J. Cent. South Univ. (2012) 19: 892-896
DOI: 10.1007/s11771-012-1089-z
Simulation calculation of solubility of
insoluble compound MmAa in complex system
YANG Tian-zu(杨天足), ZHANG Du-chao(张杜超), WU Jiang-hua(吴江华)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: A simulation calculation model for the solubility of insoluble compound MmAa in complex system was established. According to coordination equilibrium principle, relevant dissociation reaction, complexation reaction, self-complexation reaction and protonation reaction during insoluble compound dissolving were considered and then the mass balance equations about solubility calculation were obtained. In the case analysis, the solubility of silver chloride in ammonia system was obtained by simulation calculation, and curved surface charts of thermodynamic equilibrium about the total concentration of silver ions, pH and concentration of ammonia ions were drawn correspondingly. The results show that under the conditions of room temperature and 6 mol/L ammonia concentration, the calculated solubility value of silver chloride (34 g/L) is close to the actual value (31 g/L), demonstrating that this model is suitable for solubility calculation of insoluble compound MmAa in the complex system.
Key words: complex system; insoluble compound; solubility; calculation model
1 Introduction
In the modern hydrometallurgical processes and some chemical processes, appropriate ligands are often used to leach insoluble compound MmAa, for example, the hydrometallurgical process of copper/lead anode slime [1], and silver chloride leaching by ammonia or sodium sulfite [2-3]. Insoluble compound-complex system is a very important and complicated reaction system [4-6].
Therefore, using complexation principle to study the effects of complex ligands and hydrogen ion concentration on the solubility of insoluble compounds has a great theoretical and practical significance [7]. However, there are no comprehensive models for solubility calculation. And models are available only for one specific system. According to the established solubility calculation model, and taking advantage of Maple and Matlab mathematical software [8-10] in the calculation and mapping, the solubility of insoluble compounds was calculated and correspondingly curved surface charts about metal concentration-ligand concentration-hydrogen ion concentration were drawn.
The model not only can be used to analyze the concentration of various ions and ligands in the insoluble compound-complex system, but also can be used to predict the influences of the selected ligands on the solubility of insoluble materials. Therefore, the model is helpful for complex agent selection and recovery improvement of complicated materials [11]. For example, the sodium glutamate system was selected to leach low grade zinc/copper oxide ore based on the thermodynamic equilibrium analysis by the above model [12-13]. According to this model, an instance about silver chloride leaching by ammonia was analyzed to verify the reliability of this model.
2 Equilibrium equations of complex reaction
The insoluble compound MmAa and the ligand L react to form complexes into solution, and the main reactions are as follows [7]: dissociation reaction of MmAa, complexation reactions of the metal ion M and the ligand L or OH, self-complexation reaction of M and A, protonation reaction of A or L and H, and moreover H2O dissociation reaction.
