不同尺寸Mg-Gd-Y-Zn-Ag镁合金零件冷却后的腐蚀行为
来源期刊:中国有色金属学报(英文版)2021年第5期
论文作者:许诗源 刘楚明 万迎春 曾广 高永浩 蒋树农
文章页码:1291 - 1302
关键词:Mg-Gd-Y-Zn-Ag合金;冷却过程;长周期堆垛有序(LPSO)相;表面膜;微电偶腐蚀
Key words:Mg-Gd-Y-Zn-Ag alloy; cooling process; long-period stacking ordered (LPSO) phase; surface film; micro-galvanic corrosion
摘 要:研究不同尺寸的Mg-6Gd-3Y-1Zn-0.3Ag (质量分数,%)镁合金零件冷却后的腐蚀行为。小型零件冷却较快,其显微组织由镁基体和粗大的长周期堆垛有序结构(LPSO)相组成。大型零件冷却较慢,其显微组织除镁基体和粗大的LPSO相外,晶粒内部有薄片状LPSO相析出。析氢测试结果表明,大型零件的腐蚀速度高于小型零件。交流阻抗测试显示这是由于大型零件上的表面膜的保护能力较差。通过观察腐蚀后形貌发现,大型零件样品中晶粒内析出的LPSO相诱发更加严重的微区电偶腐蚀,加速样品表面保护膜的破坏。
Abstract: The corrosion behaviour of Mg-6Gd-3Y-1Zn-0.3Ag (wt.%) alloy components with different sizes after cooling was investigated. The alloys in the small components (SC) cooled fast, which were composed of α-Mg matrix and coarse long-period stacking ordered (LPSO) phases. The alloys in the large components (LC) cooled slowly, and there were thin lamellar LPSO phases precipitating inside the grains, except for α-Mg matrix and coarse LPSO phases. The hydrogen evolution test revealed that the corrosion rate of LC sample was higher than that of SC sample. Electrochemical impedance spectroscopy (EIS) test showed that the surface film on LC alloys provided worse protection. The corrosion morphologies indicated that the precipitation of the thin lamellar LPSO phases in LC sample caused severe micro-galvanic corrosion, which accelerated the rupture of the surface film.
Trans. Nonferrous Met. Soc. China 31(2021) 1291-1302
Shi-yuan XU, Chu-ming LIU, Ying-chun WAN, Guang ZENG, Yong-hao GAO, Shu-nong JIANG
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 26 May 2020; accepted 31 December 2020
Abstract: The corrosion behaviour of Mg-6Gd-3Y-1Zn-0.3Ag (wt.%) alloy components with different sizes after cooling was investigated. The alloys in the small components (SC) cooled fast, which were composed of α-Mg matrix and coarse long-period stacking ordered (LPSO) phases. The alloys in the large components (LC) cooled slowly, and there were thin lamellar LPSO phases precipitating inside the grains, except for α-Mg matrix and coarse LPSO phases. The hydrogen evolution test revealed that the corrosion rate of LC sample was higher than that of SC sample. Electrochemical impedance spectroscopy (EIS) test showed that the surface film on LC alloys provided worse protection. The corrosion morphologies indicated that the precipitation of the thin lamellar LPSO phases in LC sample caused severe micro-galvanic corrosion, which accelerated the rupture of the surface film.
Key words: Mg-Gd-Y-Zn-Ag alloy; cooling process; long-period stacking ordered (LPSO) phase; surface film; micro-galvanic corrosion
1 Introduction
The demand for high energy efficiency and low emission vehicles is rising with the global energy crisis and environmental pollution becoming more and more serious, and the lightweight design is an efficient way to achieve this demand. The consumption of magnesium (Mg) alloys as the lightest structure metal in the vehicle industry steadily has increased in recent years, and the application in the automobile industry has also developed from small components, such as steering wheels, steering column parts and instrument panels to large body parts [1-3]. Compared with the small components, Mg in large components might exhibit different cooling processes. Mg in small components might cool down to room temperature within dozens of minutes, and the microstructure at high temperature might be still maintained during the process. However, the same cooling process takes more than ten hours for the large components, and the precipitation would occur due to the decomposition of supersaturate Mg matrix during the period. The properties of Mg alloys are also affected by the different microstructures. Thus, the research on the effect of cooling processes is beneficial to the development of high-performance Mg alloys.
Mg-RE-Zn alloy with long-period stacking ordered (LPSO) phases is a kind of magnesium alloy with high mechanical properties [4-7]. The addition of silver (Ag) to Mg-RE-Zn alloy induced the additional precipitation or segregation of Ag atoms at the interfaces, which enhanced the age-hardening response and further improved the mechanical properties of alloy [8-12]. LPSO phases were also observed in Mg-RE-Zn-Ag alloys [8]. Thus, the variation in LPSO phases affects the property of the alloys. YAMASAKI et al [13] found that the precipitation of LPSO phases was observed in the Mg-Gd-Zn alloys after aging at 400 °C for 10 h. LIU et al [14] found that lamellar 14H phase was formed in the α-Mg matrix during the high temperature annealing at 773 K. BI et al [15] also found that there was LPSO precipitation in homogenized Mg-Dy-Zn alloys with low cooling rate (2 °C/min). These studies indicate that cooling processes affect the precipitation of LPSO phases in Mg-RE-Zn alloys.
