
Characteristics of graphite felt electrode electrochemically oxidized for vanadium redox battery application
LI Xiao-gang(李晓刚)1, HUANG Ke-long(黄可龙)1, LIU Su-qin(刘素琴)1,
TAN Ning(谭 宁)1, CHEN Li-quan(陈立泉)1, 2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Institute of Physics, Chinese Academy of Sciences, Beijing 100080, China
Received 13 April 2006; accepted 18 September 2006
Abstract: The graphite felt was oxidized at a positive electrode potential in sulfuric acid solution. The electrochemical performance of the treated graphite felt served as electrode for vanadium redox battery was investigated with FT-IR, SEM, XPS, BET, cyclic voltammetry and testing VRB system, respectively. The results show that the molar ratio of O to C increases from 0.085 to 0.15 due to the increase of —COOH functional groups during electrochemical oxidation treatment, and the GF surface is eroded by electrochemical oxidation, resulting in the surface area increase from 0.33 m2/g to 0.49 m2/g. The VRB with modified GF electrode exhibits excellent performance under a current density of 30 mA/cm2. The average current efficiency reaches 94% and average voltage efficiency reaches 85%. The improvement of electrochemical activity for the electrode is ascribed to the increase of the number of —COOH group and the special surface of GF.
Key words: vanadium redox battery; graphite felt; electrochemical oxidation
1 Introduction
Energy storage technologies have attracted much attention with the utilization of solar energy, wind energy and some other renewable power resources. Compared with others, all vanadium redox flow battery(VRB) proposed by SUM et al[1-2] has such unique advantages as low cost, long cycle life, deep-discharge capability and clean as well as efficient generation of electricity. At present, it becomes one of the most practical candidates for energy reserving purposes[3-4].
An ideal electrode should possess higher electric conductivity and longer cyclic life in sulfuric acid containing concentrated and oxidizing pentavalent vanadium ion 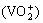 . Carbon, especially the GF, due to its greater specific surface area and good stability, is competitive when compared with metal electrode and conductive plastic composite electrode[2,6-12]. However, the lower electrochemical activity of commercial GF is still one of the major drawbacks that limit power density and voltage efficiency of VRB system. It was reported that oxygen functional groups on carbon surface behave as active sites for many electrochemical reactions[13-14]. The redox reactions would be strongly influenced by the concentration and nature of the oxygen functional groups on the electrode surface, since oxygen transfer is involved. SUN et al reported that the electrochemical activity of GF was improved by treating the GF with concentrated sulfuric acid. This result inspired researchers to develop other novel modification techniques to improve the electrochemical activity of GF material[5,15].
. Carbon, especially the GF, due to its greater specific surface area and good stability, is competitive when compared with metal electrode and conductive plastic composite electrode[2,6-12]. However, the lower electrochemical activity of commercial GF is still one of the major drawbacks that limit power density and voltage efficiency of VRB system. It was reported that oxygen functional groups on carbon surface behave as active sites for many electrochemical reactions[13-14]. The redox reactions would be strongly influenced by the concentration and nature of the oxygen functional groups on the electrode surface, since oxygen transfer is involved. SUN et al reported that the electrochemical activity of GF was improved by treating the GF with concentrated sulfuric acid. This result inspired researchers to develop other novel modification techniques to improve the electrochemical activity of GF material[5,15].
Electrochemical oxidation has been widely used in carbon material treatment, and was proved to be an effective procedure to improve the material activity [16-17]. However, the application of GF treated by electrochemical oxidation as electrode material in VRB system has not been reported yet. In current research, the electrochemical performance of GF oxidized at a positive electrode potential in VRB was investigated.
2 Experimental
2.1 Electrochemical oxidation of graphite felt
The analytical graded polyacrylonitrile-based GF(Shanghai Energy Carbon Limited Co., China) was cut into a size of 12 cm×18 cm before oxidation. The GF plate was used as anode, and Ti plate was used as cathode. The electrochemical oxidation was carried out in 1 mol/L H2SO4 solution galvanically. The potential was kept at 5-15 V. After treating for a predetermined duration, the TGF was taken out, and washed thoroughly with deionized water, then dried in a vacuum oven at 120 ?C for 5 h.
