requirements. Wastewater after treatment can represent wastewater sample of the site due to little solid particles and suspends. The analysis results of water are shown in Tables 1 and 2.
Table 1 Analysis results of wastewater of Fankou Mine

Table 2 Average concentration of residual reagents in mining wastewater

2.2 Flotation
Flotation was conducted in a 40 mL hitch groove flotation machine at an rotating speed of 1 600 r/min. In flotation process, 2.0 g mineral sample was added into a CQ50 type ultrasonic cleaner in order to remove the surface oxide, then the sample was transferred into the flotation cell for further processing. The water used in the process is recycled wastewater. The sequence of reagent addition was regulator, collector and frother. The conditioning times for collector and frother were 2 min and 1 min, respectively. The flotation time was 4 min.
2.3 Infrared spectrum examination
1.0 g sample was immersed in 25 mL collector solution, and mortar hand-ground for 15 min using an agate pestle, then settled for 30 min, filtrated, flushed 2-3 times using corresponding recycled water. After vacuum drying, the solid was obtained. Infrared spectrum examination was performed by reflect method in NEXUS-470 infrared spectrometer.
2.4 Electrochemistry test
A conventional three-electrode cell was used in experiments. A piece of platinum was severed as the counter electrode, and Ag/AgCl electrode as the reference electrode. The working electrode (galena) was prepared from pure crystal, which was from Fankou lead-zinc mine. In order to renew the working surface, the working electrode was gently polished with 600 grit silicon carbide papers and then rinsed with distilled water before every measurement.
Electrochemistry test was conducted by the Potentiostat/Galvanostat Model 273A system from EG&GPARC. Polarization curves were measured by M352 soft system.
3 Results and discussion
3.1 Effects of mineral processing wastewater on flotation of sulfide minerals
To evaluate the effect of recycling mineral processing wastewater on flotation of galena and pyrite, flotation experiments were conducted. The results are presented in Fig.1 and Fig.2.

Fig.1 Flotation of galena in distilled water and recycled wastewater (c(KBX)=1×10-4mol/L)

Fig.2 Flotation of pyrite in distilled water and recycled wastewater (c(KBX)=1×10-4mol/L)
Fig.1 shows that the recovery of galena decreases with the increase of pulp pH in the system of distilled water. When pH<8, the recovery is close to 90%. When pH increases from 8 to 12, the recovery of galena decreases from 90% to 80%. It is clear that two kinds of recycled wastewater have different effects on flotation of galena. Compared with distilled water, lead concentrate water can improve the flotation of galena, while the sulfur concentrate water has negative effect on flotation of galena.
It can be seen from Fig.2 that, compared with the recovery in distilled water, the pyrite flotation is improved in sulfur-concentrate water, but it is depressed in lead-concentrate water. This indicates that if lead-concentrate water is reused in the separation of galena and pyrite, it can enhance the separation efficiency. Therefore, in recycling of mineral processing water, the origin of wastewater must be taken into account.
3.2 Infrared spectra of sulfide minerals in mineral processing wastewater
The infrared spectra of galena in xanthate solution and in wastewater-xanthate solution are shown in Fig.3. The results indicate that the surfaces of galena in three water systems give similar adsorption peak. Absorption peaks of 2 955 cm-1 and 2 870 cm-1 contribute to the C—H asymmetry and symmetry stretching vibration. Peak at 1 466 cm-1 is derived from symmetry bend vibration of —CH3 or shear swing vibration of —CH2. Peak at 1 405 cm-1 is caused by —CH3 bend vibration, and peaks of 1 206 cm-1 and 1 029 cm-1 are stretching vibration absorption peaks of C—O—C and —C=S, respectively. It also can be seen from Fig.3 that surface absorption characteristic peak of galena is broader and stronger in mineral processing wastewater. The order of infrared absorption intensity of galena in three kinds of water systems is lead-concentrate water>distilled water>sulfur-concentrate water. This phenomenon is due to the fact that lead-concentrate water has residual collector, which is beneficial to the formation of lead xanthate on the surface of galena. This also indicates that collector is concerned with physical and chemical reaction of galena surface. This result is in good agreement with flotation result.

Fig.3 Infrared spectra of galena in three kinds of water system (c(KBX)=10-4mol/L)
Infrared spectra of pyrite interacted with three kinds of water are present in Fig.4. It can be seen that there are characteristic absorption peaks of methyl and methylene at 2 963 cm-1 and 2 880 cm-1. In addition, there appear characteristic absorption peaks at 1 290 cm-1 and 1 033 cm-1. They are caused by C=S and C—O—C stretching vibration of dixanthogen. According to XU et al[16] that C=S stretching vibration peak decreases from 1 049 cm-1 to 1 019 cm-1 and C—O—C stretching vibration increases to 1 240-1 290 cm-1 when xanthate is oxidized to dixanthogen. This results are in correspondence with the literature report and above flotation results. This can infer that infrared absorption intensity is in the order of sulfur-concentrate water>distilled water>lead- concentrate water.

