
Microstructure and properties of Cu-0.6Cr alloy after vacuum continuous casting
TSAI De-chang(蔡德昌)1, HWANG Weng-sing(黄文星)1, CHEN Siang-yong(陈向永)2,
KANG Jin-sing(康进兴)2, JIANG Cheng-xue(蒋承学)2
1. Department of Materials Science and Engineering, National Cheng Kung University, Tainan 70101, China;
2. Metal Industries Research & Development Centre, Kaohsiung 81160, China
Received 15 July 2007; accepted 10 September 2007
Abstract: To promote the solution of chromium in copper and the purity of copper-chromium (Cu-Cr) alloy, vacuum continuous casting (VCC) process was employed to fabricate the Cu-Cr alloy required for electrode material. Cu-0.6Cr (containing 0.6%(mass fraction) chromium) alloy bar with a diameter of 12 mm was cast with a constant rate of 250 mm/min. The microstructure, mechanical properties, and physical properties were discussed. After solid solution strengthening and aging treatments, Cu-0.6Cr alloy fabricated by VCC process has higher tensile strength (σ≥314 MPa), elongation rate (δ≥34%), conductivity (≥80.5% IACS), and hardness (80 HRB) compared to the as-cast alloy.
Key words: Cu-Cr alloy; vacuum continuous casting; solid solution treatment; aging treatment; conductivity
1 Introduction
Cu-Cr alloy series are often used as electrode material for the welding of resistors and widely applied for spot welding material. Electrode is a key component that determines the quality and lifespan of the electric resistance welding[1]. Consequently, looking for a better electrode, traditional copper alloy is replaced by a heat and wear resistant copper-chromium (Cu-Cr) alloy to improve the number and performance of the spot welding. After heat treatment[2-3], Cu-Cr alloy (containing 0.6%-1.2%(mass fraction) chromium) has good mechanical properties and conductivity. Therefore, it can be used as conductive material such as connector, switch, lead frame, electrical conductor for electric cars and trains, direction indicator of electric tools, large-scale high-speed turbine generator rotor conductor and ring collector that require high conductivity and high tensile strength. In particular, Cu-Cr alloy has great potential as a high-power and vacuum-high-pressure switch. It can also be used as wear resistant material such as bearing, carriage, screw bolt, and high tensile-strength cable.
Cu-Cr alloy is often melted in vacuum[4] because chromium is easy to oxidize and absorb gas. Also, the solubility of chromium in copper is very low (only about 0.6%-0.7%) due to the high melting point of chromium. For these reasons, it is difficult to produce Cu-Cr alloys. Thus, it is very desirable to combine the vacuum furnace and continuous caster to integrate melting and continuous casting into one process[5]. The advantages of the apparatus are good quality and high cleanliness because it combines the benefits of vacuum furnace and continuous caster, and symmetrical shape as well as homogeneous structure of the cast due to the vertical continuous casting process[6]. It can drastically reduce the contamination of the alloy and improve its yield. It is anticipated that the incorporation of various melting techniques with vacuum will become a very important manufacturing process for a wide variety of alloys in the future.
2 Experimental
The experiments conducted in this study used Cu-Cr alloy and vacuum continuous casting equipment as shown in Fig.1. Pure Cu and mother alloy of Cu-Cr were placed in the crucible and melted under nitrogen gas atmosphere. A mandrel was used as the withdrawal facility to manufacture a 12 mm-diameter Cu-0.6Cr alloy cylindrical bar. The cast bar was cut into several small pieces. The ingots were then subjected to various solid solution treatment (960 ℃, 1.5 h) and aging treatment (480 ℃, 1, 4, 6, 8, 10 h). After the heat treatments, samples with 2-3 cm in length were then ground, polished, and etched with a solution (2 g K2Cr2O4+8 mL H2SO4+4 mL HCl+100 mL H2O) for 3 s. Microstructures of the samples were observed by optical microscope (OM). A Rockwell hardness tester and specific conductivity meter were also used to measure the hardness and electrical conductivity.