The oxidation degree (σ) of GF, which denoted oxidation coulomb quantity per gram GF, was determined according to the following equation:
σ=JSt/m (1)
where J is the oxidation current density, and S is the apparent area of GF, t is the oxidation time, m is the mass of GF.
2.2 Characterizations
XPS was performed on Escalab MK-II electron energy instrument (Vacuum Generator Co., England). The radiation was Mg Kα. The surface of TGF specimen was treated by Ar ions. The analyzer channel energy and multiplier voltage were set at 100 eV and 3.0 kV respectively. Monosorb direct reading specific surface analyzer (Quantachrome Co., America) was employed for BET measurement in He+30%N2 atmosphere under 0.1 MPa pressure at 150 ℃. The Fourier transform infrared spectra (FT-IR) were recorded from KBr disks containing the TGF powder on an AVATAR-360 instrument (Licolet Co., Ltd, USA). Scanning Electron Microscopy (SEM) was performed on a S-2700 instrument (HITACHI, Japan).
2.3 Electrochemical measurement
The CV measurement was carried out on CHI660 electrochemical workstation (CH Instruments Inc., America) with a ternate electrode system using TGF with area of 1.0 cm×1.5 cm as working electrode, Pt as counter electrode, and Standard Calomel Electrode(SCE) with Luggin Capillary as reference electrode.
2.4 Charge-discharge performance
Constant current charge-discharge tests for the redox flow battery were carried out on a battery test system PCBT-188-1D (Wuhan Lixing Co., China). Two pieces of TGF with area of 216 cm2 (12 cm×18 cm) were served as positive and negative electrodes respectively; PE-01 cation exchange membrane (Hangzhou Qianqiu Water Treatment Co., China) was served as separator. 200 mL anolyte of 1.6 mol/L V(Ⅲ) in 3 mol/L H2SO4 solution and 200 mL catholyte of 1.6 mol/L V(Ⅳ) in 3 mol/L H2SO4 solution[2] were stored separately in two tanks and were pumped into the positive and negative compartments of the battery during testing by two ZT60-600 pumps (Baoding Longer Precision Pump Co., China) respectively. The flow rate was kept at 25 mL/min, and the test temperature was 22 ℃.
3 Results and discussion
3.1 FT-IR analysis
FT-IR spectra of GF samples are shown in Fig.1. The peaks at 1 654 cm-1 and 3 421 cm-1 are assigned to the stretching vibration of C=O and —OH, respectively. The peak at 1 400 cm-1 is attributed to the curving vibration of —OH. The peaks at 1 049 cm-1 and 1 250 cm-1 correspond to the stretching vibration of C—O. No new absorbing peaks appear on the TGF sample, while some functional groups present the different absorbing intensity. The characteristic peak of —OH (3 421 cm-1) is obviously broadened and intensified. The peak intensity of C—H (at 2 975 cm-1) decreases with the increase of oxidation degree, and disappears at σ=3 000 C/g. The peak intensity of C=O (1 654 cm-1) increases with the increasing of σ value. The intensity of the —OH curving vibration peak presented (1 400 cm-1) decreases slightly. The intensity of stretching vibration peaks of C—O at 1 049 cm-1 and 1 250 cm-1 reduces rapidly with the increase of oxidation degree.

Fig.1 FT-IR spectra of GF with various oxidation degrees
The FT-IR results indicate that the electrochemical oxidation reduces the content of C—O groups and increases the number of —OH and C=O functional groups on the GF surface. It may be caused by the elimination of impurity of alcohol or aether from the surface of the GF and the formation of —COOH groups on the GF surface during oxidation treatment.