Numerous previous studies indicated that the second phases played an important role in the corrosion behavior of magnesium alloys [16,17], which exhibited the double effects on the corrosion property. SONG et al [18-20] reported that the corrosion mechanism of Mg-Al alloys was influenced by the volume fraction and distribution of β-phase (Mg17Al12). When the volume fraction of β-phase was low, the phase formed discontinuously in the α-Mg grain boundary region, which acted as the galvanic cathode and accelerated the degradation of α-Mg matrix. In contrast, the β-phase with high volume fraction formed the interconnected network composed of the eutectic phases, which acted as the barrier and inhibited the propagation of corrosion on the α-Mg matrix. Similar phenomena were also observed in other Mg alloys [21-23]. The volume fraction and morphology of second phases have great influence on the corrosion resistance of Mg alloys. LPSO phases as other second phases showed the complex effects on corrosion resistance due to the difference in the volume fraction, morphology and distribution. In general, the existence of LPSO phases accelerated the dissolution of Mg matrix by causing the micro-galvanic corrosion [24-26]. However, some recent studies presented the opposite results. ZHANG et al [27] found that the Mg-11.3Gd- 2.5Zn-0.7Zr (wt.%) alloy with LPSO phases exhibited a better corrosion resistance than Mg-10.2Gd-3.3Y-0.6Zr (wt.%) alloy without LPSO phase. PENG et al [28] reported that the 14H-LPSO phase enhanced the corrosion resistance of Mg-2Dy-0.5Zn (at.%) alloy through improving remediation ability. The influences of LPSO phases on the corrosion resistance were still unclear, and the micro-mechanism requires more investigation.
Herein, the homogenized Mg-6Gd-3Y-1Zn-0.3Ag (all in wt.% unless specified separately) alloys were cooled with different methods to simulate the cooling process in small and large components. The corrosion behaviour of the two Mg-6Gd-3Y-1Zn-0.3Ag components in 3.5 wt.% sodium chloride (NaCl) solution was investigated. The aims were to investigate the difference in microstructure between small and large Mg-6Gd- 3Y-1Zn-0.3Ag components, the corrosion property of the two Mg-6Gd-3Y-1Zn-0.3Ag components, and the influence of cooling processes on the corrosion property and mechanism. It is expected to provide some ideas for the development of Mg-Re-Zn-Ag components with high corrosion resistance.
2 Experimental
2.1 Material preparation
As-cast Mg-6Gd-3Y-1Zn-0.3Ag alloy was prepared by semi-continuous casting method. Pure magnesium (99.96%), pure zinc (99.99%), pure silver (99.96%), magnesium gadolinium master alloy (25% gadolinium) and magnesium yttrium master alloy (25% yttrium) as the raw materials were melted in a resistance furnace under the mixed protective atmosphere of carbon dioxide and sulfur hexafluoride. The composition of the alloy is listed in Table 1.
Table 1 Chemical composition of Mg-6Gd-3Y-1Zn- 0.3Ag alloy (wt.%)

The as-cast ingot was cut into 10 mm × 10 mm × 10 mm blocks, and then the blocks were homogenized at 510 °C for 120 h and cooled by different methods. Subsequently, some blocks were cooled by water quenching cooling method to room temperature to simulate the rapid cooling process (the cooling rate is about 25.5 °C/min) in small components (SC). The other blocks were treated by furnace cooling to room temperature for 12 h to simulate the slow cooling process (the cooling rate is about 0.7 °C/min) in large components (LC).
2.2 Microstructure observation
The phase analysis of Mg-6Gd-3Y- 1Zn-0.3Ag alloy was performed using Rigaku D/Max 2500 X-ray diffraction (XRD) with copper target, and the obtained XRD patterns were analyzed by MDI Jade 6.0 software. The morphologies of alloys were observed by a Quantan-200 scanning electron microscopy (SEM). The area fraction of second phases was calculated by image-pro plus software.
The samples for corrosion morphology observation were immersed in 3.5 wt.% NaCl solution at 25 °C for different time, and then dried in a cool air stream. The corrosion morphologies of samples were recorded by the optical microscope and SEM. The severe corrosion areas on samples turned black under the optical microscope, and the fraction of the severe corrosion areas was calculated by the image-pro plus software. Three samples of each alloy (SC and LC) were calculated to minimize the deviations. The morphologies of corrosion products were observed by SEM after spray-gold, and the corrosion morphologies of alloy surface were also observed by SEM after the removal of corrosion products through 180 g/L chromium trioxide solution at 25 °C for 15 min.
2.3 Hydrogen evolution test
The corrosion rate of samples was evaluated by hydrogen evolution test [29]. The cubic samples for the corrosion rate test were successively ground to 1500 grit SiC paper before testing. The samples were encapsulated in epoxy resin with the 10 mm × 10 mm surface exposed to the electrolyte. The samples were immersed in 140 mL of 3.5 wt.% NaCl at 25 °C in beakers. The hydrogen bubbles generated from the sample surfaces were collected by a burette. The decrease of the solution level in the burette reflected the amount of hydrogen generated. The hydrogen levels were recorded every 12 h, and the total immersion time was 48 h. The corrosion rate, RH, was evaluated according to the hydrogen evolution rate, VH, as follows [29]:
RH=2.15VH (1)
Similarly, three samples of each alloy (SC and LC) were tested to minimize the deviations.
2.4 Electrochemical impedance spectroscopy (EIS) test
The electrochemical impedance spectroscopy tests were carried out on the electrochemical workstation (CS310, Wuhan CorrTest Instruments Co., Ltd). The tests were performed in 3.5 wt.% NaCl solution. A typical three-electrode cell was used with a saturated calomel-electrode (SCE) reference electrode, Pt counter electrode and the samples as working electrode. The samples were connected with a wire to one side and encapsulated in epoxy resin with a 1 cm2 surface exposed to the electrolyte. The EIS tests were measured at the open circuit potential (OCP) with amplitude of 10 mV. The scan frequency ranged from 100 kHz to 0.01 Hz. The EIS tests were performed on the different samples after immersion for 30 min, 12 and 24 h, respectively.
