J. Cent. South Univ. Technol. (2008) 15: 329-333
DOI: 10.1007/s11771-008-0062-3
Synthesis and extractive properties of bisthiophosphorylimines
TANG Rui-ren(唐瑞仁)1, SHI Xiao-ming(石晓明)1, ZHANG Qi-xiu(张启修)2, TANG Zi-long(唐子龙)3
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
3. School of Chemistry and Chemical Engineering, Hunan University of Science and Technology,
Xiangtan 411201, China)
Abstract: Novel transition metal complexes of bis(diarylsubstitutedthiophosphoryl)imines ligand derived from O,O-di(p- methoxylphenyl)thiophosphoryl chloride and O,O-di-(p-methoxylphenyl)thiophosphoryl amine with Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Fe(Ⅱ) and Mn(Ⅱ) were synthesized. The formation mechanism of complexes and their stereochemistry structures were investigated according to elemental analysis, infrared spectra and 31P-nuclear magnetic resonance spectra. The extractions of the ligand for different divalent metal ions, such as Zn(Ⅱ), Cd(Ⅱ), Cu(Ⅱ), Ni(Ⅱ), Fe(Ⅱ), Sn(Ⅱ), Mn(Ⅱ), Pd(Ⅱ), Hg(Ⅱ) and Fe(Ⅲ), were investigated in sulphate solution, respectively. The results show the metal atom is coordinated by 4 sulfur atoms in a square-planar fashion, and the titled compound has not only powerful ability to coordinate with cadmium from aqueous solution with a high extractive rate about 61.20% and a relatively weak complexation for other divalent metals with the extractive rate from 2.46% to 36.66%, but also a good selectivity to Fe(Ⅲ).
Key words: bis(thiophosphoryl)imines; transition metal complexes; synthesis; extraction
1 Introduction
Extensive crystallographic studies during the last few years have revealed the existence of M—S bonds in the active site of many metalloenzymes, involving sulfide ions (S2-), cys-thiolate, met-thioether, dithiolenic moieties, most prominently with the transition metals M (Fe, Ni, Zn, etc). The remarkable ability of enzymes is to catalyze organic reaction and to regulate their occurrence, which challenges the chemist to devise simpler organic compounds that will perform similar functions. Only the structures of active sites of enzymes are well understood, can it provide models of the synthesis of non-pepide organic systems that may simulate enzymatic behavior[1]. The complexes of organic compounds and metals cations can simulate the substrate selectivity of enzymes[2-4]. Bis-(thiophosphoryl)imines, (R2XPNXPR2)-, where R= —CH3 or —C6H5 and X=S, were first synthesized by SCHMIDPETER et al[5], and their derivatives were reported to possess important structures of targeting metalloenzymes that can form bis-coordinated complexes with divalent metals (such as Mn, Fe, Co, Ni)[6-11].
On the other hand, the commercial success for the selective solvent extraction of copper from aqueous acidic solution derived from oxide ores using ring alkylated o-hydrobenzophenone oxime[12] and salicyladoxime[13] has led a great interest in related processes that could be used for the selective solvent extraction of other metals. This interest is particularly true for cases where the concentration of the required metal in solution is smaller than main elements. Typical examples include attempts to selectively extract gallium from solutions containing high concentrations of aluminum[14], and zinc from solutions containing high concentrations iron(Ⅲ)[12]. It has been reported that ligands based on bis(dialkylsubstitutedthiophosphoryl)- imine are powerful complexing agents for zinc from aqueous solution and exhibit good selectivity to iron[12,14]. But there are few reports about the aryl substituted bis(thiophosphoryl)-imines and their complexes in literatures.
