State-of-art on corrosion and protection of magnesium alloys based on patent literatures
来源期刊:中国有色金属学报(英文版)2011年第4期
论文作者:吴超云 张津
文章页码:892 - 902
关键词:腐蚀;防护;镁合金;表面处理;专利
Key words:corrosion; protection; magnesium alloys; surface treatment; patent
摘 要:对镁合金腐蚀与防护的国内外相关专利文献进行分析,总结最新的表面防护技术,包括转化膜、电镀、表面涂层和多种复合处理技术,此外还介绍一些新的处理技术。发现转化膜技术在所有专利文献中占有极大的比例,说明在实际的工业应用中转化膜技术非常重要,且占主导地位。由于镁合金零部件形状和使用特性的多样性,单一的表面技术难以满足性能所需,越来越多的复合表面处理技术、环保型的新技术被发明用来进行镁合金的防护处理。此外,专利技术的投资成本、操作的难易程度、涂层的性能等因素极大地影响该专利技术的工业应用。
Abstract: The state-of-art of patented technologies for surface treatment of magnesium alloys including chemical conversion, electrochemical plating, surface coating, and multiple-step surface treatment technologies was reviewed and analyzed. Some new techniques were introduced. It was found that conversion film technologies account for a large amount of proportion among the patents of surface treatment. These technologies are also the main technologies used in industry. As the structures and service conditions of Mg alloy parts are of variety, a single surface-treatment process can not satisfy all requirements. Combined surface-treatment techniques can meet the needs in different applications. More and more new and environmental friendly techniques were invented. Factors such as capital investment, ease of manufacturing, and coating performances have to be considered when developing a coating technology for the industrial application.

WU Chao-yun1, ZHANG Jin1, 2
1. Institute of Advanced Materials and Technology,
University of Science and Technology Beijing, Beijing 100083, China;
2. Beijing Key Lab for Corrosion, Erosion and Surface Technology,
University of Science and Technology Beijing, Beijing 100083, China
Received 23 September 2010; accepted 20 December 2010
Abstract: The state-of-art of patented technologies for surface treatment of magnesium alloys including chemical conversion, electrochemical plating, surface coating, and multiple-step surface treatment technologies was reviewed and analyzed. Some new techniques were introduced. It was found that conversion film technologies account for a large amount of proportion among the patents of surface treatment. These technologies are also the main technologies used in industry. As the structures and service conditions of Mg alloy parts are of variety, a single surface-treatment process can not satisfy all requirements. Combined surface-treatment techniques can meet the needs in different applications. More and more new and environmental friendly techniques were invented. Factors such as capital investment, ease of manufacturing, and coating performances have to be considered when developing a coating technology for the industrial application.
Key words: corrosion; protection; magnesium alloys; surface treatment; patent
1 Introduction
The low density and high specific stiffness of magnesium-based alloys make them attractive for the aerospace and automobile industries, although they must be balanced by their susceptibility of corrosion in aqueous environment. A number of methods to prevent magnesium alloys from corrosion or delay the evolution of corrosion are used. The corrosion of magnesium alloys can be controlled by changing the environmental factors since it usually occurs in the pH<12 moist conditions. However, environmental factors cannot be controlled in most of the time. Consequently, numerous coating technologies have been used to protect the magnesium from corrosion.
GRAY and LUAN[1] reviewed the protection of magnesium alloys in 2001. They reported in details the electrochemical plating, conversion films, anodizing, gas-phase deposition, laser surface alloying/cladding and organic coatings. Since then, the research and development on Mg alloys have been developed rapidly in the world, particularly in China. It is beneficial to reveal the state of the art of corrosion and protection of magnesium alloys. Thus, more than 400 patents in seven countries have been searched from Espacenet data (http://v3.espacenet.com/), Wanfang data (http://s.g.wanfangdata.com.cn/Patent.aspx) and other resources using key words “magnesium, protection, coating and surface”. This paper mainly reviewed the patents on the protection of magnesium alloys. It was found that magnesium alloy-related patents increased every year in the last 20 years. The first patent on Mg alloy in the world was granted in USA in 1928. The number of such patents in Japan and USA appeared a peak during 1990-2000 and began to reduce in 2001, and then kept a relative constant. However, patents increased rapidly after 2001 in China and reached the peak in 2008, which is higher than the sum of Japan and USA peaks in the last 10 years. Chinese patents on corrosion and protection of Mg alloys published in recent 9 years account for the No.1 in quantity, which illustrates the prosperous research and development on Mg alloys in China.
2 Outline of magnesium alloy patents
Magnesium and its alloys have been extensively studied in many countries such as USA, Japan, China, and Germany. A great number of techniques have been invented for corrosion protection and surface treatment of Mg alloys. The distribution of the patents in different countries is shown in Fig.1. Based on the searched patents, it can be seen that 199 (47.6%) patents have been granted in China, followed by 131 (31.3%) in Japan, 76 (18.2%) in USA, and 12 (2.9%) in Germany.
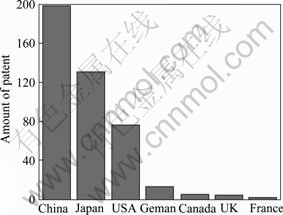
Fig.1 Distribution of patent number in different countries
Figure 2 shows the distribution of the number of patents versus year. In order to reveal the recent progress of the patent, the abscissa axis (year) is divided into three sections: before 1990, 1990-2000, and 2001-June 2010. It can be seen that there were only a few patents granted before 1990. The patents in USA and Japan concentrated in 1990s and kept the relatively constant numbers since then. Though the first Chinese patent was granted in 2001, 73 years behind the USA (first patent granted in 1928) and 21 years behind Japan (first patent granted in 1980), the quantity of patents in China has been increased tremendously since 2001. The number of patents has been increasing every year. Especially after 2004, the number of patents in China was much more than that of other countries. In addition, the number of patents in USA and Japan varied slightly after 2001, indicating that the technology of magnesium protection has been matured compared to that in China. China is one of the richest countries of magnesium-resource, not only the quantity of its minerals, but also the quality. The R&D on magnesium alloys has received great attention from Chinese government since the end of 1990s. And more than 40 millions RMB was distributed to industry, institutes and universities. Therefore, the R&D developed very fast in China in these years.