3 Results
3.1 Microstructure and phase composition
Figure 1 shows the SEM images and XRD patterns of alloys with different cooling processes. As shown in Figs. 1(a, b), coarse gray phases were distributed at the grain boundaries, and fine cubic white phases were randomly distributed both at the grain boundaries and inside the grains. Compared with SC sample, thin lamellar gray phases were precipitated in the grain interior of LC sample (Figs. 1(c, d)). According to the XRD patterns (Fig. 1(e)) and previous works [30,31], the coarse gray phases at grain boundaries and thin lamellar gray phases in the grain interior are LPSO phases. The fine cubic white phases are RE-rich phases, which were not detected in the XRD patterns due to their small volume fractions. Figure 1(f) presents the area fraction of coarse LPSO phases, and the area fractions of coarse LPSO phases are similar in SC and LC samples. These results indicate that the slow cooling process in LC sample leads to the precipitation of thin lamellar LPSO phases in the grain interior.
3.2 Hydrogen evolution
Figure 2 shows the hydrogen evolution test results of SC and LC samples after immersion in 3.5 wt.% NaCl for 48 h. The corrosion rate curves reveal that the corrosion rates of both samples increase with the increase of immersion time, and the corrosion rate of LC sample is higher than that of SC sample.
3.3 EIS test results
The EIS tests were carried out to investigate the effect of cooling process on the corrosion behaviour, and the results are presented in Fig. 3.
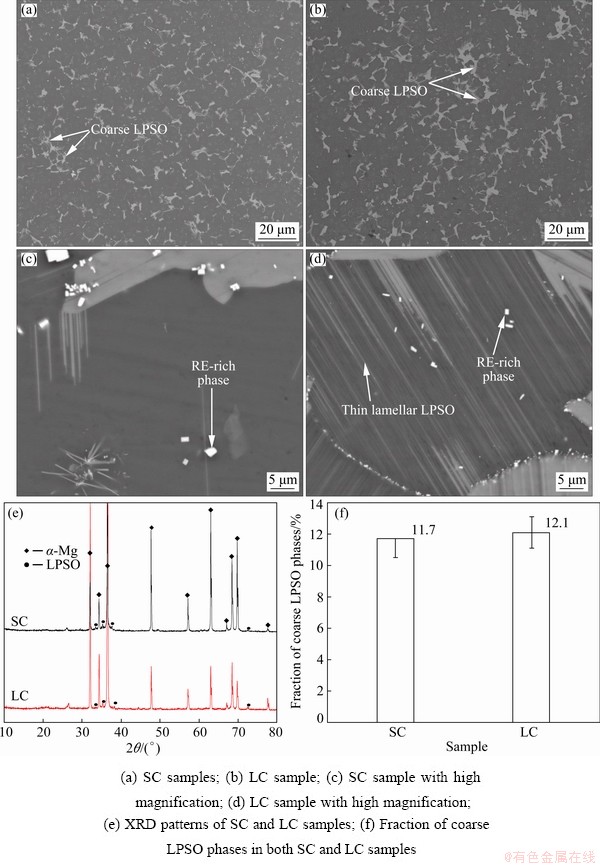
Fig. 1 SEM images of Mg-6Gd-3Y-1Zn-0.3Ag sample
The Nyquist plots shapes of SC and LC samples are similar. The Nyquist plots of both samples consisted of two capacitance loops and an inductance loop after immersion for 30 min. The capacitance loop at high frequencies was related to the charge transfer resistance and the electric double layer between the α-Mg matrix and solution [32-35]. The capacitance loop at low frequencies presented the diffusion process in the surface film [35]. The inductance loop at low frequencies presented the relaxation process of the absorbed monovalent magnesium ions (Mg+) [36-38]. The equivalent circuit is shown in Fig. 3(c), where Rs is the solution resistance, Rt is the charge transfer resistance, Rf is the film resistance, constant phase elements (CPE) were used to replace capacitances, CPEf (Qf and nf) is the capacitance of surface film, CPEdl (Qdl and ndl) is the capacitance of double electric layer, RL is the resistance of absorbed Mg+ relaxation process, and L is the inductance of absorbed Mg+ relaxation process. After immersion for 12 and 24 h, the Nyquist plots consisted of a capacitance loop at high frequencies and an inductive loop at low frequencies. The capacitance loop at high frequency presented the charge transfer resistance and the double electric layer. The inductive loop presented the relaxation process of absorbed Mg+. Thus, the charge transfer process and inductance behaviour were considered in the equivalent circuit (Fig. 3(d)). The similar shapes of Nyquist plots in SC and LC samples indicate that the corrosion processes of both samples are similar.
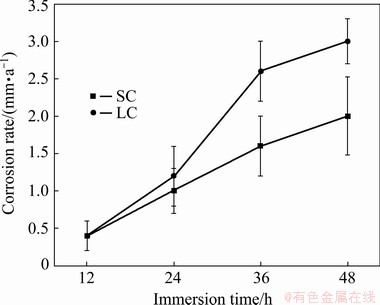
Fig. 2 Corrosion rate as function of immersion time in 3.5 wt.% NaCl solution
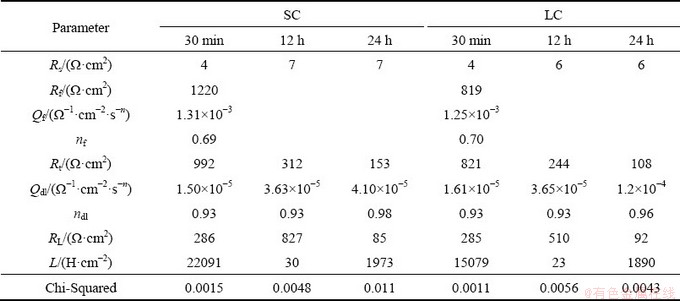
The fitting results are listed in Table 2. The Rt values of both samples decrease with the increase of immersion time and LC sample have lower Rt values. These tendencies indicate that the corrosion rates of both samples increase with prolonging immersion time, and the corrosion rate of LC sample is higher than that of SC sample. These results are in good agreement with the hydrogen evolution test results.