Fig.1 Vertical vacuum continuous casting equipment
3 Results and discussion
Traditional manufacturing methods for Cu-Cr alloy[7] such as mechanical alloying method[8], laser surface alloying method[9] are not suitable for this purpose. Therefore, the vacuum continuous casting process is used to fabricate the Cu-Cr alloy. Fig.2 shows the microstructures of the horizontal and vertical sections of the Cu-0.6Cr cast bar fabricated by the vertical vacuum continuous casting process [10] at a constant casting rate of 250 mm/min. The volume of cooling water is 8 L/min. After grinding, polishing, and etching, the horizontal cross section is shown on the left and vertical section on the right. The metallograph clearly indicates that the grains structure is columnar, which is a more ideal grain structure. Fig.3 shows the OM micrograph of the vertical section of the Cu-0.6Cr cast bar fabricated by the vacuum continuous casting process and a columnar grain structure is also found.
MA et al[11] indicated that when melting Cu-Cr alloy, the evaporation loss of Cr easily occurred due to the high melting point of Cr and the recovery rate of Cr was then reduced rather significantly. In this study, a quantitative analysis of the chemical composition of the Cu-Cr alloy cast bar was conducted and the results are shown in Table 1. In the experiments, 0.6% of Cr is added. When it is compared with the content of Cr in Table 1, the recovery rate of Cr is closed to 94%. This is much higher than that fabricated by traditional Cu-Cr alloying methods, where the recovery rates are 80%- 90%.

Fig.2 Microstructures of horizontal and vertical sections of Cu-0.6Cr cast bar fabricated by vacuum continuous casting process

Fig.3 Metallograph of vertical section of Cu-0.6Cr cast bar fabricated by vacuum continuous casting process
Table 1 Chemical composition of copper-chromium alloy (mass fraction, %)

The strengthening of Cu-Cr alloy mainly results from solid solution strengthening and precipitation strengthening mechanisms[12-14]. Solid solution strengthening improves the strength of the Cu-Cr alloy by increasing the solution content of Cr. Precipitation strengthening makes use of aging treatment to precipitate Cr particles from the over-saturated Cu-Cr alloy via either natural aging or artificial aging treatment. The purpose of precipitation strengthening is achieved by producing a uniform distribution of Cr particles. This study used solid solution treatment (960 ℃, 1.5 h) and various aging treatments (480 ℃, 1, 4, 6, 8, 10 h) to improve the mechanical and physical properties of Cu-0.6Cr alloy cast bar. Firstly, solid solution and quenching treatment make the Cu-Cr alloy to reach a over-saturated state. After aging treatment at constant temperature, Cr particles are precipitated from the Cu-Cr alloy. Fig.4 shows the microstructure observed after solid solution treatment and the aging treatment under 480 ℃ for 4 h. It shows that the Cr particles are precipitated and distributed evenly.
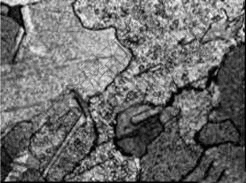
Fig.4 Micrograph after solid solution and aging treatment under 480 ℃ for 4 h
Figs.5 and 6 illustrate the relationships between hardness and electrical conductivity with respect to holding time of the aging treatment. As shown in Fig.5, the hardness increases with the increase of holding time during aging in the beginning. When the holding time reaches 6 h, the maximum hardness is obtained. After 6 h, the hardness decreases with the increase of holding time. This is mainly because of the increase in the size of the precipitates. Reducing the distance among the precipitates results in an increase in hardness for the short aging time. When the holding time reaches 6 h, the precipitates have the optimal distribution and maximum resistance to the dislocation movement. When aging time exceeds 6 h, over-aging phenomenon is observed and precipitates begin to coarsen. It is because the precipitates try to decrease the surface area to reduce the surface energy. Large precipitates grow larger and small precipitates reduce in size and finally disappear. Eventually, it results in larger size and smaller number of the precipitates as well as wider distance among the precipitates. Then, lower hardness is observed because of the lower resistance to the dislocation movement. In terms of electrical conductivity, it increases with the increase in the holding time of aging as shown in Fig.6. The electrical conductivity increases mainly because the over-saturated Cr precipitates effectively in the solution to reduce lattice distortion and the scattering ratio of electrons is decreased. Therefore, the electrical conductivity is improved[15-16].