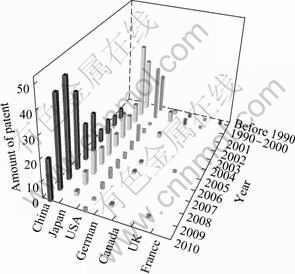
Fig.2 Distribution of patent number in different year
There are a number of coating technologies related to the corrosion protection of magnesium and its alloys. These include conversion films[2-5], electrochemical plating[6], surface coatings[7-9] and multiple surface treatments[10-11]. Figure 3 shows the distribution of the numbers of patents for different coating technologies. It is shown that there are 164 (39%) conversion film technique patents, the largest share of the technologies. It is the hotspot due to its excellent binding strength and low cost. The organic coating and anodizing account for 74 (18%), followed by multiple surface treatment technique for 73 (17%), and plating for 63 (15%), surface modification (29) 7%. There are only 16 (4%) vapor deposition since the cost and sample size restrictions hamper its development. Each of these will be described in the following sections.
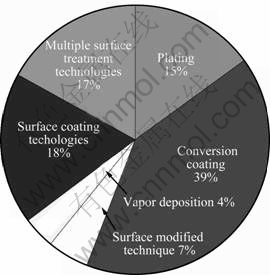
Fig.3 Distribution of patent number for different coating technologies
3 State-of-art of coating technologies
3.1 Chemical conversion film
Conversion films are superficial films of substrate metal oxides[5, 12-13], chromates[14], phosphates [15-16] or other compounds[17-19] produced by chemical or electrochemical treating on a metal surface. The superficial films are chemically bonded to the metal surfaces. The films provide magnesium alloys not only corrosion protection but also good paint-base properties.
There are many different types of conversion films, mainly including chromate[14, 20-24], phosphate, and anodizing films. The chromate conversion technique, described in details by GRAY and LUAN[1], is the most effective and mature process. It has been widely exploited in industry due to its excellent adhesion and corrosion resistance. After immersion in chromate solution, a protective coating can be obtained with uniform iridescent brassy color and free of any gray powdery material. Besides, there is no sparking when the work piece is subjected to wire brushing grinding operation[20]. However, the Cr6+ in chromate bath is highly toxic carcinogen and is gradually facing compulsory forbidding. The development of an environmental friendly process[25] is necessary due to the more and more stringent environment protection laws currently in effect or being proposed.
Phosphate treatments[15, 25-27] are explored as an alternative to conventional chromate conversion films. Phosphate films are formed when Mg substrates react with dihydrogen phosphates (M(H2PO4)2, where M represents metal elements or chemical groups such as Mn, Fe, K, Zn, Na, and NH4.
Table 1 lists the typical magnesium patents for phosphate treatments. The earliest patent was granted in 1928[3]. It shows that the phosphate solution has been changed from the simple salt of phosphate to more complicated solution consisting of manganese ions, phosphate ions[28] and permanganate ions[29]. The uniform phosphate films[30] of deeper colors formed in the manganese phosphate solutions can improve the wear resistance and corrosion resistance of Mg alloys. A chromate-free phosphate-fluoride conversion film[16, 31] was invented to improve the conversion rate and compactness of phosphate films. An active corrosion inhibitor consisting of organophosphonic acids was added in the conversion solution. The phosphoric acid group reacts with the magnesium metal substrate to form an insoluble salt. Later, nitric acid and tannin[32] were added respectively in the phosphate-permanganate solution to improve the corrosion resistance and bonding strength. The results showed that the corrosion resistances of the treated magnesium alloys were all above grade 9 after 5% NaCl salt spray test for 8 h, the surface electrical resistance of the samples was less than 2 Ω, and the bonding strength was above 3B[32].
Although the phosphate treatment has been used in industry to magnesium alloy parts such as 3C products, there are also many challenges for this technology. Firstly, the grains of phosphating film are coarse and crack occurs in the grains due to the high activity of magnesium alloys. The composition of phosphate solution, therefore, should be improved to obtain fine grains. Secondly, heavy metal ions in phosphate solution can cause environmental pollution and thus increases the cost of waste treatment.
Anodizing technology[39-44], an electrolytic process producing a thick and stable oxide film on metal and alloys, is another widely used technique in industry. The anodic oxide coating can be used to improve paint adhesion to metals[45-46], as a key for dyeing[5, 47-48] or as a passivity treatment.
Table 1 Typical phosphate treatment patents on magnesium

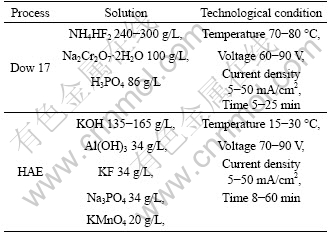
Generally, there are two basic processes for anodizing, i.e. oxygen precipitation and film forming. The growth of anodizing coatings can be divided into three stages, i.e. the forming of compact layer, then porous layer, and the growth of the porous layer, as shown in Fig.4. The properties of the coating depend on various parameters such as electrolyte composition, voltage and time. Chemical treatment Dow 17, invented by Dow Chemicals[49], and HAE process can be applied to all magnesium alloys.
The chemical solutions and process parameters are listed in Table 2. As can be seen, the Dow 17 solution contains toxic chromate. Therefore, the application of this technique has been limited. The improvement of this technique was conducted mostly from the power supply and electrolytes. Table 3 lists the typical patents covering anodizing technology. It is shown that power supply of anodizing technology has been gradually changed from DC to pulsed DC power, which can effectively control and maintain the appropriate cation-anion ratio near the anode. The anodizing coating becomes more colorful and protective. Meanwhile, the development of power supply is towards low-voltage. One of the main challenges for anodic coatings on magnesium results from the electrochemical inhomogeneity due to the phase separation in alloys. Another disadvantage of this technique is that the coatings constitute a brittle ceramic material prone to cracking or shedding after collision.
