
Influence of nitrogen gas on structure and properties of DLC films prepared by XeCl pulsed laser deposition
CHEN Yu-qiang(陈玉强)1,PENG Hong-yan(彭鸿雁)1, 3, ZHAO Li-xin(赵立新)2, LI Min-jun(李敏君)1
XIA Yi-ben(夏义本)3,WANG Lin-jun(王林军)3, XU Run(徐 闰)3, LIU Jian-min(刘建敏)3
1. Department of Physics, Mudanjiang Normal College, Mudanjiang 157012, China;
2. Department of Machinery and Electricity, Mudanjiang University, Mudanjiang 157011, China;
3. Department of Electronic Materials, Shanghai University, Shanghai 200072, China
Received 10 April 2006; accepted 25 April 2006
Abstract: Diamond-like carbon (DLC) films were prepared by PLD process using 308 nm(XeCl) laser beam with high power (500 W) and high frequency(300 Hz). The effects of nitrogen pressure on the structure and properties of the DLC films under such extremely high power and repetition rate were studied. The results indicate that the microstructures of the films are varied from amorphous carbon to graphitized carbon in long-order with the increase of N2 pressure, and the optical properties of the films are deteriorated as compared to that of DLC films without nitrogen.
Key words: DLC films; PLD; nitrogen pressure DLC films; PLD; nitrogen pressure
1 Introduction
In the past years, diamond-like carbon (DLC) films have been widely investigated because of their unique and superior properties such as extremely high hardness, good transparency in the IR region, excellent chemical stability. The combination of these virtues makes them very attractive for versatile applications, such as protective coatings of the mechanical tools and anti-reflection coatings for IR optics. Incorporation of nitrogen atoms in DLC and production of carbon nitride CN alloy have attracted much attention for its use in the preparation of n-type doping carbon.
Pulsed laser deposition is a suitable method for preparation of DLC films with large a sp3 and sp2 ratio, showing good mechanical and optical properties. The C:N films prepared by laser ablation of graphite in nitrogen atmosphere has been described to possess a great variety of properties of both fundamental and technological interest[1-4].
Most PLD experiments reported that the as-grown DLC films were essentially amorphous [5, 6]. In previous studies, we presented unique results that DLC films exhibited microstructures with small diamond crystals (a few nanometers in size) embedded in a sp2 bonded graphitic matrix deposited by using high power and high frequency XeCl excimer laser ablation of graphite at room temperature in vacuum and hydrogen ambient [7-9]. The effects of some parameters such as the laser power density, the repetition rate and the hydrogen ambient on the structures and properties of the DLC films under such extreme conditions were studied. In this paper, we investigate the effect of the nitrogen gas on the structure and properties of the films grown at high power and high frequency. The analyses were performed by Raman, XPS, FTIR spectrometer and UV-visible light spectrometer.
2 Experimental
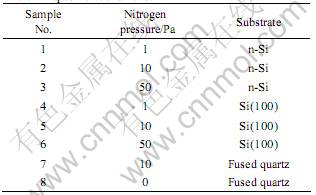
DLC films with the incorporation of nitrogen were deposited on highly doped n-Si, single crystalline silicon(100) and fused quartz substrates at room temperature by pulsed XeCl excimer laser (λ=308 nm) ablation of high-purity pyrolytic graphite targets at different nitrogen pressure. The deposition system had a high-vacuum stainless steel chamber that was evacuated down to 10-5 Pa prior to each deposition by a turbomolecular pump. The substrate was mounted parallel to the target at a distance of 30 mm, both the target and substrate holders can rotate about its main axis during the irradiation to make the films uniform. A novel XeCl laser beam with laser pulse energy up to 0.9 J was used for ablation, which was steered into the chamber using lenses and fused quartz window and focused on the target with the spot size less than 1 mm2 at an angle of 45? with the target normal. The pulsed laser was irradiated to the target with the average power of 500 W to give the laser power density of 1010 W/cm2 in the experiments. The pulse repetition rate of the laser was as considerably high as 300 Hz with 25 ns pulse duration. All of the substrates are ultrasonically cleaned in ethyl alcohol and acetone, nitrogen dried, then placed into the chamber and etched for 10 min in an 1 kV Ar glow discharge before deposition. The deposition conditions are listed in Table 1.
Table 1 Deposition conditions
The Raman measurements were carried out using Renishaw in Via Micro-Raman Spectroscopy System in the backscattering geometry using green radiation (λ=514.5 nm) from an argon-ion laser as an excitation source. The structure of the films was determined by FTIR(IFS66V) and XPS(VG-ESCALABMKⅡ). The UV-Vis transparency and the optical band gap of the films measurements were made on samples deposited on the fused quartz substrates using a SHIMADZU UV-3100 spectrophotometer.
3 Results and discussion
Fig.1 shows the UV-visible transparencies of the samples 7 and 8. It can be seen that the incorporation of nitrogen has a strong effect on the films’ optical properties, for the transparency of the film deposited in nitrogen ambient (Sample 7) decreases from 80% to about 40% as compared to that of the film deposited in vacuum (Sample 8), and the absorption edge shifts to longer wavelength (240 nm to 350 nm), indicating the absorb coefficient increases and the band gap decreases, i.e. graphitization occurs in the DLC films deposited in nitrogen ambient.