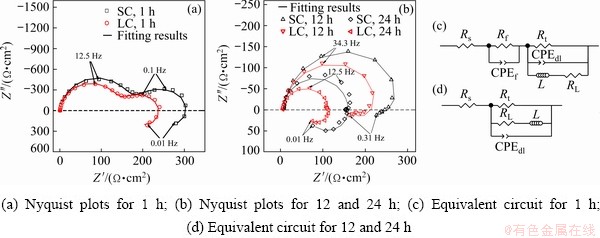
Fig. 3 EIS results
Table 2 Fitting results of EIS for SC and LC samples
3.4 Corrosion morphology
The optical images of SC and LC samples as function of immersion time are shown in Figs. 4(a) and (b). The alloy surface was covered by a gray surface film after immersion for 30 min. With the increase of immersion time, the gray areas and black areas were observed on the alloy surface. Figure 4(c) presents the relation between the fraction of black areas and corrosion rates. Two obvious trends were concluded from these results: (1) corrosion rates of both samples increased with the propagation of black areas, which meant that the propagation of black areas deteriorated the corrosion resistance of SC and LC samples; (2) the propagation rate of black areas in LC sample was faster than that in SC sample.
The microscopic corrosion morphologies of both samples were observed by SEM to further understand the effect of cooling process on corrosion behaviour, and the morphologies of surface films are shown in Fig. 5. Both of the Mg matrix and second phases were covered by a smooth surface film after immersion for 30 min (Figs. 5(a) and (b)). After immersion for 24 h, the smooth surface films on the gray areas (Figs. 5(c) and (d)) are similar to the surface film after immersion for 30 min, the morphology of surface film on the black areas exhibits different morphologies. There are many spongy corrosion products on the film surface of the black areas (Figs. 5(e) and (f)), and the rough surface of spongy corrosion products causes the diffuse reflection of light on the surface film, making these areas black under optical microscope. Some cracks were observed on the corrosion product films. SONG et al [39] indicated that these cracks were caused by the effect of vacuum during SEM, which had no connection with the corrosion process. The EDS results are listed in Table 3, and all surface films were enriched with Mg and O elements. There were the peaks for α-Mg and Mg(OH)2 in the XRD results (Fig. 6). The EDS and XRD results reveal that the main composition of surface film is Mg(OH)2.
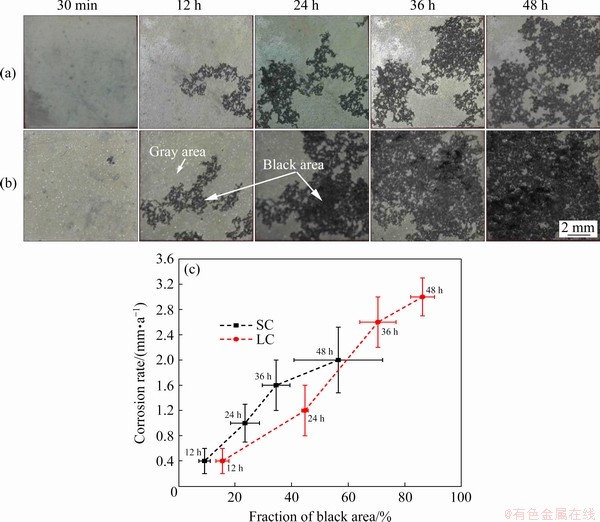
Fig. 4 Optical images (a, b) of SC (a) and LC (b) samples after immersion in 3.5 wt.% NaCl solution for different time, and corrosion rate as function of fraction of black areas (c)
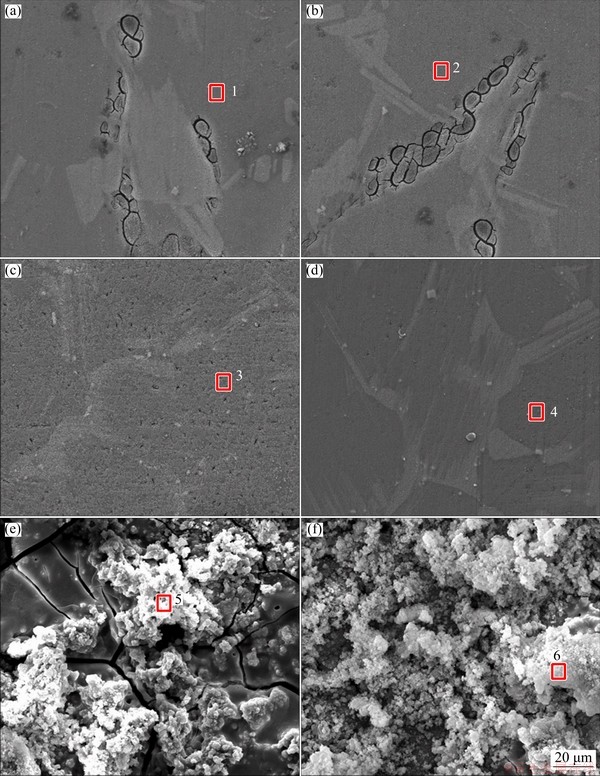
Fig. 5 SEM images of SC (a, c, e) and LC (b, d, f) samples showing surface film after immersion for 30 min (a, b), surface film on gray areas after immersion for 24 h (c, d), and surface film on black areas after immersion for 24 h (e, f)
Table 3 EDS results of surface film (at.%)
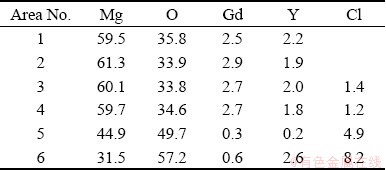
Figure 7 presents the corrosion morphologies of the alloy surface after removing the corrosion products. The corrosion pits were detected in the vicinity of second phases in both samples after immersion for 30 min (Figs. 7(a) and (b)). The corrosion pits in SC sample were located in the vicinity of coarse LPSO phases. The corrosion pits in LC sample appeared not only in the vicinity of coarse LPSO phases but also in the vicinity of thin lamellar LPSO phases. These results indicated that LPSO phases caused micro-galvanic corrosion and acted as the galvanic cathode, the micro-galvanic corrosion ruptured the surface films on anodic α-Mg matrixes. Figures 5(c)-(f) show the morphologies after 24 h immersion. The α-Mg matrix on the gray area was slightly corroded, only shallow corrosion pits were observed, which indicated that the surface films on the gray areas provided effective protection. The α-Mg matrix on black area was severely corroded, which meant that the surface films on the black areas were severely ruptured.
