流速对船舶海底阀箱5083-H116铝合金电化学行为的影响
来源期刊:中国有色金属学报(英文版)2011年第8期
论文作者:Seung-Jun LEE Seong-Jong KIM
文章页码:1703 - 1709
关键词:铝制船舶;过度保护;船舶附生物预防系统;海底阀箱;流速
Key words:aluminium ships; over-protection; marine growth prevention system; sea-chest; flow velocity
摘 要:研究流速对铝制船舶的海底阀箱材料5083-H116铝合金电化学行为的影响。为检测电化学特性和流速对其性能的影响,实验在静态和通过搅拌仪产生的4个不同流速下进行,并使用超声波振荡器利用压电效应进行空化实验。结果表明,合金在施加流速后其腐蚀电流密度和损害程度较在静态环境下增加,更易于发生腐蚀。
Abstract:
Electrochemical behavior of 5083-H116 Al alloy with flow velocity of seachest material for Al ship was evaluated. To examine the electrochemical characteristics of flow velocity and its effects on the performance of the alloy, experiments were conducted at four flow velocity variables using static state with an agitator. An ultrasonic vibration generator using piezoelectric effect was used in cavitation test according to the requirements of in ASTM-G32. The results show that the corrosion current density and damage were increased by applying the flow velocity compared to static state. Therefore, it is determined that the case of applying flow velocity is weaker to the corrosion.

Seung-Jun LEE, Seong-Jong KIM
Division of Marine Engineering, Mokpo Maritime University, Mokpo 530729, Korea
Received 25 September 2010; accepted 24 December 2010
Abstract: Electrochemical behavior of 5083-H116 Al alloy with flow velocity of seachest material for Al ship was evaluated. To examine the electrochemical characteristics of flow velocity and its effects on the performance of the alloy, experiments were conducted at four flow velocity variables using static state with an agitator. An ultrasonic vibration generator using piezoelectric effect was used in cavitation test according to the requirements of in ASTM-G32. The results show that the corrosion current density and damage were increased by applying the flow velocity compared to static state. Therefore, it is determined that the case of applying flow velocity is weaker to the corrosion.
Key words: aluminium ships; over-protection; marine growth prevention system; sea-chest; flow velocity
1 Introduction
Seawater supply to a ship is essential for engine cooling and balancing of the ship. Engine cooling efficiency tends to decrease the seawater inflow quantity due to the impossibility of effective seawater flow resulting from the proliferation of marine growths. In addition to the decreasing efficiency of engine cooling due to marine growths, the pipeline and seachest are frequently corroded by seawater. To kill these marine growths and prevent corrosion, marine growth prevention system (MGPS) is installed to improve the efficiency of ship operations. The principle of MGPS is to apply current to copper and Al anodes. Then the copper anode electrolyzes seawater and generates sodium hypochlorite which kills marine growths. The Al anode forms thin Al hydroxide films on pipelines and coolers to prevent corrosion. Furthermore, as this system can be installed not only in new ships but also in existing ships, it has been applied to numerous ships since 1980s with excellent effects. Recently, MGPS has been installed in the seachest of Al ships as well as steel ships to prevent the corrosion of seawater pipelines for cooling and the attachment of marine growths. Despite its excellent effects, however, the Al anode used for anticorrosion may cause large accidents resulting from corrosion due to inappropriate operation following the application of the same conditions as steel ships which exhibit fundamentally different electrochemical behaviors in seawater. Lately, the seachest of an Al ship which was less than a year old was cracked due to corrosion. Since the crack of the seachest meant the submersion of the engine room, it was discovered right before sinking in this case. Seachest is the part through which seawater first flows into the ship and it is always exposed to the environment of seawater flow, and this flow has considerable influence on the erosion and corrosion of materials. Therefore, a basic study on the prevention of the damage of seachest due to inadequate MGPS for Al ships which is the main type of medium and small ships is needed. The electrochemical characteristics of 5083-H116 Al alloy were examined in seawater with flow velocity.