Table 2 Typical processes of Dow 17 and HAE[49]
To further improve the corrosion resistance and abrasion resistance of anodizing coatings, a technology called micro-arc oxidation (MAO)[44, 50-53] or plasma electrolytic oxidation (PEO) was invented based on the anodizing technology. It is similar to anodizing, but it employs higher voltage, so that discharges occur and the resulting plasma modifies (and enhances) the structure of the oxide layer. This process can be used to grow thick (tens or hundreds of micrometers), large crystalline oxide coatings that can offer protection against wear, corrosion and heat, as well as electrical insulation. LIU et al[51] reported the patent progress on MAO before 2008. There are about ten new patents appeared in recent two years, which mainly dedicated to reduce the voltage to save energy and equipment costs[54-55]. The trend of MAO development is to improve the coating appearance, adhesion strength, corrosion resistance and hydrophobic properties[56-58]. A micro-arc oxidation process for forming a coating with excellent corrosion resistance on Mg alloys surfaces has been disclosed[55]. TiO2 nanoparticles are added into the electrolyte using sol-gel method. In addition, a superhydrophobic coating[56-57] can be prepared by adding the polytetrafluor ethylene (PTFE) into the electrolyte; a self-lubricating surface can also be obtained.
In addition to the traditional chemical conversion treatment, molybdate conversion technology[65], vanadate[17] and tartrate chemical conversion technology[66] have been invented for corrosion protection of magnesium alloys due to their friendly environmental. A method[17] for improving corrosion resistance and paint adhesion of magnesium alloys has been invented by adding silane in vanadate solution. The result showed that the sample surface with silane was still intact after 5% NaCl salt-spray test for 300 h and the paint adhesion was excellent[17].
3.2 Plating
The plating process can be subdivided into two categories: electroplating and electroless plating. In both cases a metal salt in solution is reduced to its metallic form and deposited on the surface of work piece. In electroplating, the electrons are supplied by an external power source. In electroless plating, the electrons are supplied by a chemical reducing agent in the solution.
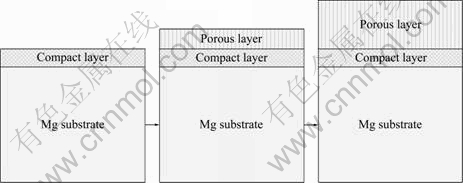
Fig.4 Schematic of growth of anodizing coating
Table 3 Typical magnesium patents covering anodizing technology

There are three major steps in a plating process, as shown in Fig.5. Firstly, the cations are gathered at the cathode surface by concentration diffusion. Secondly, the displacement reaction occurs at the cathode and the cations are consumed in the meantime. Finally, a film is formed by the deposition of metal crystal from displacement reaction on the substrate surface.
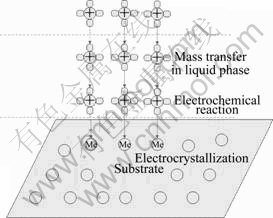
Fig.5 Schematic of electroplating process
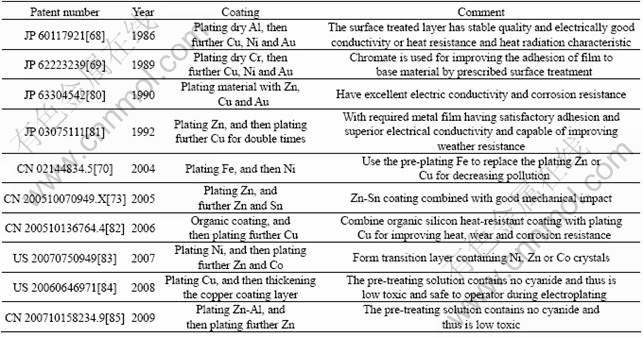
Plating on Mg alloy surfaces has shown to be useful in applications. However, due to the high chemical activity of magnesium, a strong replacement reaction occurs in plating process accompanied by a large number of hydrogen evolution. The plating films, therefore, have weak adhesion to magnesium alloys. Generally, the pretreatment is complicated for plating on magnesium alloys. Thus, standard ASTM B 480-1988[67] was established for preparation in electroplating. Improved methods were proposed to make the process more simple and lower the cost. Table 4 lists typical patents covering electrochemical plating. Ni, Cu or Au plating with dry Al pretreatment shows excellent electrical conductivity and heat radiation characteristics of the surface of Mg or Mg alloy material[68]. To improve the adhesion of the film to the substrate material by prescribed surface treatment, a pretreatment of Cr plating was invented[69]. The Cr plating solution, however, contains toxic substances. A solution containing fluoride, iron compounds and complex agents was invented as an alternative to toxic cyanide solutions[70]. A simplified process in Ref.[71] showed capability to make the coating integrity after salt spray test for 1 000 h. A method was invented for plating Zn-Ni alloys[71-72] on Mg alloys. The plated films have smooth surfaces, free striking, and good bonding to the substrates. Additionally, electroplating or electroless plating of alloys such as Zn-Sn[73], Ni-P[74-76], Ni-Cu-P[77], Ni-W-P[78], Ni-P-V[79] can produce surface films to increase the corrosion resistance, surface hardness and thermal conductivity.
As we known, magnesium and its alloys are prone to have galvanic corrosion while they are in touch with other metals due to their high reactivity and low open circuit potentials. General corrosion rate can be accelerated by galvanic corrosion once the plated films rupture. The existence of Ni as an impurity in Mg alloys will reduce corrosion resistance severely. Hence, Ni is a disastrous element to the corrosion resistance of Mg alloys. However, most coatings contain Ni, which has to be carefully removed when Mg alloys are recycled. In addition, the electrolytes have a limited life, which is a serious limitation from both coating and environmental perspectives. There are many challenges to be overcome in order to develop a versatile plating process.