In order to explore the structures and the electronic properties of the ligands and metal complexes, a novel aryl substituted bis(p-methoxylphenyl thiophosphoryl)- imines (HL) and its transition metal complexes with Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Fe(Ⅱ) and Mn(Ⅱ) were synthesized. Their structures and the formation mechanism of the complexes were proposed according to the infrared(IR) and 31P-nuclear magnetic resonance (31P- NMR) spectra; the extractive behavior of the prepared ligand for Zn(Ⅱ), Cd(Ⅱ), Cu(Ⅱ), Fe(Ⅱ), Ni(Ⅱ), Pd(Ⅱ), Sn(Ⅱ), Mn(Ⅱ), Hg(Ⅱ), and Fe(Ⅲ) were also investigated.
2 Experimental
2.1 Reagent and instrument
All reagents used were commercially available and were purified and dried by the standard procedures. Uncorrected melting points were determined on an XRC-1 apparatus. Infrared(IR) spectra were recorded on a Perkin-Elmer1420 spectrophotometer with KBr plates. 1H-NMR and 31P-NMR spectra were recorded with a INOVA-400MHz apparatus in CDCl3. Elemental analyses(EA) were performed on a Perkin-Elmer 2400 elemental analyzer. Mass spectra(MS) were measured with an HP 5988 instrument. The pH values were measured using a PHS-3C pH meter.
2.2 Synthetic route for ligand
The synthetic route for ligand(HL) is shown in Fig.1.
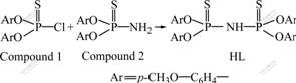
Fig.1 Synthetic route of ligand(HL)
2.3 Synthesis of ligand(HL)
The suspension of 0.4 mol phenol and sodium hydroxide solution (16.0 g, 0.4 mol in 24 mL water) was added into 80 mL benzene under vigorous stirring, then 1.2 mmol benzyl triethyl ammonium chloride(BTAC) was added to the reaction mixture, followed by dropwise addition of PSCl3 (34 g, 0.2 mol) from the addition funnel over a period of 30 min. The mixture was stirred until thin layer chromatography(TLC) showed no starting materials. 100 mL water was added and the organic phase was separated. The aqueous phase was extracted with 50 mL benzene for 3 times. The combined organic phase was washed with water until pH=7, dried with anhydrous Na2SO4 and evaporated, and the crude products was purified by recrystalization to give stable crystals compound 1 with a yield of 77.1%.
Ammonia gas was bubbled through a solution of 35.0 g (0.1 mol) O,O-di(p-methoxylphenyl)phosphoro- chlorothionate[15] (compound 1) in 250 mL benzene while keeping the reaction temperature below 40 ℃ for 2 h. The reaction mixture was transferred to separatory funnel and washed with water to remove ammonium chloride. The organic layer was separated and dried with Na2SO4 and then evaporated to give crude compound 2.
6.0 g (0.2 mol) sodium hydride in parafine (80%) was slowly added to a well stirred solution of 0.1 mol compound 2 in 150 mL anhydrous tetrahydrofuran (THF). After refluxed for 2 h, the monosodium salt of compound 2 was formed. Then a solution of 0.1 mol compound 1 in 100 mL anhydrous THF was added dropwise to the suspension reaction mixture. The mixture was stirred and refluxed for about 6 h. The THF was evaporated and the residue was diluted with 200 mL benzene, washed by diluted hydrochloric acid and then water. The mixture was dried with Na2SO4 and then evaporated, and 40.5 g white crystals were obtained after recrystallization from ethanol and benzene with yield of 64.0%; melting point: 98-101 ℃; IR: 3 258, 1 590, 1 488, 880, 1 017, 618, 748 cm-1; 1HNMR: δ=5.82(d, 1H, br, NH), 8.23-8.30(dd, 16H, aryl-H), 4.13(s, 12H, CH3); 31PNMR, δ=50.5; MS(fast atom bombardment mass spectrometry): m/z=633(M+, 95%); chemical formula is C28H29NO8P2S2; element analysis results(%): 1) calculated, C 53.08, H 4.49, N 2.21; 2) found, C 52.94, H 4.38, N 2.29.