2.1 Dissociation reaction of MmAa
The insoluble compound MmAa dissolves into aqueous solution according to the following reaction:
MmAa mM+aA (I)
mM+aA (I)
Corresponding solubility product constant is
Ksp=c(M)m·c(A)a (1)
Since A comes from the dissolution of MmAa, there is a relationship as follows:
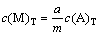 (2)
(2)
2.2 Complexation reaction of metal ion M and ligand L
The equilibrium equation of the complexation reaction of M and L is
M+iL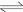 MLi (i=1, 2, …, x) (II)
MLi (i=1, 2, …, x) (II)
2.3 Complexation reaction of metal ion M and OH
The equilibrium equation of the complexation reaction of M and OH is
M+iOH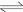 M(OH)i (i=1, 2, …, y) (III)
M(OH)i (i=1, 2, …, y) (III)
2.4 Self-complexation reaction of M and A
The possible self-complexation reaction of M and A is
M+iA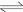 MAi (i=1, 2, …, z) (IV)
MAi (i=1, 2, …, z) (IV)
2.5 Protonation reaction of A and H
If A is a weak acid anion, then the protonation reaction may occur between A and H:
A+iH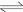 HiA (i=1, 2, …, n) (V)
HiA (i=1, 2, …, n) (V)
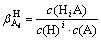
2.6 Protonation reaction of ligand L and H
The balance equation of the protonation reaction between ligand L and H is
L+iH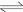 HiL (i=1, 2, …, m) (VI)
HiL (i=1, 2, …, m) (VI)
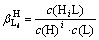
2.7 Other reactions
The equilibrium equation of H2O dissociation reaction is
H2O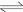 H+OH (VII)
H+OH (VII)
KW=c(H)·c(OH)
3 Establishment of mathematical model
According to mass balance principle and the above equilibrium reactions (I)-(VII), the mass balance equations are obtained as follows:
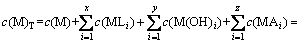
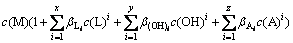 (3)
(3)

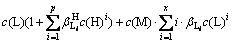 (4)
(4)

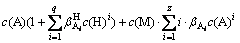 (5)
(5)
There are seven variables of c(M)T, c(M), c(L)T, c(L), c(A)T, c(A) and pH in the Eqs. (1)-(5). c(M)T, c(L)T and c(A)T are respectively the total ions concentrations of M, L and A; c(M), c(L) and c(A) are the free ions concentrations. Once c(L)T and pH are fixed, the other variables can be calculated according to these five equations.
In Eqs. (1)-(5), i, m, a, 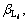
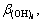
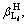
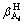 and Ksp are constants, therefore these equations can be simplified for convenience. The specific simplification steps are as follows:
and Ksp are constants, therefore these equations can be simplified for convenience. The specific simplification steps are as follows:
According to Eq. (1), Eq. (6) can be obtained:
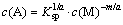 (6)
(6)
According to Eq. (4), Eq. (7) can be obtained:
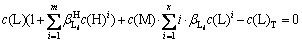 (7)
(7)
Combining Eqs .(2), (3), (5) and (6), Eqs. (8) can be obtained:
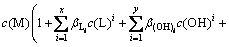

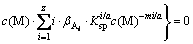 (8)
(8)
Now, the calculating idea should be discussed. There are four variables of c(M), c(L)T, c(L) and pH in Eqs. (7) and (8), where c(L)T and pH are fixed. Combining these two equations, c(L) and c(M) can be obtained according to the solved function from Maple software. Then c(A) can be gained by putting c(M) into Eq. (6), and c(M)T can be calculated by putting c(M), c(A) and c(L) into Eq. (3).
In fact, the self-complexation reaction of M and A rarely occurs in aqueous solution. If ignoring the self-complexation reaction of M and A, Eqs. (7) and (8) can be simplified as follows:
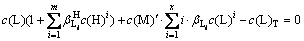 (9)
(9)
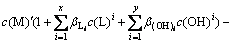
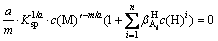 (10)
(10)
4 Case analysis
There are several ligands to leach silver from the refractory materials such as ammonia, sodium sulfite, thiourea and ammonium thiocyanate [14-15]. Especially, silver chloride leaching by ammonia is widely used in practice. Therefore, it was selected as an example to verify this mathematical model. The possible complexation reactions and corresponding cumulative equilibrium constants during silver chloride leaching [16-17] are shown in Table 1.