Fig.5 Relationship of hardness and aging time after solid solution and aging treatment
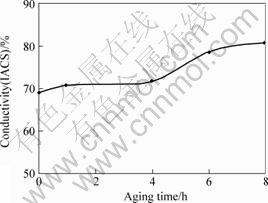
Fig.6 Relationship of electrical conductivity and aging time after solid solution and aging treatment
4 Conclusions
1) By employing the vacuum continuous casting process, higher Cr recovery rate and Cu-Cr purity are achieved.
2) After solid solution treatment and aging treatment, the mechanical strength and electrical conductivity of Cu-Cr alloy are improved.
References
[1] ZHANG Jing, YU Wei-yuan, LU Wen-jiang. Study on microstructure and mechanical proper ties of vacuum brazing joints of copper[J]. Welding Technology, 2006, 35(6): 17-19.
[2] GAO Hua-wei. Effect of heat treatment on hardness and impact toughness of polynary copper alloy[J]. Physics Examination and Testing, 2007, 25(1): 17-19.
[3] XIAO Dai-hong, CHEN Kang-hua, SONG Min. Effect of heat treatment on microstructure and properties of Al-5.3Cu-0.8Mg-0.5 Mn-0.6Ag alloy[J]. Material & Heat Treatment, 2007, 36(8): 52-57.
[4] LI Hua-qing, XIE Shui-sheng, MI Xu-jun, LI Yan-feng. Defects analyzing and prevention of Cu-Cr-Zr alloy ingots[J]. Foundry Technology, 2006, 27(11): 1205-1209.
[5] WEI Jun-wei, WANG Xin-zheng. Analysis and preventive measures of common defects of copper alloy extruded products[J]. Sichuan Nonferrous Metals, 2006, (4): 30-33. (in Chinese)
[6] MAE Y, IWAI R. Development of the vacuum melting pressurized upward continuous casting process[J]. Special Melting and Processing Technologies, 1990, 223-231.
[7] MA Feng-cang, NI Feng, YANG Di-xin. The present situation of preparation methods of Cu-Cr alloy materials[J]. Material Development and Application, 2002, 17(3): 35-38.
[8] MORRIS D G, MORRIS M A. Rapid solidification and mechanical alloying techniques applied to Cu-Cr alloys[J]. Material Science and Engineering A, 1988, 40(3): 201-221.
[9] HIROSE A, KOBAYASHI K F. Surface alloying of copper with chromium by CO2 laser[J]. Material Science and Engineering A, 1994, 46(3): 174-199.
[10] OZBERK E, GUTHIRE R I L. Application of vacuum refining in copper production[J]. Materials Science and Technology, 1985(1): 12-18.
[11] MA Feng-cang, NI Feng, YANG Dixin. Vacuum melting of high chromium Cu-Cr alloy[J]. Material Development and Application, 2003, 18(1): 8-11.
[12] HUANG Xiong-hui, Heat treatment of copper-chromium-zirconium electrode alloy[J]. China Academic Journal, 1986, 7(6): 16-21.
[13] SHEN Ding-jie, XIONG Wei-hao, YANG Qing-qing, YANG Zhen, WANG Rong-hui. Effect of heat treatment on mechanical properties and microstructures of 925Ag alloys of Ag-Cu-Zn (-Sn) system[J]. Materials Review, 2007, 21(8): 436-438.
[14] LI Qiang, CHEN Jing-chao, SUN Jia-lin. The meta-stable structure of aging Cu-Cr alloy[J]. Rare Metals and Cemented Carbides, 2006, 34(4): 1-5. (in Chinese)
[15] ZHANG Gou-feng, LI Zhi-min, WANG Er-de, LIANG Guo-xian. Structure and properties of the mechanically alloyed Cu-5Cr alloy[J]. Powder Metallurgy Technology, 1996, 14 (3): 175-180. (in Chinese)
[16] XIE Chun-sheng, ZHAI Qi-ming, XU Wen-qing, WANG Ji-heng. Study and application development of strengthening theory of copper alloy with high strength and high conductivity[J]. Heat Treatment of Metals, 2007, 32(1): 12-20.
(Edited by ZHAO Jun)
Corresponding author: HWANG Weng-sing; Tel: +886-6-234-4393; E-mail: wshwang@mail.ncku.edu.tw