2.4 Preparation of complexes [ML2, M=Cu(Ⅱ), Mn(Ⅱ), Fe(Ⅱ), Co(Ⅱ), Ni(Ⅱ)]
Bis(p-methoxylphenyl thiophosphoryl)imines, HL, was converted to the sodium salt, NaL, by reaction with Na in anhydrous THF and recrystallized from dioxolane. The obtained NaL 1.3 g (0.002 mol) in 20 mL water was added dropwise into a solution of 0.001 mol of MCl2 in water. The formed precipitate was filtered and the solid was dried in vacuum below 50 ℃. The crude product was dissolved in benzene and purified by flash chromatography on positive ion exchange resin column using water as eluent. The first fraction was evaporated and precipitated with petroleum ether (boiling point, 30-60 ℃) and dried under vacuum to give the complexes ML2.
2.5 Extraction
10 mL 0.2 mol/L organic solution of HL in benzene and 10 mL aqueous sulphate solution respectively containing 7.0 g/L Fe(Ⅲ) and 5 g/L other metal ions at pH=2.0 were mechanically shaken in a separation funnel until the equilibrium was attained (about 20 min). After phase separation, the pH value was measured while the analysis of metal ions in aqueous was carried out by the methods in Ref.[13] and the metal in organic phase was calculated out with subtractive methods[16].
3 Results and discussion
3.1 Preparation of compound 1
The O,O-di(p-methoxylphenyl)phosphorochloro- thionate (compound 1) was prepared by treatment of thiophosphyl chloride (PSCl3) with p-methoxylphenol directly, but PSCl3 did not react with phenol even under forcing conditions. In the presence of aluminum chloride, p-methoxylphenol and PSCl3 remained unchangeable after reflux for 2 h. But in the presence of NaOH or triethylamine as an acid acceptor PSCl3 reacted with two equivalent p-methoxylphenol, and O,O-di(p-metho- xylphenyl)phosphorochlorothionate (compound 1) was prepared in moderate yield. Furthermore, the formation of by-products such as mono-aryl derivatives [(ArO)P(S)Cl2] and thiophosphates [(ArO)3P(S)] was difficult to avoid. In an attempt to improve the yields of the reactions, phase-transfer-catalysts(PTCs), such as tetra butyl ammonium bromide(TBAB), and benzyl triethyl ammonium chloride(BTAC) were used. As expected, the yield of compound 1 was increased up to about 80%.
3.2 Elemental analysis
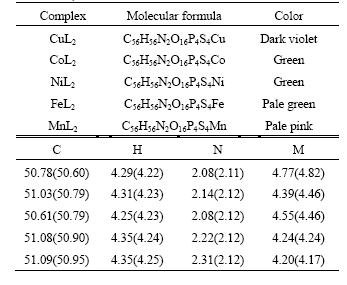
The elemental analysis results of compositions of the complexes obtained are listed in Table 1, where the values in the parentheses are the theoretical ones. The measurement values of the elements are basically in accordance with the theoretical ones with tolerances less than 0.8%. The results indicate that the complexes of HL with Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Fe(Ⅱ), Mn(Ⅱ) can be formulated as ML2.
Table 1 Results of elemental analysis of complexes (mass fraction, %)
3.3 31P-NMR of HL and its complexes
Just like acetylacetonate with two tautomeric forms, it will be appreciated that the structure of the titled compound may have two tautomeric forms, namely, SS and SH as shown in Fig.2. Although SS and HS are correctly orientated to facilitate the formation of ML2 by a single-step mechanism, the tautomer is likely to be the active chelating agent with M2+ by replacing the active hydrogen connected with the sulfur atom.
The proton-decoupled 31P-NMR spectra (Fig.3) of free ligand and its complexes in CDCl3 show just one signal at about δ=45-50, indicating that the major isomer is SS, in which P-atom is in the same chemical environments and not the tautomer SH where 31P-NMR spectra should show two signals for two magnetically non-equivalent P-nuclear. So it can be concluded that the ligand reacts with M2+, SS tautomerizes into SH, which is a more active chelating agent with M2+ to form the stable complexes. The mechanism of the formation of complexes can be proposed, as outlined in Fig.2.