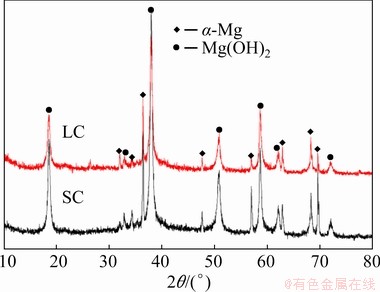
Fig. 6 XRD patterns of SC and LC samples after 24 h immersion in 3.5 wt.% NaCl solution
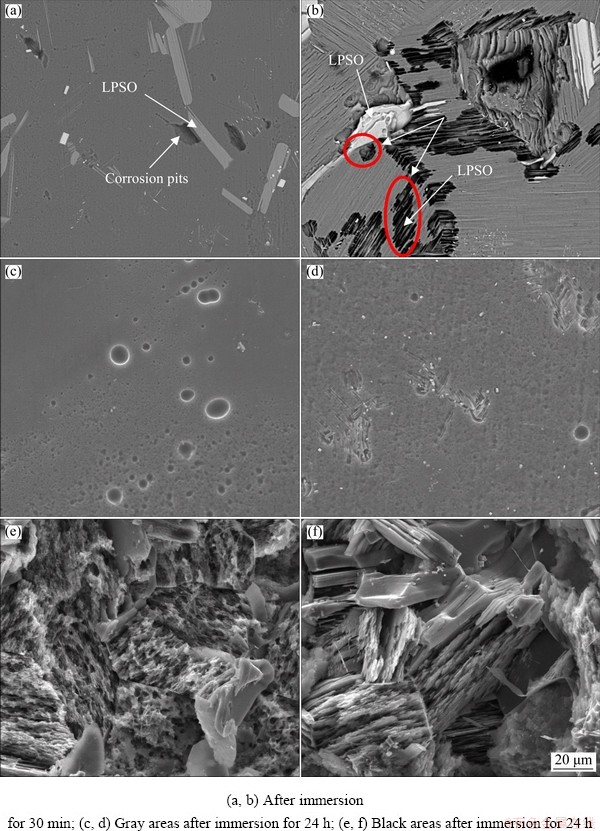
Fig. 7 SEM images of SC (a, c, e) and LC (b, d, f) samples after removal of corrosion products
4 Discussion
The hydrogen evolution test and EIS test showed that the corrosion rate of LC sample with slow cooling process is higher than that of SC sample with rapid cooling process. The EIS test also provided information about the influence of surface films on corrosion behaviour. On the basis of literature data [40,41], the interface of Mg alloys was presented as show in Fig. 8. The Mg matrix was covered by a duplex film: a thin compact MgO film contacted with the Mg matrix and a relatively thick porous Mg(OH)2 film in the outer. The compact MgO film effectively protected the Mg matrix from the corrosion of NaCl solution. The dissolution of Mg matrix only occurred on the film-free areas.
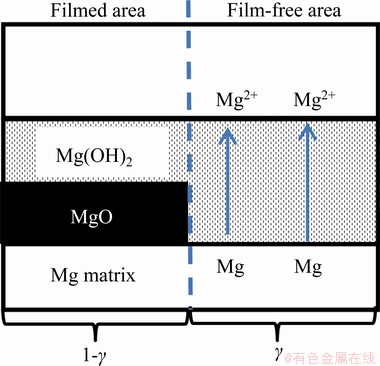
Fig. 8 Illustration of Mg interface in NaCl solution
On the film-free areas, the dissolution of Mg matrixes occurred as follows:
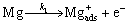 (2)
(2)
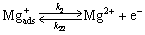 (3)
(3)
The high-frequency capacitive loop corresponded to the charge transfer resistance of Reactions (2) and (3), which was in parallel to the double electric layer capacitance. According to the reaction of Mg dissolution, EIS results can be calculated and simulated by assuming that the adsorbate 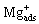 obeys the Langmuir’s isotherm and the rate constants of the electrochemical reactions follow the Tafel’s law.
obeys the Langmuir’s isotherm and the rate constants of the electrochemical reactions follow the Tafel’s law.
The normalized time constant Ki corresponds to its rate constant ki by
Ki=kiexp[bi(φ-φcorr)] (4)
where bi is the charge transfer coefficient, and φcorr is the corrosion potential obtained at the steady- state in solution.
The charge balance can be expressed as
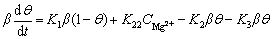 (5)
(5)
where β is the maximum number of adsorption site for 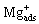 per unit surface, θ is the surface coverage fraction of
per unit surface, θ is the surface coverage fraction of 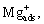
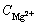 is the interfacial concentration of Mg2+ in solution.
is the interfacial concentration of Mg2+ in solution.
The faradic current density can be expressed as
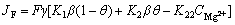 (6)
(6)
where F is the faraday constant, γ is the fraction of film-free area.