2 Experimental
Al-Mg series 5083-H116 Al alloy (Fe 0.25%, Mn 0.72%, Si 0.11%, Cu 0.08%, Cr 0.09%, Mg 4.74%, Ti 0.01%, Zn 0.13% and balance Al, mass fraction) was used as hull material. In general, the Al-Mg series alloys are used in applications that require corrosion resistance, formability, surface treatment characteristics and weldability while the moderate strength and applications are very broad. The 5000 series Al alloys have a shortcoming in that their rolling workability decreases if Mg content is higher than 5%. In addition, it is usually cold-rolled to further improve strength. Since it is a non heat-treated alloy that has excellent strength and weldability and is used in welding structures of ships, vehicles and marine plants, its usability is very high. To examine the electrochemical characteristics of flow velocity and its effects on performance, experiments were conducted at four flow velocity variables using in static state with an agitator. The electrochemical test specimens were mounted with epoxy to expose an area of 1 cm2, and all the specimens were ground with No. 2000 emery paper before testing, washed with ethanol and distilled water, and then dried. The electrochemical experiment measured a potential in nature sea water solution, anodic and cathodic polarization trend were tested from open circuit potential from +3.0 to -2.0 V at a scan rate of 2 mV/s. A silver/silver chloride electrode was used as reference electrode. A platinum electrode was used as counter electrode applied. Tafel analysis was used to obtain corrosion potential and corrosion current density by polarizing voltage of ± 250 mV based on the open circuit potential. Moreover, galvanostatic experiment used scanning electron microscope (SEM) to observe the specimen after applying constant current for 3 600 s in sea water. For potentiostatic experiment, corrosion current density after 1 200 s was compared under various applied potential conditions and the damage surface for 3D microscope was observed. Cavitation test with an ultrasonic vibration generator using piezoelectric effect was used in accordance with the requirements of ASTM-G32. It generated a rated output from 20 kHz to 60 Hz, 220 V power through an electronic circuit and supplied it to the vibrator, and the amplitude was set to 50 μm by static amplitude automatic control. For generation of vibration by piezoelectric device, electric AC was applied to a conical horn to generate vibration in the axial direction. The sample was set to face the horn of the vibrator, and a distance of 1 mm was maintained using a filler gauge. The mass of the sample was calculated before and after test. The specimen after test was treated cleaning with an ultrasonic cleaner and dried in a vacuum dryer for 24 h. 3D microscopy was used to observe the damage of the surface after experiment.
3 Results and discussion
Figure 1 presents the surface of the 5083-H116 Al alloy in static state after applying various current densities for 3 600 s. At the current density of 1×10-6 A/cm2, a little dissolution reaction and pitting were observed around the scratches formed during polishing. At 5×10-6 A/cm2, both the corrosion and pitting by dissolution reaction of the scratches formed during polishing were observed, and the union of pitting was also observed. At current density of 1×10-5 A/cm2, pitting and corrosion by dissolution reaction were observed, which considerably increased compared with that at current density of 5×10-6 A/cm2. Furthermore, at 5×10-5 and 1×10-4 A/cm2, the dissolution reaction and the pitting occurred simultaneously, and as the pitting gradually increased, they were jointed. Therefore, as the current density increased, the corrosion and pitting grew and joined by the effect of the active dissolution reaction, thus increased their depth and distribution.
Figure 2 compares the current density after a potentiostatic experiment of the 5083-H116 Al alloy for 1 200 s. In zone III(-1.25 to -0.75 V), the current densities exhibit similar values although there is a little difference after several experiments. The lowest mean current is 1.18×10-6 A/cm2 at -0.75 V, which seems due to the concentration polarization caused by the reduction of dissolved oxygen because of a potential close to the open circuit potential. At potential between -0.75 and -0.7 V, the current density increases very sharply, and it also increases considerably as the potential rose in zone IV(-0.7 to -0.5 V). In this zone, it is expected that the passive film will be destroyed by excessive active dissolution due to the chlorine ions of seawater solution and stress corrosion cracking will appear in the existence of external stress. In zone II(-1. 5 to -1.3 V), current density gradually increased as potential turned to the active direction due to the effect of atomic hydrogen. Potential between -1.3 and -1.25 V corresponds to the protection zone and activation reaction range. The current density is a little low because the effect of atomic hydrogen is more dominant than that of molecular hydrogen. Furthermore, in zone I(-1.9 to -1.55 V), the current density is higher at a more active potential, because the effect of molecular hydrogen is greater than that of atomic hydrogen.