Table 4 Typical magnesium patents covering electrochemical plating
3.3 Surface coating
Surface coating technologies were used to form protective layers on substrates via thermal spraying[86], overlaying welding[87-88] and hot-dipping[89]. Coating layers can be metal alloys[90-91], ceramics[92], paints[93], etc.
In a thermal spraying process, the coating materials, which can be metal, ceramic, cement or polymeric, are fed to a torch or a gun and heated to above or near its melting point. The resulting droplets are accelerated by a gas stream and sprayed into thin lamellar particles, which adhere to a substrate. An Al or Al/Zn thermal spraying coating was invented for improving the corrosion resistance of Mg alloys[91, 94]. The elements of Al and Zn are the main constitution of Mg alloys. They make the recycling of Mg alloys with these coatings much easier, compared to metal plating such as Ni and Cr plating. However, the spray coatings are porous and need to be sealed or reheated before the parts are exposed to humid environment. The coating quality can be improved by further heating at 380-420 °C to enable atom inter- diffusion at the coating and substrate interface. The coating-substrate adhesion can be improved by forming new Al-Mg or Mg-Zn phases. The coating is of good impact resistance and corrosion resistance (Fig.6 and Fig.7)[95]. Surface nanocrystallization process was invented to reduce the diffusion temperature[90-91]. Additionally, thermal spraying of ceramics[92] such as A12O3, Cr2O3, TiC, Si3N4, ZrO2, SiC, TiO2, Cr3C2, MgO was also invented to improve the surface hardness, abrasion resistance and thermal resistance of magnesium alloys for applications such as motorcycle and motor spare parts.
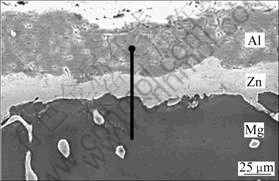
Fig.6 Cross-section morphology of as-Al sprayed specimen
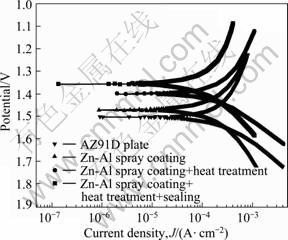
Fig.7 Polarization curves of thermal sprayed Zn-Al coatings with different post-treatments
3.4 Organic coatings
An organic coating is typically used in the final stage of a coating process. It can enhance corrosion resistance or specific decoration[1]. Organic coating involves a variety of processes such as painting, powder coating, cathodic electrocoating (E-coating) and the application of lacquers, enamels and varnishes. Table 5 lists the typical magnesium patents covering organic coating technologies. It can be seen that silane plays an important role in organic coatings. Silane is a class of environmentally-benign organic-inorganic hybrids, which has a great potential to replace toxic chromates in industries. An independent silane film[8-9] without top coating was initially used to improve the corrosion resistance of magnesium and its alloys. However, the silane film is too thin to protect the magnesium alloys for long time. Therefore, as a paint primer, the silane film provides excellent paint adhesion between metal and painting (Fig.8)[96-97].
3.5 Multiple surface coatings
A single surface treating might not meet the requirements of magnesium alloys in some working conditions. Therefore, surface treatment technologies [102] that combine two or more kinds of surface treatment together to form multi-layer surface coating have been developed rapidly in recent years.
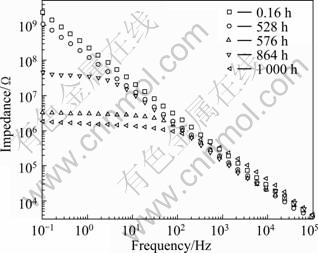
Fig.8 Bode plots of Mg/silane/E-coating in 3.5% NaCl solution after immersion for different time
The benefits of this type of technology are not a simple addition of the properties of multiple layers but the synergistic effects. For example, an insoluble fluoromagnesium film is formed on the surface of magnesium alloy, followed by immersing the coated metal in a metallic container comprising an aqueous solution of alkali metal silicate and an aqueous solution of alkali metal hydroxide[103]. Corrosion and abrasion resistance of magnesium alloys were improved by fluoromagnesium film and top anodizing coating, respectively. Additionally, this invention exhibited greater resistance to strong acids and alkali. Another example: an oxide film is formed by anodic oxidation, a thermosetting resin film is then formed on top of it, and finally a metallic conductive film is formed on the surface by a vapor deposition method[104-106]. This can enhance surface characteristics such as corrosion resistance and conductivity. HOSHI et al[107] invented a three-layer structure, in which nickel/copper/aluminum were plated successively on magnesium alloy and then an anodized aluminum coating was formed on the outer surface. The internal stress generated in the nickel plating film and the aluminum plating film is relaxed by forming a copper plating film between them. This, therefore, improves the adhesion of the films over the entire part.
However, a typical multi-layer surface treatment technology involves chemical conversion or anodizing and paint coating to the conversion or anodizing film on magnesium alloys[29, 108-109].
3.6 Other technologies
Many other protective technologies such as surface heat treatment[110], vapor deposition[111-113], ion implantation[114-115] were invented. They are excellent alternatives with respect to environmental impact. However, these technologies usually involved more capital investment and power consumption. The corrosion and adhesion properties of these coatings on magnesium are not satisfactory yet.
Table 5 Typical magnesium patents covering organic coating technologies
4 Summary
The technologies mentioned above reveal that almost all surface technique including pretreatment can be used for the corrosion protection of Mg alloys. It seems that the corrosion protection to Mg alloys is not a key point for their applications. However, up to now, no single technique or a multi-layer coating technique can meet the industry requirements for Mg alloys in different service conditions. As Mg alloys have low open circuit potential and negative differential effect, coating techniques suitable for Al or Fe or their alloys can not be applied directly to Mg alloys. Some corrosion protection techniques for materials other than Mg alloys may be useful for Mg alloy corrosion protection. However, the process parameters and/or solution compositions may have to be modified before they can be used to treat the components of Mg alloys. Meanwhile, recycling of Mg alloys should get rid of Ni, Fe, Cr, Cu coatings.