3.4 Analysis of infrared spectra
The IR spectra of the ligand and the complexes are listed in Table 2.
Table 2 IR spectra of HL and ML2

Spectral differences in the free ligand HL and deprotonated ligand, L-1, of complexes(ML2), are marked by P—N, P—O—C, P—S vibrations as well as the presence (or lack) of N—H group vibrations. The disappearance of N—H at 3 258 cm-1(νN—H) in the IR spectra of the metal complexes indicates that the N—H group is deprotonated and coordinated with the metal ion. On the other hand, the P=S stretching mode is shifted to a lower frequency by about 50 cm-1, while the P—N stretching mode is shifted to a higher frequency by about
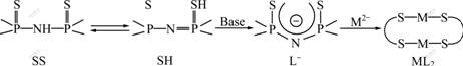
Fig.2 Proposed mechanism of formation of ML2
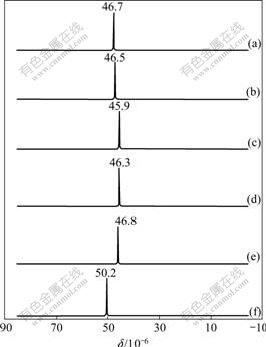
Fig.3 31P-NMR spectra of HL and its complexes: (a) HL; (b) CuL2; (c) CoL2; (d) NiL2; (e) FeL2; (f)MnL2
50 cm-1 compared to the free ligand HL, for the reason that the double bond P=S and the single bond P—N—P in the free ligand HL are converted to the P—S (with partly single bond) and P—N—P (partly double bond) respectively (see Fig.2). The new IR bands appearing at 400-410 cm-1 are assigned to ν(M—S). These IR results indicate that the ligand is coordinated with Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Fe(Ⅱ) and Mn(Ⅱ) through two S atoms. This is suggested that the complexes are square planar or nearly square planar, according to the common stereochemistry of this kind of compounds. The structure of the complexes prepared is thus proposed, as shown in Fig.4. In Fig.4, M=Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Fe(Ⅱ), Mn(Ⅱ) and Cd (Ⅱ).
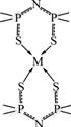
Fig.4 Structure of prepared complex
3.5 Extraction behavior of HL
Preliminary experiment was carried out to investigate the extraction behavior of HL for different divalent metal(Ⅱ) ions and Fe(Ⅲ) ions in their sulphate solutions. The data of distribution ratio(D) and rate of extraction(q)[16] are listed in Table 3.
The results show that HL is a powerful complexing agent for cadmium with a high extraction rate of 61.20%, and a relatively weak complexing agent for the other divalent metals with a extraction rate from 2.46% to 36.66%. Particularly, HL exhibits excellent selectivity to Fe(Ⅲ) with an extraction rate of 2.46%.
4 Conclusions
1) Bis(p-methoxylphenyl thiophosphoryl)imines and its complexes with divalent metals (Cu, Co, Ni, Fe, Mn, Cd) are prepared, and the structures are characterized by IR, NMR and EA. The metal atom coordinated by 4 sulfur atoms in a square-planar fashion.
2) A small scale solvent extraction process shows that the titled compound is a powerful complexing agent for cadmium(Ⅱ) with an extractive rate of 61.20% and a relatively weak complexing agent for other divalent metals such as Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Fe(Ⅱ) and Mn(Ⅱ) with an extraction rate in the range of 2.46%- 36.66%.
Table 3 Extraction behavior of HL for different metal ions

References
[1] DAVIS J J, HILL H A. The scanning probe microscopy of metalloproteins and metalloenzymes [J]. Chemical Communications, 2002, 136(5): 393-401.
[2] RICHARD H H, EDWARD I S. Preface: Bioinorganic enzymology [J]. Chemical Reviews, 1996, 96(7): 2237-2238.