3.2 Graphite felt mass loss during oxidation
The relationship between GF mass loss and oxidation degree (σ) is displayed in Fig.2. At the beginning of the oxidation, the mass loss increases with the increase of σ values, and then experiences a stable period when σ varies in the range of 1 200-3 000 C/g. The mass loss increases quickly again when oxidation degree increases beyond 3 000 C/g. The mass loss might be due to the oxidation of the unsaturated carbon atom.

Fig.2 Mass loss of GF versus extent of electrochemical oxidation
3.3 XPS analysis
The general XPS spectra for GF and TGF samples are shown in Fig.3. It can be seen from Fig.3(a), the GF sample contains impurity elements such as Si, S and O besides the major carbon element. The molar ratio of O/C is about 0.085. The TGF sample contains only C and O elements; and the impurities Si and S disappear, as shown in Fig.4(b). The O/C ratio increases from 0.085 to 0.15.
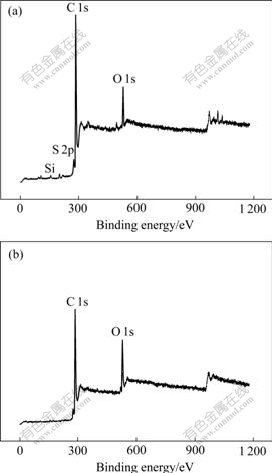
Fig.3 General spectra of major elements for different graphite felts: (a) GF; (b) TGF (Oxidation degree of 3 000 C/g)
The peak position and peak area percentage for the curve-fit of C 1s spectra are given in Table 1. The C 1s spectrum can be divided into five peaks. It can be seen from Table 1, the C—C content (Ⅰ) decreases from 58.23% to 50.80%, and COOH content (Ⅳ) increases from 11.06% to 15.89%. The absorbed CO/CO2 (Ⅴ) content rises from 6.525% to 9.51%. In addition, C=O (Ⅲ) content has a little increase from 5.93% to 6.71% and C—OH (Ⅱ) content decreases slightly from 18.25% to 17.09%. Those results are in accordance with the FT-IR analysis.
Table 1 Curve-fit data of C 1s spectra

The curve-fit data of O 1s spectra are listed in Table 2. Three peaks can fit the spectra: Peak Ⅰ(531.2-531.6 eV) and PeakⅡ(532.2-533.4 eV) are attributed to the O 1s signals from C—OH and C=O (or/and C—O—C) functional groups, respectively. Peak Ⅲ is assigning to the adsorbed water and probably some chemical absorbed oxygen (534.6-535.4 eV). When compared the spectra of GF with that of TGF, the C=O content has an obvious increase from 35.5% to 47.45%, while the C—OH content reduces sharply from 45.97% to 38.44%.
Table 2 Curve-fit data of O 1s spectra

In general, the functional groups of —COOH increase after electrochemical oxidation compared with GF sample. The increased activity of the electrochemical oxidized GF for the vanadium redox reactions can thus be attributed to the increased surface concentration of the functional groups of —COOH induced during activation. The —COOH groups on the electrode surface probably behave as active sites, catalyzing the vanadium species reactions. The mechanism of catalysis for reactions on the electrode surface can be hypothesized as follows.
On the positive half-battery, the reactions occur as
(2)
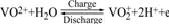
As can be seen from this reaction, the charge and discharge processes at the positive electrode involve the transfer of an oxygen atom, which is likely to be the rate determining step in the overall mechanism. The availability of oxygen groups on the electrode surface would thus be expected to affect overall rate of the reactions.
It can be concluded that electrochemical oxidation produces the functional group of —COOH, and the functional group catalyses the V(Ⅳ)/V(Ⅴ) redox reaction by producing active sites. The improvement in electrode activity is thus supposed to be due to the—COOH functional group produced by electrochemical oxidation. This is proved by the results of electrochemical experiment.