Of all the coating techniques, we can divide them into two types: dry and wet methods. Thermal spray, laser surface alloy or cladding, physical or chemical deposition, and solid diffusion are dry methods. Conversion film, electrochemical plating, anodizing or plasma oxidizing, painting or organic/polymer coating, and sol-gel belong to wet methods. Dry methods are usually environmental friendly and are suitable for treating precision or decorating parts with simple shape and small size and free of holes or grooves. Special apparatuses, which are usually very expensive, are needed for dry techniques. Wet methods, on the other hand, are easy to realize with little investment and have good throughput. Wet methods are suitable for the complex and large components used in automobiles and bicycle industries. However, great efforts are needed for waste disposal especially for solutions consisting of toxic and carcinogen components such as chromium and cyanide. Some new patents have shown techniques of chromium-free or cyanide-free coating, and lower power cost apparatus. Coating techniques for strong adhesion, corrosion- and wear-resistance, and high impact- resistance are the future R&D interest.
Magnesium alloys, known as "the green engineering materials with the greatest potential of development in the 21st century", were paid more and more attention by the majority of researchers. The number of Mg alloy related patents was increasing every year. However, only a small amount of these patents were really applied in industries. A number of factors including capital investment, ease of manufacturing, coating performances and environment issues need to be considered when developing a coating process for industrial application. Therefore, future trends for patents of magnesium alloys are predicted to be towards low cost, pollution-free, and easy to recycle.
Acknowledgements
The authors gratefully acknowledge the financial support of Beijing Key Laboratory for Corrosion, Erosion and Surface Technology and project 9140A18060409 QT0202 in China.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys — A critical review [J]. Journal of Alloy and Compounds, 2002, 336: 88-113.
[2] MACCULLOCH J A, ROSS P N, HENSHAW G S. Colouring magnesium or magnesium alloy articles. EP 19980914164 [P]. 2000.
[3] ZIMMERMAN A C. Treatment of magnesium and magnesium alloys to inhibit corrosion. US 1677667 [P]. 1928.
[4] GUO Xing-wu, DING Wen-jiang, ZHAI Chun-quan, LU Chen. Two-step colored method of green oxide film on magnesium alloy surface. CN 200410067814. 3 [P]. 2005. (in Chinese)
[5] YUKIO O, TOSHIHIKO S. Surface-treating method for magnesium or magnesium alloy. JP 53089901 [P]. 1980.
[6] MOEBIUS A P D, WANDNER K H D. Galvanic deposition of metal layers on magnesium or magnesium alloy surfaces. EP 20080016260 [P]. 2009.
[7] CUI J. Water dispersible silanes as corrosion-protection coatings and paint primers for metal pretreatment. US 20070166467 [P]. 2007.
[8] ARAKI T, TESHIGAWARA M, KIMURA T S. Magnesium alloy-bonding organopolysiloxane composition having improved chemical resistance. US 20070699994 [P]. 2007.
[9] KIMURA T S, TESHIGAWARA M. Organopolysiloxane composition for bonding to magnesium alloy. US 20050099483 [P]. 2005.
[10] BARTAK D E, LEMIEUX B E, WOOLSEY E R. Two-step chemical/electrochemical process for coating magnesium alloys. US 5240589 [P]. 1993.
[11] BARTAK D E, LEMIEUX B E, WOOLSEY E R. Two-step electrochemical process for coating magnesium alloys. US 5264113 [P]. 1993.
[12] KATAOKA A. Surface treating method of magnesium or its alloy. JP 53149330 [P]. 1980.
[13] FURUTA M, UEHORI K, KOBAYASHI W. Anodizing solution for anodic oxidation of magnesium or its alloys. US 19870030941 [P]. 1988.
[14] YOSHINORI T. Surface treatment of magnesium or magnesium alloy. JP 60132460 [P]. 1986.
[15] SADAO I, YOKICH S, MASAHIKO N. Composition and process for treating magnesium-containing metals and product therefrom. US 19970822444 [P]. 1999.
[16] TANG X, JAWOROWSKI M, HAMMERSCHMIDT K. Corrosion resistant, chromate-free conversion coating for magnesium alloys. US 20030601247 [P]. 2004.
[17] GER M D, YANG K H, SUNG Y, HWU W, LIUY. Method for treating magnesium alloy by chemical conversion. US 20030230365 [P]. 2003.
[18] KOZO I, SHUJI T, KINUE T, UENO H. Surface treatment method for magnesium alloy material and magnesium alloy material treated thereby onal institute of advanced industrial and technology. JP 2005350043 [P]. 2007.
[19] TAKENAKA S, KAWAKAMI M, ONO T, NARASAKI Y. Highly corrosion-resistant magnesium alloy and its production method. JP 2006217864 [P]. 2008.
[20] HEZBERT K, DE L. Surface treatment of magnesium alloys. US 2428749 [P]. 1947.
[21] OSAMU M, ATARU Y, SABURO S, GOHARA Y. Surface treatment of magnesium or magnesium alloy. JP 54057110 [P]. 1980.
[22] LEUZINGER J M. Conversion of coating of magnesium alloys surfaces. CA 726661[P]. 1966.
[23] HAGANS P L. Method for providing a corrosion resistant coating for magnesium containing materials. US 4569699 [P]. 1986.
[24] HELLER F P. Method of forming a chromate conversion coating on magnesium. CA 603580 [P]. 1960.
[25] SADAO I, MASAHIKO N, YOKICHI S. Composition and process for treating magnesium-containing metals and product therefrom. US 63763596A [P]. 1997.
[26] SHIKATA N, KONDOU Y, NISHIKAWA Y, NISHIZAWA Y, SAKAMOTO Y, FUJIWAKI T. Surface-treated article of magnesium or magnesium alloys, method of surface preparation and method of coating. US 20000623218 [P]. 2002.
[27] SANTI J D. Magnesium piston coated with a fuel ignition products adhesive. US 48284190A [P]. 1991.