Figure 3 shows the surface of 5083-H116 Al alloy for seachest under 3D microscope after potentiostatic experiment for 1 200 s. At -0.7 to -0.5 V, many corrosions are observed due to high current density by active dissolution. Compared with the sample at -0.7 V, the depth and distribution of corrosion by dissolution are greater than those at -0.5 V. At potential between -0.8 and -1.2 V, the surface is clean with almost no corrosions because it is in a concentration polarization range by the reduction reaction of dissolved oxygen corresponding to the protection zone. When cathodic protection is applied, a little corrosion tendency is observed at -1.4 V which is a little lower than the limit potential of -1.25 V, but there is no noticeable damage. At potential between -1.8 and -1.6 V, as the potential turns to the active direction, severe damage occurrs due to the activation polarization by the generation of hydrogen gas. It appears that in the low potential region, the reaction caused by molecular hydrogen is more dominant than that caused by the atomic hydrogen, and a large volume of hydrogen gas is generated during the experiment as well.
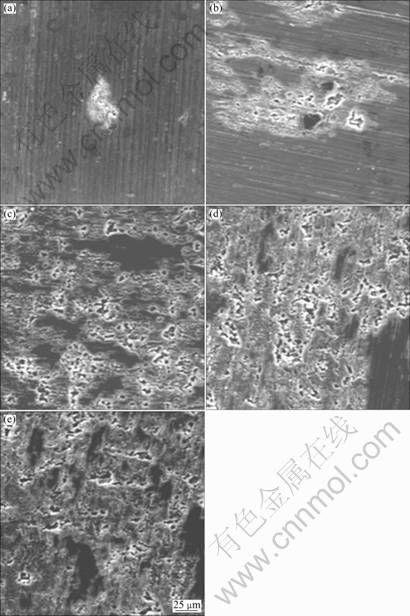
Fig. 1 Surface morphology of 5083-H116 Al alloy after galvanostatic experiment with different current densities: (a) 1×10-6 A/cm2; (b) 5×10-6 A/cm2; (c) 1×10-5 A/cm2; (d) 5×10-5 A/cm2; (e) 1×10-4 A/cm2
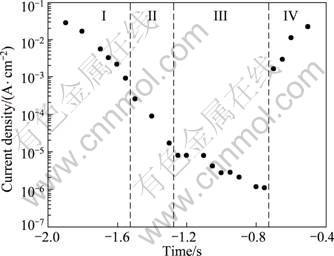
Fig. 2 Current density for 5083-H116 Al alloy after potentiostatic experiment during 1 200 s
Figure 4 shows the mass loss and damage rate (mass loss per unit time) of the 5083-H116 Al alloy for seachest after cavitation experiment with time. The mass loss is measured by cavitation erosion damage and it is found that the mass loss greatly increases during 60 to 120 min and slowly increases until 180 min. As time extends, the mass loss steadily increases. It is found that the largest damage rate appeared at 120 min. It decreases a little at 180 min but increases slightly from 240 min, and exhibits generally similar values until 300 min. This matches the erosion rate—time curve presented by TALKS [1], which is divided into incubation period, acceleration period, attenuation period and steady state period. It is regarded that the incubation period appeared before 60 min after the start of the experiment and the acceleration period appeared after 60 min. In the incubation period, it is estimated that the pits form as the plastic deformation develops on the metal surface by the impact of bubbles. At 120 min, the largest damage rate appears due to the growth and merging of pits, and the pattern of attenuation period appears as it passes 180 min. Between 240 and 300 min the number of pits decreases and the entire area becomes flat or the depth of damage increases at specific locations, indicating that it enters the steady state period.