Table 1 Possible complexation reactions and corresponding cumulative equilibrium constants during silver chloride leaching by ammonia
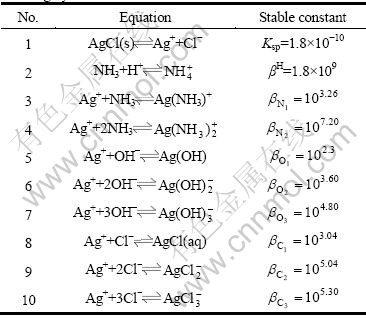
According to the principle of model construction, the mass equilibrium of the total concentration of Ag (c(Ag)T), the total concentration of ammonium ion (c(NH3)T), and the total concentration of Cl (c(Cl)T) in aqueous solution is obtained, and meanwhile considering the self- complexation reaction of Ag+ and Cl-, the mathematical models shown in Eqs. (11)-(13) are established by putting the cumulative equilibrium constants and solubility product constants listed in Table 1 into Eqs. (7) and (8):
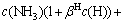
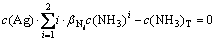 (11)
(11)
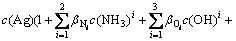

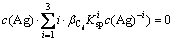 (12)
(12)
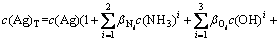
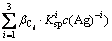 (13)
(13)
There are five variables of c(NH3), c(Ag), c(NH3)T, c(Ag)T and pH in Eqs. (11)-(13). In actual industry, the total concentration of ammonia ions is known (namely c(NH3)T is known), and pH is controlled as a constant. Therefore, the values of c(NH3), c(Ag) and c(Ag)T can be calculated through Maple software when the values of pH and c(NH3)T are given.
4.1 Effect of pH on formation distribution of ammonia in aqueous solution
In aqueous solution, the protonation reaction of ammonia ion and H+ occurs as described in equation (VI). The specific reaction equation and corresponding protonation equilibrium constant are shown in Table 1. c(NH3)T in the aqueous solutions can be expressed as follows:
c(NH3)T=c(NH3)+ (14)
(14)
And the contents of 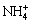 and NH3 out of total NH3 (namely Φ0 and Φ1) are
and NH3 out of total NH3 (namely Φ0 and Φ1) are
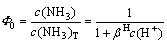 (15)
(15)
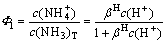 (16)
(16)
Putting the protonation constant βH into Eqs. (15) and (16), the relationship between Φ0, Φ1 and pH can be obtained and shown in Fig. 1.
Figure 1 shows that at the pH lower than 9.4,  is the dominant ion and there is little free ammonia in solution, which is extremely unfavorable for the leaching of silver. However, when pH is over 9.4, the content of NH3 gradually goes up and reaches 99% at pH 11. Therefore, it is necessary to improve pH for increasing ammonia ions in aqueous solution.
is the dominant ion and there is little free ammonia in solution, which is extremely unfavorable for the leaching of silver. However, when pH is over 9.4, the content of NH3 gradually goes up and reaches 99% at pH 11. Therefore, it is necessary to improve pH for increasing ammonia ions in aqueous solution.
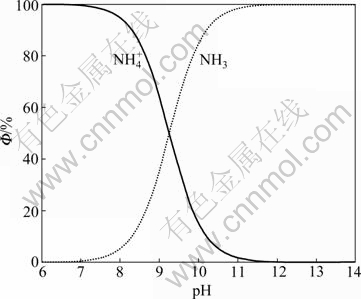
Fig. 1 Relationship between Φ0, Φ1 and pH
4.2 Effect of pH and c(NH3)T on c(Ag+)T
In the system of Ag+-NH3-H2O, when c(NH3)T is kept at 2, 4, 6 and 8 mol/L, respectively, and pH value varies from 7 to 14, the value of c(Ag)T can be calculated and the curved surface chart (Fig. 2) reflecting the effect of pH and c(NH3)T on c(Ag+)T can be drawn through Matlab software.
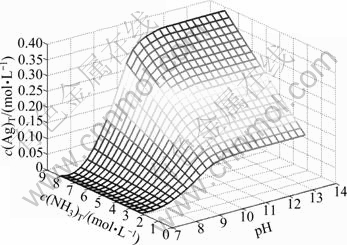
Fig. 2 Curved surface chart of c(Ag)T with pH and c(NH3)T
In Fig. 2, under the conditions of fixed c(NH3)T and pH<11, c(Ag)T increases fast first and then slow, but c(Ag)T becomes a constant at pH 11-14. As pH increases from 7 to 11, the protonation reaction of ammonia becomes weaker and weaker, and more and more free NH3 combines with Ag+, resulting in the total concentration of Ag+ increasing. When pH varies from 11 to 14, the concentration of free ammonia rises very slowly, so there is little change in the total concentration of Ag+. All of these are consistent with Fig. 1.