[28] KINUE T, KOZO I, SHUJI T, SAKAMOTO M. Magnesium alloy material and method of treating surface of magnesium alloy material onal institute of advanced industrial and technology. JP 2008147291 [P]. 2009.
[29] YOMOGIHARA M, MIYAMOTO T, YAMAZOE K, YASUHARA K. Conversion coating solution for magnesium alloy, surface treatment method and magnesium-alloy base material. JP 2001094195 [P]. 2002.
[30] FUTSUHARA M, MIYAMOTO T, YAMAZOE K, YASUHARA K. Chemical conversion reagent for magnesium alloy, surface-treating method, and magnesium alloy substrate. US 20020106098 [P]. 2002.
[31] BRILES O M, JAWOROWSKI M, KRYZMAN M A. Corrosion resistant, chromate-free for magnesium alloys. US 6887320 [P]. 2005.
[32] SHENG E H, YONG L, LI F X, JIN Z. Phophating solution and method for conversion treating surface of magnesium alloy workpiece. US 20090159158 [P]. 2009.
[33] NAOHORO Y, YOSHIAKI K, YUKIO N, SHINODA K. Surface treated magnesium or magnesium alloy product, method of surface treatment and coating method. JP 09183547 [P]. 1999.
[34] NAOHORO Y, YOSHIAKI K, YUKIO N, NISHIZAWA Y. Surface-treated article of magnesium or magnesium alloys, method of surface pretreation and method of coating. JP 9901275W [P]. 1999.
[35] NAOHORO Y, YOSHIAKI K, YUKIO N, NISHIZAWA Y. Surface treated magnesium or magnesium alloy product, primary treatment for coating and coating method. JP 11024956 [P]. 1999.
[36] YOSHIAKI N, YOSHIAKI K, YUKIO N, SHIKATA N. Surface-treated article of magnesium or magnesium alloys, method of surface preparation and method of coating. DE 19996002151 [P]. 1999.
[37] KOJI T, KATSUYOSHI O. Low pollution surface treatment method of magnesium alloy. JP 10288094 [P]. 2000.
[38] HIDEAKI M, MASAHIRO M, NOBUYOSHI K, SUZUKI M. High corrosion resistance surface treated magnesium alloy product and producing method therefor. JP 11302499 [P]. 2001.
[39] STEFAN L, WOLFGANG S, ULLRICH B, SCHMIDT J. Production of anticorrosion coating on magnesium or alloy part, used in vehicle or aircraft construction, involves oxidation in aluminum phosphate electrolyte containing vanadium, molybdenum and/or manganese compound. DE 20011027770 [P]. 2002.
[40] OSTROVSKY I. Method of anodizing of magnesium and magnesium alloys and producing conductive layers on an anodized surface. US 20030000847 [P]. 2003.
[41] GER M D, CHANG C L, SUNG Y, WEN N H, WEI K. Method for treating surface of magnesium or magnesium alloy. US 20060390206 [P]. 2006.
[42] TATSUHIKO M, KEITARO Y, TAKAYUKI K. Method for surface treatment of magnesium or magnesium alloy. JP 11306790 [P]. 2001.
[43] JIANG Bai-ling, ZHANG Shu-fen, HAO Jian-min, SUN Jun-tu, BAI Li-jing, Li Jun-ming. Process of surface treatment on magnesium alloy. CN 01106741.1 [P]. 2002. (in Chinese)
[44] ZHANG Ding-hui, LIU Yu-ping, SHEN Ying-pin. Method of surface treatment on magnesium alloy with anoding electrolyte. CN 200910103124.1 [P]. 2009. (in Chinese)
[45] OKUDA Y, SAKAI K, HINO M, HIRAMATSU M. Magnesium or magnesium alloy product having electroconductive anodic oxidation coating film on surface thereof and method for production thereof. JP 2008137326 [P]. 2008.
[46] OKUDA Y, SAKAI K, HINO M, HIRAMATSU M. Manufacturing method of magnesium or magnesium alloy product having anodic oxidation coating on surface. JP 2008137327 [P]. 2008.
[47] ATSUSHI F, MITSURU S K I, TOSHIO I. Method for forming colored protective film on surface of magnesium material. JP 56096564 [P]. 1983.
[48] TAKAHATA S, KOBAYASHI W. Aqueous anodizing solution and process for coloring article of magnesium or magnesium-base alloy. US 19840631577 [P]. 1985.
[49] CO D C. Anodizing magnesium. US 2901409 [P]. 1959.
[50] ZHANG Wei, LI Jiu-qing, LIU Yuan-gang. Method of ceramic layer using cathode and anode micro-arc electrodeposition on magnesium alloy. CN 03157173.5 [P]. 2004. (in Chinese)
[51] LIU C F, GU S H, WANG L Z. Patent progress of micro-arc oxidation on magnesium alloy [J]. Material Protection, 2008, 41(2): 53-56.
[52] LI Rong-hua. Magnesium alloy treated by micro-arc oxidation. CN 200710190847.0 [P]. 2008. (in Chinese)
[53] JING Xiao-yan, LIU Jing-yuan, LU Yi, YUAN Yi. Method of plasma electrolytic oxidation on Mg-Li alloy. CN 200810137014.2 [P]. 2009. (in Chinese)
[54] YAO Zhong-ping, JIANG Zhao-hua, WANG Fu-ping, WU Zhen-dong. Method of preparation zirconia coating on magnesium alloy surface. CN 200710072391.8 [P]. 2008. (in Chinese)
[55] ZHU Li-qun, WANG Xi-mei, LI Wei-ping. Method of preparing membrane in no-voltage and low voltage on magnesium Alloy surface. CN 200810226985.4 [P]. 2009. (in Chinese)
[56] WANG Li-ping, XUE Qun-ji, GUO Jie. Method of micro-arc oxidation coating with self-lubricating and hydrophobic structural integration on magnesium alloy surface. CN 200710078090.6 [P]. 2009. (in Chinese)
[57] WANG Li-ping, XUE Qun-ji, LIU Wei-min. Preparation method of super-hydrophobic on magnesium alloy surface. CN 200710078089.3 [P]. 2009. (in Chinese)
[58] XUE Qun-ji, WANG Li-ping, LIANG Jun. Preparing of membrane with high corrosion resistance using micro-arc oxidation method on magnesium alloy. CN 200710078091.0 [P]. 2009. (in Chinese)
[59] ATSUSHI F, KENJI S, MITSURU I, IGARASHI T. Method for forming protective film on surface of magnesium material. JP 56096562 [P]. 1983.