Fig.4 Infrared spectra of pyrite in three kinds of water system (c(KBX)=10-4mol/L)
3.3 Corrosive electrochemistry of oxidization- reduction of galena in mineral processing wastewater
Fig.5 shows the polarization curves for galena electrode in different mining recycled wastewater with 0.1 mol/L KNO3. Electrochemistry parameters by the computer PARcal are listed in Table 3. Obviously, galena corrosive potential moves by about 50 mV negatively. The corrosive current densities in lead-concentrate water and sulfur-concentrate water are 0.973 and 1.421 μA/cm2, respectively. The results indicate that, compared with the reaction in distilled water, the electrochemistry reaction on galena surface in wastewater has apparent change. The anode polarization is improved and cathode polarization is depressed. This change has relation with residual collector in wastewater.
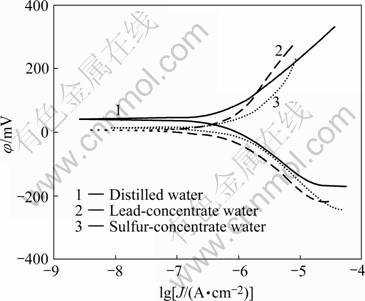
Fig.5 Polarization curves of galena electrode in mineral processing wastewater (0.1 mol/L KNO3, pH=12)
Table 3 Tafel parameters of galena electrode in 0.1 mol/L KNO3 solution at pH12

4 Conclusions
1) Lead-concentrate water can improve the flotation of galena, while the sulfur concentrate water has negative effect on flotation of galena compared with distilled water.
2) Infrared spectra indicate that surface absorption characteristic peak of galena is broadened and stronger in mineral processing wastewater. The order of infrared absorption intensity of galena in three kinds of water is lead-concentrate water>distilled water>sulfur- concentrate water. The residual collector in the lead- concentrate water is beneficial to the formation of lead xanthate on the surface of galena. For pyrite, the order of infrared absorption intensity is sulfur- concentrate water>distilled water>lead-concentrate water.
3) Electrochemistry results indicate that electrochemistry reaction on galena surface in wastewater has apparent change. The anode polarization is improved and cathode polarization is depressed. This change has relation with residual collector in wastewater.
References
[1] National Environment Protect Department. Environment statistics communique of nation (2004) [EB/OL]. http://www.zhb.gov.cn/ plan/hitj/qghjtjgb/t20050610-67563.htm.
[2] BROMAN P G. Water reuse at sulfide ore concentrators in Sweden: practice, experience and current development [C]// JONES M J. Complex Sulphide Ores. London: The Institution of Mining and Metallurgy, 1980: 28-39.
[3] FORSSBERG K S E, J?NSSON H R, P?ALSSON B I. Full scale test of process water reuse in a complex sulphide ore circuit [C]// FORSSBERG K S E. Flotation of Sulphide Minerals. Amsterdam, Netherlands: Elsevier, 1985: 197-217.
[4] RAO S R, FINCH J A. A review of water reuse in flotation [J]. Minerals Engineering, 1989, 2: 65-85.
[5] BASILIO C I, KARTIO I J, ROON R H. Lead activation of sphalerite during galena flotation [J]. Minerals Engineering, 1996, 9: 869-879.
[6] LEVAY G, SMART R ST C, SKINNER W M. The impact of water quality on flotation performance [J]. Journal of the South African Institute of Mining and Metallurgy, 2001, 101: 69-75.
[7] WU Zhao-qing, PENG Wen-sheng, YING Li-li. Study on treatment and reuse of ore-dressing wastewater for Chenzhou new-star preparation plant [J]. Hunan Nonferrous Metals, 1997, 13(4): 19-21. (in Chinese)
[8] LU Xiao-bing, ZHANG Jia-hui. Study of mineral processing process affected by recycle water [J]. Jiangxi Metallurgy, 2001, 21(2): 39-41. (in Chinese)
[9] LUO Hong-tao. Study on recycling water utilization in Huili zinc dressing plant [J]. Conservation and Utilization of Mineral Resources, 1999(4): 48-50. (in Chinese)
[10] WU Zhao-qing, YING Li-li, PENG Wen-sheng. Study on wastewater recovery in lead and zinc concentration plant [J]. Hunan Nonferrous Metals, 2003, 19(3): 8-10. (in Chinese)
[11] WANG Jian-bing, LIU Yun-jie, YANG Rong-yue. The experimental research and industrial practice on purification and recycling of a flotation plant wastewater [J]. Multipurpose Utilization of Mineral Resources, 2003(1): 14-17. (in Chinese)
[12] ZHAI Ping, LIU Ru-yi, ZHANG Zhi. The experimental research on purifying and reusing floatation wastewater [J]. Technology of Water Treatment, 2004, 30(4): 237-240.
[13] WEI Y H, ZHOU G Y, ROELF F S. Effects of recycled water on flotation of a complex sulphide ore [J]. Nonferrous Metals, 2006, 58(2): 82-85.
[14] XIE Guang-yan, SUN Shui-yu, NING Xun-an, LIU Ru-yi. Treatment and reuse of wastewater from a sulphide ore flotation plant [J]. Techniques and Equipment for Environmental Pollution Control, 2002, 3(2): 67-70. (in Chinese)
[15] YUAN Zeng-wei, ZHAO Yong-bin, DAI Wen-can, SUN Shui-yu, LIU Ru-yi. Purification of flotation wastewater and reuse [J]. Technology of Water Treatment, 2002, 28(4): 232-234. (in Chinese)
[16] XU Jing, SUN Wei, ZHANG Qin, LIU Hui, HU Yue-hua. Research on depression mechanism of pyrite and pyrrhotite by new organic depressant RC [J]. Mining and Metallurgical Engineering, 2003, 23(6): 29-32. (in Chinese)
Foundation item: Project(2006AA06Z120) supported by the Hi-tech Research and Development Program of China; Project(50874117) supported by the National Natural Science Foundation of China
Corresponding author: LIU Run-qing; Tel: +86-13875851194; E-mail: liurunqing@126.com
DOI: 10.1016/S1003-6326(08)60294-0
(Edited by YANG Bing)