When pH is fixed, c(Ag)T goes up with the increase of c(NH3)T. Especially, when pH varies from 11 to 14, the concentration of free ammonia combined with Ag+ nearly shows a linear increase with the rise of c(NH3)T, and then there is a linear relationship between c(Ag)T and c(NH3)T.
4.3 Distribution of silver species under condition of pH=12
Under the condition of pH=12 and c(NH3)T=2- 8 mol/L, the dissolution of silver chloride reaches equilibrium and there are nine species of silver i.e. Ag+, Ag(NH3)+, 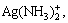 AgOH,
AgOH, 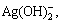
 AgCl,
AgCl, 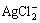 and
and 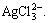 The concentrations of various silver species vary with the change of c(NH3)T, as shown in Fig. 3. It is known from Fig. 3 that under equilibrium the main complex species of silver is Ag(NH3)2+, while all the other species are in very low concentration.
The concentrations of various silver species vary with the change of c(NH3)T, as shown in Fig. 3. It is known from Fig. 3 that under equilibrium the main complex species of silver is Ag(NH3)2+, while all the other species are in very low concentration.
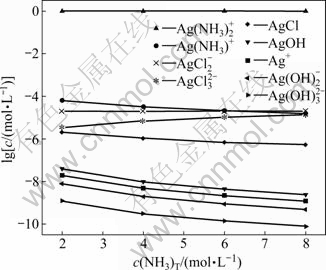
Fig. 3 Distribution of silver species under condition of pH=12
4.4 Data analysis
Some values were taken out of the results from Maple calculation, and then corresponding two- dimensional chart was drawn in Fig. 4 about the relation- ship between c(Ag)T and c(NH3)T or pH. By comparing Fig. 4 with the relevant figure in Ref. [2] drawn according to the values from literature, it is easy to find that they are completely consistent.
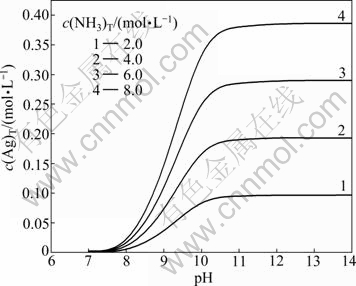
Fig. 4 Relationship between c(Ag)T and pH
In industrial practice of silver chloride leaching by ammonia, the concentration of ammonia is controlled at 6 mol/L, pH value is 12.5, reaction temperature and time are room temperature and 4 h, and liquid-to-solid ratio is controlled to maintain the total concentration of silver at 34 g/L [1], meaning that the concentration of silver is about 0.315 mol/L after silver completely dissolves into the aqueous solution. Under the above conditions, the theoretical concentration of silver after completely dissolving into the aqueous solution is 0.289 mol/L, namely 31g/L, according to the results from calculation model. Theoretical value agrees well with the actual value, which demonstrates that it is reliable to use this calculation model to analyze the solubility of insoluble compound in complex system.
5 Conclusions
1) According to the principle of mass equilibrium and considering relevant dissociation reaction, complexation reaction, self-complexation reaction and protonation reaction during insoluble compound dissolving, the model for solubility calculation of insoluble compound is established. After fixing two variables (usually choosing the known variables in industrial production), it is easy to solve the equations and gain the solubility value of insoluble compound MmAa. The curved surface charts of thermodynamic equilibrium of the total concentration of M, pH and concentration of ligand ions are drawn correspondingly.
2) The verification results from silver chloride leaching in ammonia system show that the theoretical value of silver chloride solubility (34 g/L) is close to the actual value (31 g/L), which demonstrates that this calculation model has a very high reliability.