[60] MATSUFUMI T. Surface treatment of magnesium and its alloy. JP 59154069 [P]. 1986.
[61] MATSUFUMI T. Surface treatment of magnesium, aluminum and alloy thereof. JP 60077724 [P]. 1986.
[62] BARTAK D E, LEMIEUX B E, WOOLSEY E R. Hard anodic coating for magnesium alloys. US 5470664 [P]. 1995.
[63] OSTROVSKY I. Method of anode on magnesium and its alloy surface and the method to generate conductive layer. CN 02816684.1 [P]. 2004. (in Chinese)
[64] AKIMOTO M. Magnesium metallic material having excellent corrosion resistance, and method for producing the same. JP 2005112939 [P]. 2006.
[65] RO B, ARAI T, FUJIMURA T. Surface treatment agent for magnesium or magnesium alloy product. JP 2005355759 [P]. 2007.
[66] JIANG Yong-feng, ZHOU Hai-tao, YI Dan-qing, ZENG Su-min. Tartrate chemical conversion treatment on magnesium alloy surface. CN 200710035567.2 [P]. 2008. (in Chinese)
[67] ASTM B 480—1988. Standard guide for preparation of magnesium and magnesium alloys for electroplating [S].
[68] YOSHINORI T. Surface treatment of magnesium or magnesium alloy. JP 60117921 [P]. 1986.
[69] YOSHINORI T. Surface treatment of magnesium or magnesium alloy. JP 62223239 [P]. 1989.
[70] SHAN Da-yong, ZHOU Wan-qiu, HAN En-hou, KE Wei. Method of electroless nickel plating on magnesium alloy. CN 02144834.5 [P]. 2004. (in Chinese)
[71] LIAN Jian-she, LI Guang-yu, NIU Li-yuan, JIANG Zhong-hao. Method of electroless plating nickel of magnesium alloy. CN 200410011014.X [P]. 2005. (in Chinese)
[72] ZHU Ping, ZHOU Ming, WU Jin-hua, ZHOU Jin. Method of direct electrodeposited zinc-nickel alloy on magnesium alloy. CN 200710037391.4 [P]. 2007. (in Chinese)
[73] ZHU Li-qun, LI Wei-ping, PAN Bo. Process composite mechanical plating tin plating on the magnesium alloy surface. CN 200510070949.X [P]. 2005. (in Chinese)
[74] WANG Zhou-cheng, TANG Yi. Method of plating Ni-B alloy on magnesium alloy. CN 200610005330.5 [P]. 2006. (in Chinese)
[75] WANG Zhou-cheng, TANG Yi. Method of plating Ni-B alloy on magnesium alloy surface. CN 200610070858.0 [P]. 2006. (in Chinese)
[76] SUN Dong-bai, YU Hong-ying, MENG Hui-min, WANG Xu-dong. Method of electroless plating Ni-P alloy on magnesium alloy. CN 200610113781.0 [P]. 2007. (in Chinese)
[77] WANG Jiang-feng. Surface treatment of electroless Ni-Cu-P alloy on magnesium alloys. CN 200610033119.4 [P]. 2007. (in Chinese)
[78] ZHANG Tao, YOU Zhong, SHAO Ya-wei, MENG Guo-zhe. Ni-W-P electroless plating bath for protecting magnesium alloy. CN 200810064719.6 [P]. 2008. (in Chinese)
[79] LIU Sheng-xin, GUAN Shao-kang, MIAO Jin-qi. Method of improving the surface hardness of magnesium alloy. CN 200810230741.3 [P]. 2009. (in Chinese)
[80] MASAKI T. Surface treatment of magnesium material. JP 63304542 [P]. 1990.
[81] NORIO I. Method for treating and condition surface of magnesium alloy. JP 03075111 [P]. 1992.
[82] CUI Jiang-zhong, ZHAO Long-chang, ZHAO Hui. Method of electroless plating Cu on magnesium and its alloy surface. CN 200510136764.4 [P]. 2006. (in Chinese)
[83] CHING H, TE L W. Method for forming a nickel-based layered structure on a magnesium alloy substrate, a surface-treated magnesium alloy article made therefrom. US 20070750949 [P]. 2007.
[84] CHING H, TSUNG W, KUNG C. Cyanide-free pre-treating solution for electroplating copper coating layer on magnesium alloy surface and a pre-treating method thereof. US 20060646971 [P]. 2008.
[85] SONG Gui-hong, LI De-gao, CHEN Li-jia, LI Feng. Method of non-cyanide electroplating metal layer on magnesium alloy. CN 200710158234.9 [P]. 2009. (in Chinese)
[86] NISHIO K, UCHIBAYASHI T. Magnesium material having excellent corrosion resistance. JP 2004172850 [P]. 2005.
[87] DING Wen-bin, JIANG Hai-yan, YAO Shou-shan, ZENG Xiao-qin, DING Wen-jiang, WU Guo-song. Method of SiC + Al welding on magnesium alloy surface. CN CN200410089271.5 [P]. 2005. (in Chinese)
[88] DING Wen-bin, JIANG Hai-yan, YAO Shou-shan. Method of B4C+Al remelting on magnesium alloy surface. CN 200510025065.2 [P]. 2005. (in Chinese)
[89] SKACH J E J, COBEL G B. Salt-coated magnesium granules. US 47158183A [P]. 1985-12-17.