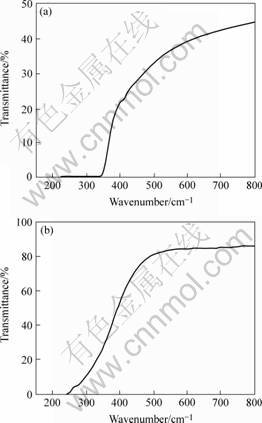
Fig.1 UV-visible spectra of samples 7(a) and 8(b)
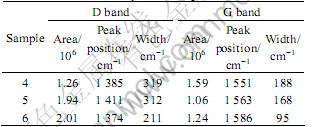
Fig.2 shows the Raman spectra of the samples 4, 5 and 6, the detailed information of both G band and D band in addition to the bandwidth of the peaks for each sample is listed in Table 2. The G band shifts to higher wave numbers, from 1 551 cm-1 to 1 586 cm-1, and meanwhile the bandwidth becomes narrower (1 Pa: 188 cm-1-50 Pa: 95 cm-1), which is accompanied by the gradual increasing of the density of the D band when the nitrogen pressure increases from 1 Pa to 50 Pa, indicating the development of long-range order in the DLC or disordering of the graphitic structure[1] .
In DLC Raman spectra the G band corresponds to graphite-like layer of sp2 micro domains in the films, while the D band is attributed to the bond angle disorder in the films, induced by the linking with sp3 carbon atoms, the finite crystalline size of sp2 micro domains and the substitutional N atoms. The increased ID suggests the disordering progressive due to the increased incorporation of N atoms in the films, and meanwhile the G band shifts to the position of graphite with narrowing the bandwidth, meaning graphitization in the films, which can be partly explained by the increased collision cooling of the ablation plume by the heavier nitrogen molecules leading to graphitized films.
FTIR absorption spectra are usually used to determine the structure of the C:N films. Generally there are three absorption bands in the CN films, which are C—N stretching modes from 1 212 to 1 265 cm-1, C ≡≡ N triple stretching modes from 2 065
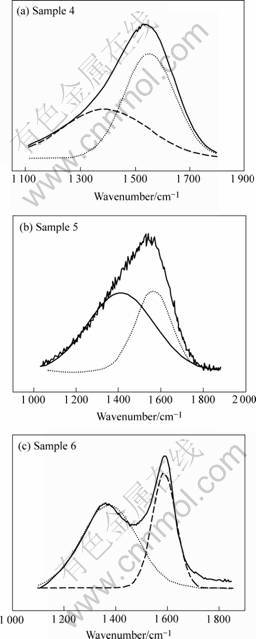
Fig.2 Raman spectra of samples 4, 5, 6
Table 2 Data of Raman spectra in Fig.2
to 2 260 cm-1, and C == N double mode at approximately 2 200 cm-1 [10].
Fig.3 shows the FTIR absorption spectra of the samples 4, 5 and 6. In addition to a weak absorption at 2 220 cm-1, the films exhibit two intensive peaks at approximately 1 550 cm-1 and 1 260 cm-1. With increasing nitrogen pressure, the intensities of both these peaks are increased notably and the 1 260 cm-1 peak increases slightly. The results suggest that the amount of nitrogen atoms in the DLC films increases with the nitrogen pressure increasing, which is associated with carbon atoms with the chemical bonds of C == N, preferentially. Such results are consistent with the conclusion of Raman spectra.
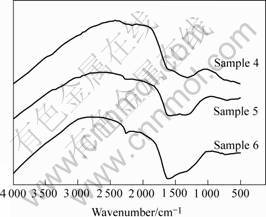
Fig.3 FTIR absorption spectra of films deposited in different N2 pressures: Sample 4, 1 Pa; Sample 5, 10 Pa; Sample 6, 50 Pa
XPS analyses are usually used to determine the nitrogen concentration and bonds condition of the films. Fig.4 shows the XPS spectrum of the film of sample 5. A weak O1s(533 eV) peak is detected in addition to the predominant C1s(285.28 eV) and N1s(399 eV) peaks. The existence of O1s should result from the absorbing of the oxygen or the moisture in the atmosphere. The atomic fraction of nitrogen [N]/([N]+[C]) in the films can be determined from the XPS:
[N]/([N]+[C])=(AN/0.42)/(AC/0.25+AN/0.42)
where AN and AC are the areas under the N1s and C1s peaks, and the constants 0.42 and 0.25 are the sensitivity factors of nitrogen and carbon respectively [11]. The measurement of N concentration using this method is 5%,13%,32% for the samples 4, 5 and 6, respectively. It was mentioned [10] that N does act as an effective n-type dopant below and up to a threshold of 1%, at higher concentration of N, carbon nitride CN films are formed. According to this comment, CN films are formed in our case.