References
[1] YANG Tian-zu. Metallurgy and chemicals of precious metals [M]. Changsha: Central South University Press, 2005: 386-388. (in Chinese)
[2] YANG Tian-zu, DOU Ai-chun, JIANG Ming-xi, CHU Guang. Influence of concentration of chlorine ion on leaching of silver chloride by ammonia [J]. Precious Metals, 2006, 27(4): 6-11. (in Chinese)
[3] ZHANG Du-chao, DU Xin-ling, YANG Tian-zu, DU Zuo-juan, DOU Ai-chun. Study on leaching of silver chloride in sodium sulfite solution containing chloride ions [J]. Precious Metals, 2007, 28(3): 10-14. (in Chinese)
[4] MCCLEVERTY J A, MEYER T J. Comprehensive coordination chemistry II [M]. New York: Pergamon Press, 2004: 763-768.
[5] RACHEL C. Traversing the coordination chemistry and chemical biology of hydroxamic acids [J]. Coordination Chemistry Reviews, 2008, 252: 1387-1408.
[6] TIAN Guo-cai, LI Jian, HUA Yi-xin. Application of ionic liquids in hydrometallurgy of nonferrous metals [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(3): 513-520.
[7] ZHANG Xiang-lin, KANG Heng. Coordination chemistry [M]. Changsha: Central South University Press, 1986: 93-120. (in Chinese)
[8] JACQUES C, MICHAEL K. Partial evaluation of maple [J]. Science of Computer Programming, 2011, 76: 469-491.
[9] YUE Chong-shan, JING Hai-bin, ZHANG He. Application of maple in solving nonlinear equation [J]. Journal of Shijiazhuang University, 2007, 9(6): 5-7. (in Chinese)
[10] LIU Wei, TANG Mo-tang, TANG Chao-bo, HE Jing, YANG Sheng-hai, YANG Jian-guang. Thermodynamics of solubility of Cu2(OH)2CO3 in ammonia-ammonium chloride-ethylenediamine- (En)-water system [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 336-343.
[11] YANG Tian-zu, DOU Ai-chun, LEI Chun-mao, REN Jin, LIU Zhen-zhen. Ligand selection for complex-leaching valuable metals in hydrometallurgy [J]. Transactions of Nonferrous Metals Society of China, 2010, (20): 1148-1153.
[12] YANG Tian-zu, REN Jin, LIU Wei-feng, DOU Ai-chun, LIU Wei, ZHANG Du-chao. Thermodynamics equilibrium analysis of Zn(II)- Glu2--CO32--H2O system [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(6): 1155-1161. (in Chinese)
[13] YANG Tian-zu, LIU Zhen-zhen, REN Jin, DOU Ai-chun, LIU Wei, LIU Wei-feng, ZHANG Du-chao. Thermodynamic equilibrium analysis on Cu(II)-Glu2--CO32--H2O system [J]. The Chinese Journal of Process Engineering, 2009, 9(4): 745-749. (in Chinese)
[14] BRIONES R, LAPIDUS G T. The leaching of silver sulfide with the thiosulfate-ammonia-cupric ion system [J]. Hydrometallurgy, 1998, 50: 243-260.
[15] YANG Sheng-hai, CHEN Geng-tao, CAI Ya-nan, GUO Huan, YAO Wei-yi and TANG Mo-tang. Selective leaching of silver-rich residue in NH4SCN solution under oxygen pressure [J]. Separation and Purification Technology, 2011, 1(77): 1-6.
[16] FU Chong-yue, ZHENG Di-ji. Thermodynamics study of Cu(II)-NH4+-H2O system [J]. Journal of Central South University of Technology, 1989, 20(1): 37-42. (in Chinese)
[17] ZHU Yuan-bao, SHEN Zhi-chen, ZHANG Chuan-fu. Handbook of Electrochemical data [M]. Changsha: Hunan Science and Technical Press, 1985: 132-151. (in Chinese)
(Edited by HE Yun-bin)
Foundation item: Project(2007CB613604) supported by the National Basic Research Program of China
Received date: 2011-05-12; Accepted date: 2011-07-19
Corresponding author: YANG Tian-zu, Professor, PhD;Tel: +86-731-88836791; E-mail: tianzuyang@163.com