At steady state, the charge transfer resistance Rt can be calculated by [42]
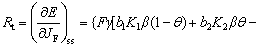
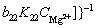 (7)
(7)
The Eq. (7) reveals that the Rt is inversely proportional to the fraction of film-free areas γ. The fitting results show that the Rt in SC and LC samples decreased with the increase of immersion time, which indicated that the protective compact MgO films were gradually ruptured by NaCl solution and the film-free areas propagated during the whole immersion process. The propagation of the film-free areas offered more sites for the dissolution of Mg matrix. As a result, the corrosion rates increased with the propagation of film-free areas. At the same time, the fitting results show that the Rt in LC sample is lower than that in SC sample, which means that the film-free area on LC sample is larger than that on SC sample.
The corrosion morphology confirmed the EIS results. Figure 4 shows that the black area on LC sample is larger than that on SC sample. The SEM images in Fig. 5 and Fig. 7 reveal that the protection performance of surface films on the gray areas is much better than that on the black areas. It can be inferred that the fraction of the film-free areas on the black areas is much higher than that on the gray areas. The film-free areas on the gray areas are negligible with respect to the film-free areas on the black areas. The fraction of the film-free areas (γ) can be calculated by
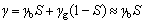 (8)
(8)
where γb is the fraction of film-free areas on the black areas, γg is the faction of film-free areas on the gray areas, and S is the fraction of black areas. Equation (8) reveals that the fraction of film-free areas is inversely proportional to the fraction of black areas. The larger black areas on LC sample meant that there were larger film-free areas on LC sample than SC sample. Therefore, the surface film of LC sample provided worse protection performance.
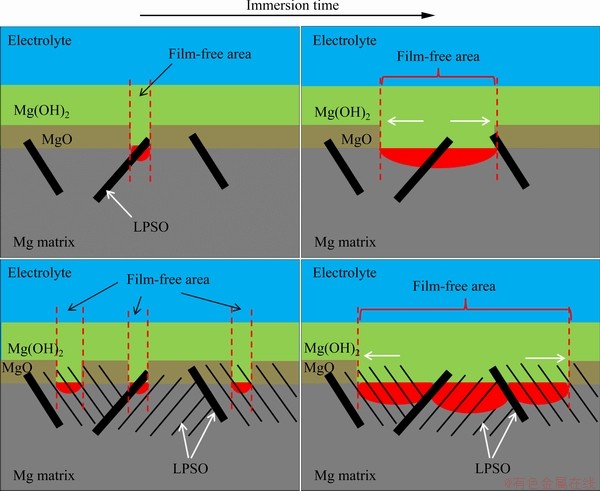
Fig. 9 Schematic illustration showing influence of thin lamellar LPSO precipitation on surface film
The corrosion morphology images in Figs. 7(a) and (b) indicate that the larger film-free areas on LC sample are related to the precipitation of thin lamellar LPSO phases in grain interior, and the influence of thin lamellar LPSO is schematically shown in Fig. 9. Both of the coarse and thin lamellar LPSO phases caused micro-galvanic corrosion and acted as galvanic cathodes. The α-Mg matrix around LPSO phases acted as galvanic anodes. The micro-galvanic corrosion accelerated the rupture of the surface film on anodic sites, which was due to the concentration of chloride ions and acidic pH [43]. The original duplex film was replaced by porous Mg(OH)2 film [41,44]. More importantly, these small local anodes propagated away from their initiation sites and continually ruptured the surface film [45,46]. In LC sample, the extra thin lamellar LPSO phases offered more galvanic cathodes. LC sample was subject to severe micro-galvanic corrosion, and there were more local anodes propagating on the LC sample surface, which accelerated the rupture of the surface film. As a result, the corrosion resistance of LC sample is worse than SC sample.
5 Conclusions
(1) The slow cooling process in LC sample leads to the precipitation of thin lamellar LPSO phases in the grain interior.
(2) The corrosion rate of LC sample in 3.5 wt.% NaCl solution is higher than that of SC sample, which is due to the worse protection of surface film in LC sample.
(3) The precipitation of thin lamellar LPSO phases in LC sample causes severe micro-galvanic corrosion, which accelerates the rupture of surface films on LC sample.
Acknowledgments
The authors are grateful for the financial supports from the National Natural Science foundation of China (Nos. 51574291, 51874367).
References
[1] MORDIKE B L, EBERT T. Magnesium: Properties— applications—potential [J]. Materials Science and Engineering A, 2001, 302(1): 37-45.
[2] ABBOTT T B. Magnesium: Industrial and research developments over the last 15 years [J]. Corrosion, 2014, 71(2): 120-127.
[3] ESMAILY M, SVENSSON J E, FAJARDO S, BIRBILIS N, FRANKEL G S, VIRTANEN S, ARRABAL R, THOMAS S, JOHANSSON L G. Fundamentals and advances in magnesium alloy corrosion [J]. Progress in Materials Science, 2017, 89: 92-193.
[4] LIAO Hong-xin, KIM Jongh-yun, LV Jin-bei, JIANG Bin, CHEN Xian-hua, PAN Fu-sheng. Microstructure and mechanical properties with various pre-treatment and Zn content in Mg-Gd-Y-Zn alloys [J]. Journal of Alloys and Compounds, 2020, 831: 154873.
[5] SHI Fei, WANG Chun-qing, ZHANG Zhong-ming. Microstructures, corrosion and mechanical properties of as-cast Mg-Zn-Y-(Gd) alloys [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(7): 2172-2180.
[6] XU C, ZHENG M Y, XU S W, WU K, WANG E D, KAMADO S, WANG G J, LV X Y. Ultra high-strength Mg-Gd-Y-Zn-Zr alloy sheets processed by large-strain hot rolling and ageing [J]. Materials Science and Engineering A, 2012, 547: 93-98.