[90] MA Yin, LI Yuan-dong, CHEN Ti-jun, LIANG Yong-zheng. Surface treatment methods for the magnesium alloy workpiece. CN 200410026088.0 [P]. 2005. (in Chinese)
[91] ZHANG Jin, OU Xin-bing, MA Yan-long, WANG Ying, YANG Dong-hua, GONG Xi-bin. Method of reducing diffusion temperature of the spraying coating on magnesium alloy surface. CN 200710092486.6 [P]. 2008. (in Chinese)
[92] SUN Zhi-fu, TU Jian-hua, CAO Jian-yong, ZHANG Zhong-he, ZHANG Jin. Surface modification method of magnesium alloy products. CN 03102219.7 [P]. 2003-06-25. (in Chinese)
[93] YUAN Xiao-guang, LIU Yan-xue, LV Nan, HUANG Hong-jun. Spraying process of protective layer coating on a magnesium alloy surface. CN 200510045984.6 [P]. 2005. (in Chinese)
[94] ZHANG Jin, SUN Zhi-fu. A kind of corrosion resistance of Al-Zn coating on the surface of automobile or motorcycle magnesium alloy wheel. CN 200420060580.5 [P]. 2005. (in Chinese)
[95] ZHANG J, WANG Y. Effect of heat treatment on microstructures and properties of zinc-aluminum coating on AZ91D magnesium alloy [J]. Key Engineering Materials, 2008, 373-374: 55-58.
[96] ZHANG Jin, SUN Zhi-fu. Method of improving corrosion resistance of magnesium alloy surface treatment. CN 200510057166.8 [P]. 2005. (in Chinese)
[97] ZHANG Jin, WU Chao-yun, HUANG Fu-xiang, ZHANG Wei, MA Yan-long, WANG Ying. Method of cathodic electrophoretic coating enhanced by silane on magnesium alloy surface. CN 200610095111.0 [P]. 2007. (in Chinese)
[98] TAKAYUKI F, TERU N. Surface processing method of magnesium alloy article and reflection mirror formed by the same. JP 2000275616 [P]. 2002.
[99] TAKAYUKI F, TERU N. Surface treating method of zinc-containing magnesium-lithium alloy. JP 2000127827 [P]. 2002.
[100] ARAKI T, KIMURA T S. Magnesium alloy-bonding organopolysiloxane composition and composite article. US 20050214810 [P]. 2006. (in Chinese)
[101] MMOTOJIMA Y, ODA H. Anticorrosive coating material composition for magnesium alloy and article having coating film made from the same. JP 2004329050 [P]. 2006.
[102] OSTROVSKY I. Treatment for improved magnesium surface corrosion-resistance. US 20030641133 [P]. 2004.
[103] KOZAK O. Anti-corrosive coating on magnesium and its alloys. US 4184926 [P]. 1980.
[104] MASATO M, ICHI S J, GORO Y, ARITA K. Surface-treated magnesium or magnesium alloy, and surface treatment process therefor. DE 19853576834 [P]. 1990.
[105] MASATO M, GORO Y, KISHIO A. Surface-treated magnesium or magnesium-alloy and process for surface treatment of magnesium or magnesium alloy. US 41124389A [P]. 1990.
[106] YAMAUCHI G, ARITA K, SEKI J, SAKIDA E, MINO M. Surface treatment of magnesium and magnesium alloy. JP 59215110 [P]. 1986.
[107] HOSHI H, YAMAGUCHI H, OKAMOTO A, ANDO S. Method of forming highly corrosion resistant film of magnesium alloy. JP 2005325416 [P]. 2006.
[108] TANAKA K, ASAMI T, HIROCHI M. Surface treating method for magnesium alloy. JP 11333739 [P]. 2001.
[109] FUKUDA S. Coating method of magnesium alloy molded product. JP 2004345978 [P]. 2006.
[110] SUZUKI H, SATO K, OKAZAKI K, et al. Surface treatment method for magnesium base material, and method for manufacturing magnesium shaped article. JP 2004204957 [P]. 2006.
[111] YAMANISHI T, TSUBAKINO H, YAMAMOTO A, TAKATANI Y. Method for surface treating Mg alloy. JP 11367394 [P]. 2001.
[112] INAGAWA K, TANI N, FUJIMOTO S. Method and device for surface treatment of Mg alloy member. JP 2004048142[P]. 2005.
[113] FUJIMOTO S, TANI N, INAGAWA K. Surface treatment method and surface treatment apparatus for Mg alloy member. JP 2004067175 [P]. 2005.
[114] WANG Xue-min, ZENG Xiao-qin, WU Guo-song, YAO Shou-shan. Modification method of ion implantation on magnesium alloy surface. CN 200610027164.9 [P]. 2006. (in Chinese)
[115] WANG Xue-min, ZENG Xiao-qin, WU Guo-song, YAO Shou-shan. Ion implantation method to improve oxidation resistance on magnesium alloy surface. CN 200610027991.8 [P]. 2006. (in Chinese)
吴超云1, 张 津1, 2
1. 北京科技大学 新材料技术研究院,北京 100083;
2. 北京科技大学 北京市腐蚀、磨蚀与表面技术重点实验室,北京 100083
摘 要:对镁合金腐蚀与防护的国内外相关专利文献进行分析,总结最新的表面防护技术,包括转化膜、电镀、表面涂层和多种复合处理技术,此外还介绍一些新的处理技术。发现转化膜技术在所有专利文献中占有极大的比例,说明在实际的工业应用中转化膜技术非常重要,且占主导地位。由于镁合金零部件形状和使用特性的多样性,单一的表面技术难以满足性能所需,越来越多的复合表面处理技术、环保型的新技术被发明用来进行镁合金的防护处理。此外,专利技术的投资成本、操作的难易程度、涂层的性能等因素极大地影响该专利技术的工业应用。
关键词:腐蚀;防护;镁合金;表面处理;专利
(Edited by YUAN Sai-qian)
Corresponding author: ZHANG Jin: Tel: +86-10-82377393;E-mail:zhangjin@ustb.edu.cn
DOI: 10.1016/S1003-6326(11)60799-1