3.4 SEM and BET analysis
The SEM images of GF and TGF are shown in Fig.4. As can be seen in Fig.4, the GF sample surface is clean, while the TGF sample has some layered solid material clung on the graphite fiber surface, which is supposed to be the products of graphite crystallite corrosion due to electrochemical oxidation. The specific areas of GF sample and TGF sample obtained by BET single-point adsorption method are 0.33 m2/g and 0.49 m2/g, respectively. The increase of specific area is also related with the electrochemical oxidation process.

Fig.4 SEM images of different graphites: (a) GF; (b) TGF (Oxidation degree of 3 000 C/g)
3.5 Electrochemical property
The CV curves of GF are shown in Fig.5. Compared with untreated material, the TGF exhibits a great improvement of electrochemical activity for the V(Ⅳ)/ V(Ⅴ) couple. The graph shows a significant increase in redox peak area. The peak potential separation (Δφp) of 70 mV for TGF indicates that the electrochemical reaction on TGF is a quasi-reversible process.

Fig.5 Cyclic voltammograms at GF electrode before and after electro-oxidation in 3.0 mol/L H2SO4, V(Ⅳ) 0.008 7 mol/L, and V(Ⅴ) 0.008 7 mol/L (Sweep rate 0.001 V/s at 25 ℃).
An assembled test battery with TGF as electrode shows good charge and discharge property at 30 mA/cm2 as shown in Fig.6. The battery has 1.30-1.75 V charge voltage plateau and 1.50-1.25 V discharge voltage plateau, while the battery with GF as electrode has no charge voltage plateau and discharge voltage plateau at all. It is noticeable that the TGF performance depends on oxidation extent. Compared with the battery using TGF electrode treated with the extent of 1 200 C/g, the cell with TGF electrode treated with the extent of 3 000 C/g has a lower charge voltage plateau of 1.30-1.55 V and higher discharge voltage plateau of 1.45-1.25 V. This indicates that the TGF electrode treated with the extent of 3 000 C/g has lower polarization in VRB.

Fig.6 Charge-discharge curves of TGF at 30 mA/cm2 under different oxidation extents
The average charge voltage and discharge voltage of VRB at a current density of 30 mA/cm2 are 1.47 V and 1.34 V, respectively. The current efficiency is 94%, and the voltage efficiency is 84%.
3.6 Charge and discharge performance
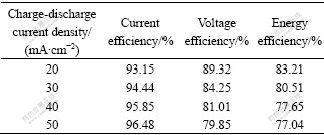
The current efficiency, voltage efficiency and energy efficiency at different charge/discharge current densities are listed in Table 3. It can be seen that the cell has good charge and discharge property. The current efficiency increases from 93% to 96% when the current density increases from 20 to 50 mA/cm2, while the voltage efficiency and energy efficiency decrease slightly. The energy efficiency of VRB at current density of 50 mA/cm2 is above 77%.
Table 3 Average efficiency values of 20 cycles for battery with TGF as electrodes under various current densities
4 Conclusions
1) The GF electrochemically oxidized in 1.0 mol/L H2SO4 exhibits a great improvement in performance in the VRB. The electrochemical activity of the TGF is enhanced due to the electrochemical oxidation, which increases the molar ratio of O to C and the number of—COOH functional groups on the surface.
2) The surface of TGF is eroded. As a result, the surface area increases.
3) The improvement of electrochemical activity is ascribed to two mechanisms. On one hand, the increase in the number of —COOH groups is benefit to the transfer of electron and oxygen element between VO2+ and  , which catalyzes the redox reaction on the surface of electrode; on the other hand, the real reaction area is increased by electrochemical oxidation.
, which catalyzes the redox reaction on the surface of electrode; on the other hand, the real reaction area is increased by electrochemical oxidation.
4) The VRB with TGF as electrode displays excellent charge-discharge property at current density of 20-50 mA/cm2.
References
[1] SUM E, SKYLLAS-KAZACOS M. A study of the V(Ⅱ)/V(Ⅲ) redox couple for redox flow cell applications [J]. J Power Sources, 1985, 15: 179-190.