[7] KAWAMURA Y, HAYASHI K, INOUE A, MASUMOTO T. Rapidly solidified powder metallurgy Mg97Zn1Y2Alloys with excellent tensile yield strength above 600 MPa [J]. Materials Transactions, 2001, 42(7): 1172-1176.
[8] ZHU Y M, MORTON A J, NIE J F. Improvement in the age-hardening response of Mg-Y-Zn alloys by Ag additions [J]. Scripta Materialia, 2008, 58(7): 525-528.
[9] ZHANG Yu, WU Yu-juan, PENG Li-ming, FU Peng-huai, HUANG Fei, DING Wen-jiang. Microstructure evolution and mechanical properties of an ultra-high strength casting Mg-15.6Gd-1.8Ag-0.4Zr alloy [J]. Journal of Alloys and Compounds, 2014, 615: 703-711.
[10] YU Shi-lun, GAO Yong-hao, LIU Chu-ming, HAN Xiu-zhu. Effect of aging temperature on precipitation behavior and mechanical properties of extruded AZ80-Ag alloy [J]. Journal of Alloys and Compounds, 2015, 646: 431-436.
[11] LV Shu-hui, LU Xiao-ling, LI Yan-wei, MENG Fan-zhi, HUA Xi-ru, YANG Qiang, QIU Xin, MENG Jian, DUAN Qian. Crystallographic orientation relationships between the aggregated intermetallic phases in a casting Mg-Ag-Al alloy [J]. Materials & Design, 2020, 190: 108561.
[12] MENG Fan-zhi, LV Shu-hui, QIU Xin, YANG Qiang, MENG Jian, DUAN Qian. Interphase precipitation in an Ag-modified Mg-Al-La casting alloy [J]. Materials Characterization, 2020, 161: 110144.
[13] YAMASAKI M, SASAKI M, NISHIJIMA M, HIRAGA K, KAWAMURA Y. Formation of 14H long period stacking ordered structure and profuse stacking faults in Mg-Zn-Gd alloys during isothermal aging at high temperature [J]. Acta Materialia, 2007, 55(20): 6798-6805.
[14] LIU Huan, YAN Kai, YAN Jing-li, XUE Feng, SUN Jia-peng, JIANG Jing-hua, MA Ai-bin. Precipitation behavior of 14H LPSO structure in single 18R phase Mg-Y-Zn alloy during annealing at 773 K [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(1): 63-72.
[15] BI Guang-li, JIANG Jing, ZHANG Fan, FANG Da-qing, LI Yuan-dong, MA Ying, HAO Yuan. Microstructure evolution and corrosion properties of Mg-Dy-Zn alloy during cooling after solution treatment [J]. Journal of Rare Earths, 2016, 34(9): 931-937.
[16] ATRENS A, SONG Guang-ling, CAO Fu-yong, SHI Zhi-ming, BOWEN P K. Advances in Mg corrosion and research suggestions [J]. Journal of Magnesium and Alloys, 2013, 1(3): 177-200.
[17] ATRENS A, SONG Guang-ling, LIU Ming, SHI Zhi-ming, CAO Fu-yong, DARGUSCH M S. Review of recent developments in the field of magnesium corrosion [J]. Advanced Engineering Materials, 2015, 17(4): 400-453.
[18] SONG Guang-ling, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of diecast AZ91D [J]. Corrosion Science, 1998, 41(2): 249-273.
[19] SONG Guang-ling, ATRENS A, WU Xian-liang, ZHANG Bo. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride [J]. Corrosion Science, 1998, 40(10): 1769-1791.
[20] SONG Guang-ling, BOWLES A L, STJOHN D H. Corrosion resistance of aged die cast magnesium alloy AZ91D [J]. Materials Science and Engineering A, 2004, 366(1): 74-86.
[21] SONG Ying-wei, HAN En-hou, SHAN Da-yong, YIM Chang-dong, YOU Bong-sun. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys [J]. Corrosion Science, 2012, 65: 322-330.
[22] WU Peng-peng, XU Fang-jun, DENG Kun-kun, HAN Fu-yin, ZHANG Zhong-zhong, GAO Rui. Effect of extrusion on corrosion properties of Mg-2Ca-xAl (x=0, 2, 3, 5) alloys [J]. Corrosion Science, 2017, 127: 280-290.
[23] LIU Ming, SCHMUTZ P, UGGOWITZER P J, SONG Guang-ling, ATRENS A. The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys [J]. Corrosion Science, 2010, 52(11): 3687-3701.
[24] SRINIVASAN A, HUANG Y, MENDIS C L, BLAWERT C, KAINER K U, HORT N. Investigations on microstructures, mechanical and corrosion properties of Mg–Gd–Zn alloys [J]. Materials Science and Engineering A, 2014, 595: 224-234.
[25] ZHANG Xiao-bo, BA Zhi-xin, WANG Zhang-zhong, XUE Ya-jun. Microstructures and corrosion behavior of biodegradable Mg-6Gd-xZn-0.4Zr alloys with and without long period stacking ordered structure [J]. Corrosion Science, 2016, 105: 68-77.
[26] ZONG Xi-mei, ZHANG Jin-shan, LIU Wei, ZHANG Ya-tong, YOU Zhi-yong, XU Chun-xiang. Corrosion behaviors of long-period stacking ordered structure in Mg Alloys used in biomaterials: A review [J]. Advanced Engineering Materials, 2018, 20(7): 1800017.
[27] ZHANG Xiao-bo, WU Yu-juan, XUE Ya-jun, WANG Zhang-zhong, YANG Lei. Biocorrosion behavior and cytotoxicity of a Mg-Gd-Zn-Zr alloy with long period stacking ordered structure [J]. Materials Letters, 2012, 86: 42-45.