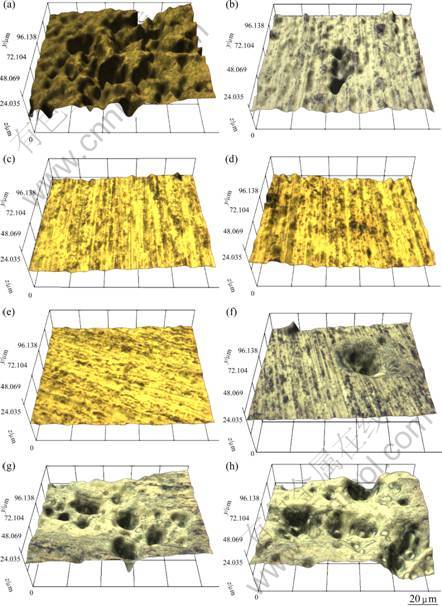
Fig. 3 3D analysis of 5083-H116 Al alloy after potentiostatic experiment for 1 200 s at different voltages: (a) -0.5 V; (b) -0.7 V; (c) -0.8 V; (d) -1.2 V; (e) -1.4 V; (f) -1.6 V; (g) -1.7 V; (h) -1.8 V
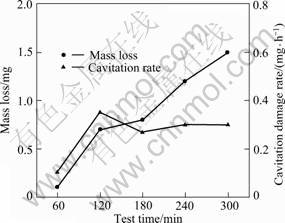
Fig. 4 Mass loss and cavitation rate of 5083-H116 Al alloy after cavitation test with time
Figure 5 shows the analysis of the damaged surface and the damage depth of the 5083-H116 Al alloy for seachest after cavitation experiment for 60-300 min observed under a 3D microscope. In single metal, small indentations appear on the surface in the first stage of erosion damage by cavitation, and then pits and cracks turn out. In the last stage, pits combine and grow [2]. The base metal shows flat shape with roughness of 0.184 5 μm. As shown in Fig. 6, at 60 min, rough surface forms, pits with maximum depth of 18 μm and the mean depth of 6 μm appear. At 120 min, pits grow in depth and combine with one another, making the surface flat, however, deeper than those formed at 60 min with maximum depth of 30 μm and mean depth of 11 μm. At 180 and 240 min, pits combine and overall erosion appear corresponding to the attenuation period and the steady state period, respectively. At 300 min, pits concentrate around the center of the specimen and a large pit of 71 μm in depth form. The region around the center is eroded flatly with mean depth of 20 μm.
Figure 7 shows the potential results of the 5083- H116 Al alloy with flow velocity for seachest in seawater. In the static state, an active potential appears during the early stage of immersion, and the potential exhibits a tendency of quickly moving to the noble direction due to the formation of protective film on Al alloy. Then, the potential almost remained constant when slightly rises or declined by turns. The reason for this seems due to the destruction and generation of the protective film on the surface right after immersion by the chlorine ions in the seawater. The potential at the end of experiment is -0.735 V. In general, Al in neutral solution forms a film of Al2O3 or Al2O3?3H2O and the potential moves to the noble direction. As the corrosion resistance is improved by this film, current density decreases during the polarization experiment [3]. At 400 r/min, the potential quickly moves to the noble direction during the early stage of immersion and gradually increases to a stable value within a short time. At 800 and 1 100 r/min, on the other hand, the potential slowly increases from the early stage of immersion and maintained a constant value around 4 000 s. At the end of the experiment, a similar potential of -0.882 V is obtained. Consequently, an active potential appears when there is a flow rather than in the static state. Most metals form passive film or corrosion products on the surface in corrosion environment and these films are destroyed by flow. As a result, the base metal is exposed to corrosion environment and the corrosion develops. The reason that a higher potential appeared at 1 100 r/min than at 800 r/min is that even though oxygen supply increases due to the generation of bubbles by the vortex resulting from the increased rotation speed, the effective area required for reaction between solution and metals decreases [4-5].