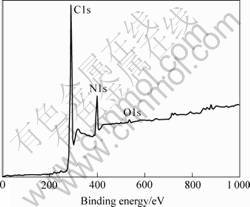
Fig.4 XPS spectrum of sample 5
Fig.5 shows the C1s peaks and N1s peaks of the samples 4, 5 and 6, respectively. Some information about the film chemical structure could be derived from the spectra. In Fig.5(a), the C1s peak position locates constantly at about 284 eV for the three samples. In turn, the width at half maximum increases from 2.4 eV to 4.0 eV with the nitrogen pressure increasing from 1 Pa to 50 Pa, and meanwhile asymmetric increases towards the higher binding energy side, which indicates the ratio of bonding configurations with high energy enhances
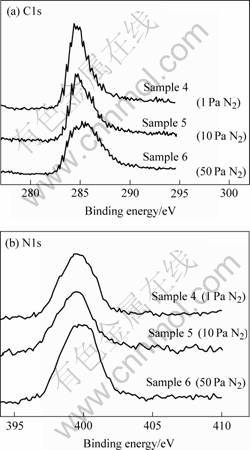
Fig.5 C1s(a) and N1s(b) peaks of samples 4, 5 and 6
with the nitrogen pressure increasing. The conclusion agrees well with the FTIR results. The deconvoluted peaks best fits to N1s envelopes resulted into two Gaussian peaks located at binding energies of (398.4±0.2) eV and (399.6±0.3) eV, which is related to
C—N or C ≡≡ N, C == N bonds.
4 Conclusions
The nitrogen content in the films increases with the nitrogen pressure increasing, which induces long-range order in the DLC films and graphitization as well as the formation of C ≡≡ N and C == N at the expense of C—N. The optical properties get worse with the increase of the N incorporation in the films.
References
[1] PAPAKONSTANTINOU P, ZEZE D A, KLINI A, MCLAUGHLLIN J. Chemical bonding and nanomechanical studies of carbon nitride films synthesized by reactive pulsed laser deposition [J]. Diamond and Related Materials, 2001, 10: 1109-1114.
[2] ALEXANDROU I, ZERGIOTI I, AMARATUNGA G A J, HEALY M J F, KIELY C J, HATTO P, VELEGRAKIS M, FOTAKIS C. A new reactive pulsed laser ablation technique for the deposition of hard carbon and carbon-nitride thin films [J]. Materials Letters, 1999, 39: 97-102.
[3] TSUYOSHI Y, TAKASHI N, KUNIHITO N. The role of hydrogen and oxygen gas in the growth of carbon thin films by pulsed laser deposition [J]. Diamond and Related Materials, 2000, 9: 689-692.
[4] SHARIF M M, TETSUO S, TAKASHI J, MASAYOSHI U. Diamond-like carbon by pulsed laser deposition from a camphoric carbon target: effect of phosphorus incorporation [J]. Diamond and Related Materials, 2001, 10: 1839-1842.
[5] MARTELLUCCI S, MESSINA G, RICHETTA M, SATANGELO S, SPENA A, TEBANO A, TUCCIARONE A. Pulsed laser deposition and laser machining of diamond-like carbon films [J]. SPIE, 1998, 3423: 302-308.
[6] CAPANO M A, MCDEVITT N T, SINGH R K, QIAN F. Characterization of amorphous carbon thin films [J]. J Vac Sci Technol, 1996, A14(2): 431-435.
[7] PENG H Y, JIN Z S, LI J J, SHEN H, ZHAO L X, SHEN J J. Structure and properties of diamond-like film prepared by excime laser with high power and high frequency [J]. Chem J of Chinese Universities, 2003, 24(11): 2048-2050. (in Chinese)
[8] PENG H Y, ZHAO L X, JIN Z S, CHEN B L, ZHANG B, ZHOU C S, ZHANG M H, YANG X H. Microstructures and properties of the DLC films pre pared by PLD process using high power high frequency 308nm laser beam [J]. Mater Sci Forum, 2005, 475-479: 3631-3634.
[9] PENG H Y, ZHOU C S, ZHAO L X, JIN Z S, ZHANG B, CHEN B L, CHEN Y Q, LI M J. Effect of the laser power density on the properties and structures of the diamond-like carbon films deposited by pulsed laser ablation of graphite [J]. Acta Physica Sinica, 2005, 54(9): 4294-4299.
[10] EBIHARA K, NAKAMIYQ T, OHSHIMA T, IKEGAMI T, AOQUI S. Influence of ambient gas on diamond-like carbon films prepared by KrF pulsed laser deposition [J]. Diamond and Related Materials, 2001,10: 900-904.
[11] ONG C W, ZHAO X A, TSANG Y C, CHOY C L, CHAN P W. Effects of substrate temperature on the structure and properties of reactive pulsed laser deposited CNx films [J]. Thin Solid Films, 1996, 280: 1-4.
(Edited by YUAN Sai-qian)
Corresponding author: PENG Hong-yan; Tel: +86-453-6511275; Fax: +86-453-6511275; E-mail: mdjphy@163.com