[28] PENG Qiu-ming, GUO Jian-xin, FU Hui, CAI Xue-cheng, WANG Yan-an, LIU Bao-zhong, XU Zhi-gang. Degradation behavior of Mg-based biomaterials containing different long-period stacking ordered phases [J]. Scientific Reports, 2014, 4: 1-8.
[29] SHI Zhi-ming, LIU Ming, ATRENS A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation [J]. Corrosion Science, 2010, 52(2): 579-588.
[30] XU C, ZHENG M Y, WU K, WANG E D, FAN G H, XU S W, KAMADO S, LIU X D, WANG G J, LV X Y. Effect of cooling rate on the microstructure evolution and mechanical properties of homogenized Mg–Gd–Y–Zn–Zr alloy [J]. Materials Science and Engineering A, 2013, 559: 364-370.
[31] ZHOU Xiao-jie, LIU Chu-ming, GAO Yong-hao, JIANG Shu-nong, HAN Xiu-zhu, CHEN Zhi-yong. Evolution of LPSO phases and their effect on dynamic recrystallization in a Mg-Gd-Y-Zn-Zr alloy [J]. Metallurgical and Materials Transactions A, 2017, 48(6): 3060-3072.
[32] ZHANG Jian-qing. Electrochemical measurement technology [M]. Beijing: Chemical Industry Publishing House, 2010. (in Chinese)
[33] GUO Xing-Wu, CHANG Jian-Wei, HE Shang-Ming, DING Wen-Jiang, WANG Xishu. Investigation of corrosion behaviors of Mg-6Gd-3Y-0.4Zr alloy in NaCl aqueous solutions [J]. Electrochimica Acta, 2007, 52(7): 2570-2579.
[34] CHEN Jun, SONG Ying-wei, SHAN Da-yong, HAN En-hou. Study of the corrosion mechanism of the in situ grown Mg–Al–CO32- hydrotalcite film on AZ31 alloy [J]. Corrosion Science, 2012, 65: 268-277.
[35] LIU Jin-hui, SONG Ying-wei, CHEN Jia-chen, CHEN Peng, SHAN Da-yong, HAN En-hou. The special role of anodic second phases in the micro-galvanic corrosion of EW75 Mg alloy [J]. Electrochimica Acta, 2016, 189: 190-195.
[36] CAO Chu-nan. On the impedance plane displays for irreversible electrode reactions based on the stability conditions of the steady-state—I. One state variable besides electrode potential [J]. Electrochimica Acta, 1990, 35(5): 831-836.
[37] CAO Chu-nan. On the impedance plane displays for irreversible electrode reactions based on the stability conditions of the steady-state—II. Two state variables besides electrode potential [J]. Electrochimica Acta, 1990, 35(5): 837-844.
[38] SONG Ying-wei, SHAN Da-yong, CHEN Rong-shi, HAN En-hou. Corrosion characterization of Mg–8Li alloy in NaCl solution [J]. Corrosion Science, 2009, 51(5): 1087-1094.
[39] SONG Ying-wei, XU Zu-bin, DONG Kai-hui, SHAN Da-yong, HAN En-hou. Investigation of microcracks on conversion film of AZ80 Mg alloy [J]. Surface Engineering, 2019, 35(6): 527-535.
[40] BARIL G, GALICIA G, DESLOUIS C, PEBERE N, TRIBOLLET B, VIVIER V. An impedance investigation of the mechanism of pure magnesium corrosion in sodium sulfate solutions [J]. Journal of The Electrochemical Society, 2007, 154: C108-C113.
[41] TAHERI M, PHILLIPS R C, KISH J R, BOTTON G A. Analysis of the surface film formed on Mg by exposure to water using a FIB cross-section and STEM–EDS [J]. Corrosion Science, 2012, 59: 222-228.
[42] CAO Chu-nan. An introduction to electrochemical impedance spectroscopy [M]. Beijing: Science Press, 2002. (in Chinese)
[43] SONG Ying-wei, SHAN Da-yong, CHEN Rong-shi, HAN En-hou. Effect of second phases on the corrosion behaviour of wrought Mg–Zn–Y–Zr alloy [J]. Corrosion Science, 2010, 52(5): 1830-1837.
[44] NORDLIEN J H, ONO S, MASUKO N, NISANCIOGLU K. A TEM investigation of naturally formed oxide films on pure magnesium [J]. Corrosion Science, 1997, 39(8): 1397-1414.
[45] WILLIAMS G, BIRBILIS N, MCMURRAY H N. The source of hydrogen evolved from a magnesium anode [J]. Electrochemistry Communications, 2013, 36: 1-5.
[46] CURIONI M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging [J]. Electrochimica Acta, 2014, 120: 284-292.
许诗源,刘楚明,万迎春,曾 广,高永浩,蒋树农
中南大学 材料科学与工程学院,长沙 410083
摘 要:研究不同尺寸的Mg-6Gd-3Y-1Zn-0.3Ag (质量分数,%)镁合金零件冷却后的腐蚀行为。小型零件冷却较快,其显微组织由镁基体和粗大的长周期堆垛有序结构(LPSO)相组成。大型零件冷却较慢,其显微组织除镁基体和粗大的LPSO相外,晶粒内部有薄片状LPSO相析出。析氢测试结果表明,大型零件的腐蚀速度高于小型零件。交流阻抗测试显示这是由于大型零件上的表面膜的保护能力较差。通过观察腐蚀后形貌发现,大型零件样品中晶粒内析出的LPSO相诱发更加严重的微区电偶腐蚀,加速样品表面保护膜的破坏。
关键词:Mg-Gd-Y-Zn-Ag合金;冷却过程;长周期堆垛有序(LPSO)相;表面膜;微电偶腐蚀
(Edited by Xiang-qun LI)
Corresponding author: Chu-ming LIU, Tel: +86-13327212336, E-mail: cmliu803@sina.com
DOI: 10.1016/S1003-6326(21)65578-4
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press