[2] SUM E, RYCHCIK M, SKYLLAS-KAZACOS M. Investigation of the V(Ⅴ)/V(Ⅳ) system for use in the positive half-cell of a redox battery [J]. J Power Sources, 1985, 16: 85-95.
[3] FABJAN C, GACHE J, HARRER B. The vanadium redox-battery: An efficient storage unit for photovoltaic systems [J]. Electrochimica Acta, 2001, 47(5): 825-831.
[4] JOERISSEN L, GARCHE J, FABJAN C. Possible use of vanadium redox-flow batteries for energy storage in small grids and stand-alone photovoltaic systems [J]. J Power Sources, 2004, 127(1/2): 98-104.
[5] SUN B T, SKYLLAS-KAZACOS M. Chemical modification and electrochemical behaviour of graphite fibre in acidic vanadium solutions [J]. Electrochem Acta, 1991, 36: 513-517.
[6] RYCHCIK M, SKYLLAS-KAZAOCS M. Evaluation of electrode materials for all-vanadium redox flow cell [J]. J Power Sources, 1987, 19: 45-54.
[7] KAZACOS M, SKYLLAS-KAZACOS M. Performance of carbon plastic electrodes in vanadium redox cell [J]. J Electrochem Soc, 1989, 136: 2759-2760.
[8] BRUNGS A, KAZACOS M, HADDADI-ASL V. Preparation and evaluation of electrocatalytic oxide coatings on conducting carbon-polymer composite substrates for use as dimensionally stable anodes [J]. J Appl Electrochem, 1996, 26: 1117-1123.
[9] HUANG Ke-long, WU Qiu-mei, LIU Su-qin. Performance of graphite power-carbon black composite electrodes for the vanadium redox flow battery [J]. J Chinese Power Source, 2004, 2: 91-93. (in Chinese)
[10] WU Qiu-mei, HUANG Ke-long, SANG Shang-bin, LIU Su-qin, LI Xiao-gang. Study of PAN-graphite felt electrode in the vanadium redox flow battery [J]. J Chinese Power Source, 2005, 7: 456-458. (in Chinese)
[11] LI Xiao-gang, HUANG Ke-long, LIU Su-qin. Properties of the current collector of all vanadium redox flow battery [J]. J Chinese Battery Bimonthly, 2005, 2: 93-94. (in Chinese)
[12] HUANG Ke-long, TAN Ning, LIU Su-qin, LI Xiao-gang, CHANG Zhi-feng. Reaction mechanism of V(Ⅳ)/V(Ⅴ) redox couple at graphite felt electrode [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(5): 871-876. (in Chinese)
[13] LI Xu, HORITA K. Electrochemical characterization of carbon black subjected to RF oxygen plasma [J]. Carbon, 2000, 38(1): 133-138.
[14] SANTIAGO M, FORTUNY A, FABREGAT A, FONT J. Modified activated carbons for catalytic wet air oxidation of phenol [J]. Carbon, 2005, 43(10): 2134-2145.
[15] SUN B T, SKYLLAS-KAZACOS M. Chemical modification of graphite electrode materials for vanadium redox flow battery application (Part Ⅱ): Acid treatments [J]. Electrochim Acta, 1992, 37: 2459-2465.
[16] JUREWICZ K, BABEL K, LKOWSKI A, WACHOWSKA H. Ammoxidation of active carbons for improvement of supercapacitor characteristics [J]. Electrochimica Acta, 2003, 48(11): 1491-1498.
[17] JACOBSON N S, CURRY D M. Oxidation microstructure studies of reinforced carbon/carbon [J]. Carbon, 2006, 44(7): 1142-1150.
Foundation item: Project(02-09-01) supported by Pangang Group Pangzhihua Iron and Steel Research Institute, China
Corresponding author: HUANG Ke-long; Tel/Fax: +86-731-8879850; E-mail: klhuang@mail.csu.edu.cn
(Edited by YANG Bing)