[3] WHITE R J, MARGOLIS P S, TRIAS J, YUAN Z Y. Targeting metalloenzymes: A strategy that works [J]. Current Opinion in Pharmacology, 2003, 3(5): 502-507.
[4] THOMAS C M, WARD T O. Artificial metalloenzymes: Proteins as hosts for enantioselective catalysis [J]. Chemistry Society Review, 2005, 34(4): 337-346.
[5] SCHMIDPETER A, GROEGER H. Phosphazenes (II): Preparation and structure of tetraphenyldithioimidodiphosphinic acid [J]. Zeitschrift Fuer Anorganische und Allgemeine Chemie, 1966, 345(1/2): 106-118.
[6] MAGANAS D, STANILAND S S, GRIGOROPOULOS A, WHITE F, PARSONS S, ROBERTSON N, KYRITSIS P, PNEUMATIKAKIS G. Structural, spectroscopic and magnetic properties of M[R2P(E)NP(E)R′2]2 complexes, M=Co, Mn, E=S, Se and R, R′=Ph or iPr: Covalency of M—S bonds from experimental data and theoretical calculations [J]. Dalton Transactions, 2006, 19: 2301-2315.
[7] MENDEL R R. Molybenum: Biological activity and metabolism [J]. Dalton Transactions, 2005, 21: 3404-3409.
[8] PHILLIPS J R, SLAWIN A M, WHITE A J, WILLIAMS D J, WOOLLINS J D. Conformational control in PtS2(PR2)2N metallacycles via ligand-substituent effects (R=Ph or OPh) [J]. Dalton Transactions, 1995, 14: 2467-2468.
[9] ROSSI R, MARCHI A, MARVELLI L, MAGON L, PERUZZINI M, CASELLATO U, GRAZIANI R. Reactivity of the [Re.tplbond.NR]3+ and [Re.tplbond.N]2+ cores toward bis(diphenylphosphino)amine and its derivatives: Synthesis and crystal structures [J]. Dalton Transactions, 1993(5): 723-729.
[10] NOUAMAN M, ZAK Z, HERRMANN E, NAVRATIL O. The tetraphenyl of the imidodithiodiphosphoric acid and its palladium complex-crystal structures [J]. Zeitschrift fuer Anorganische und Allgemeine Chemie, 1993, 619(6): 1147-1153.
[11] LAN Zhuo-yue, HU Yue-hua, LIU Jian-she, WANG Jun. Solvent extraction of copper and zinc from bioleaching solutions with LIX984 and D2EHPA [J]. Journal of Central South University of Technology, 2005, 12(1): 45-49.
[12] CAMPBELL J, DALTON R F, QUAN P M. Solvent extraction process with thiophosphoryls for selective recovery of metal values from acidic aqueous solutions: US 5433855 [P]. 1995-07-18.
[13] ECKHARD H, OLDRICH B, PETR S. Bi- and tridentate organophosphorous compounds for extraction and complexation of metal ions [J]. Phosphorus, Sulfur and Silicon and the Related Elements, 1996, 109/110(1/4): 201-204.
[14] MRLEY J O, CHARLTON M H. Molecular modeling studies on the structure and electronic properties of bis(thiophosphoryl)amines and their zinc complexes [J]. Journal of Physical Chemistry A, 1998, 102(34): 6871-6878.
[15] TANG Rui-ren, ZHANG Qi-xiu, YANG Hua-wu. Preparation of diarylphosphorochlorothionate by phase transfer catalyst [J]. Chinese Journal of Synthetic Chemistry, 2003, 8(5): 381-382. (in Chinese)
[16] ZHANG Qi-xiu. Science and engineering in metallurgical separation [M]. Beijing: Science Press, 2004. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project([2004]52) supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars
Received date: 2007-08-30; Accepted date: 2007-11-29
Corresponding author: TANG Rui-ren, PhD, Professor; Tel: + 86-731-8836961; E-mail: trr@mail.csu.edu.cn