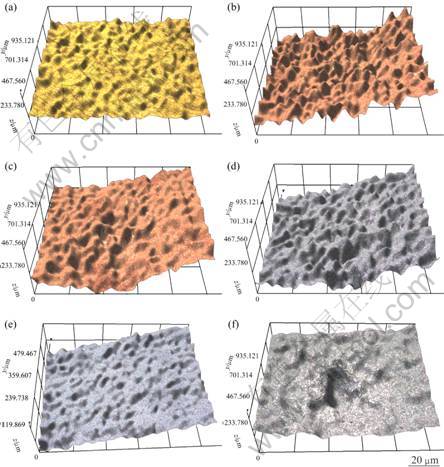
Fig. 5 3D analysis of 5083-H116 Al alloy after cavitation test with different cavitation time: (a) Base metal; (b) 60 min; (c) 120 min; (d) 180 min; (e) 240 min; (f) 300 min
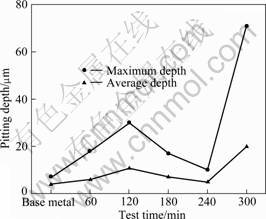
Fig. 6 Pitting depth of 5083-H116 Al alloy after cavitation test
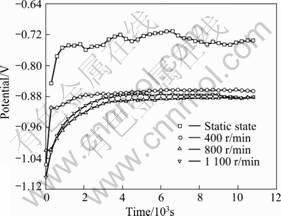
Fig. 7 Potential of 5083-H116 Al alloy with flow velocity
Figure 8 shows the passivation range of the 5083-H116 Al alloy with flow velocity for seachest in seawater and the concentration polarization range after polarization experiment. The passive range in the static state is -0.851 to -0.675 V. Passivation characteristics appear even when flow velocity is applied, but the range is larger than that in static state. The reason for this appears to be that the formation of passive film is relatively easy due to sufficient oxygen supply by the flow velocity. To compare the passivation range by flow velocity, the widest passivation range appears at 800 r/min, followed by 1 100 and 400 r/min. The reason for this seems that the effective area required for reaction between solution and metals decreases due to the generation of bubbles by vortex. As a whole, the pitting potential is almost similar at -0.67 to -0.65 V. As the potential increases after the pitting potential, the rise of current density exhibited almost identical trend. In the concentration polarization range, the potential is -1.645 to -0.895 V in static state and -1.645 V corresponding to the limit potential when the cathodic protection is applied. When this is applied to marine structures or ships, the corrosion protection range must maintain, which has such effects as improved anticorrosion characteristics and reduction of maintenance costs due to lengthened life span [6-9].
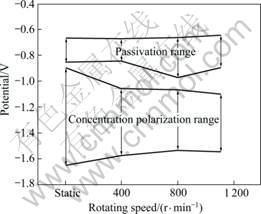
Fig. 8 Passive and concentration polarization range of 5083-H116 Al alloy with flow velocity
Figure 9 presents the polarization curves with flow velocity for the corrosion potential and corrosion current density by Tafel analysis. The corrosion potential in static state is the noblest potential at -0.790 V, but turned to the active direction as the flow velocity increased. It becomes -0.850 V at 400 r/min, -0.911 V at 800 r/min, and -0.947 V at 1 100 r/min. In general, the corrosion potential in a neutral solution is formed by concentration polarization following the reduction reaction of dissolved oxygen and by activation polarization following the oxidation of anode. To determine the corrosion rate, the limiting diffusion current density on the polarization curve is sometimes assumed as the corrosion current density [10]. The current density in the static state is low at 5.95×10-7 A/cm2, and relatively high at 400, 800 and 1 100 r/min, suggesting that it is more vulnerable to corrode than in static state. The reason that corrosion rate increases as the flow velocity increases to 800 r/min in static state appears to be that the reaction increases between metal surface and solution. Furthermore, the corrosion current density decreases a little at the flow velocity of 1 100 r/min. This is due to the fact that the corrosion behavior changed by the influence of flow velocity transforms from laminar flow to turbulent flow as the rotation speed increased, this is unlike in the static environment that only the inside of the materials is considered due to the effect of vortex. As a result, the corrosion current density increases as the flow velocity increased and it became stagnant above a certain value. The reason for this appears to be that even though the flow velocity increases in alkali environment, there is a limiting stage that prohibits the rise of corrosion current density. In general, if cathodic diffusion control is dominant, the corrosion rate increases along with the flow velocity. If the flow velocity increases further, however, the reduction becomes activation dominant. As a result, the corrosion rate becomes unrelated to flow velocity at higher velocity [11].
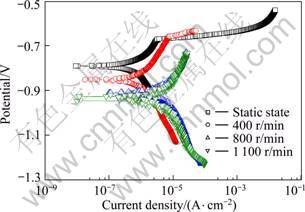
Fig. 9 Polarization curves of 5083-H116 Al alloy for Tafel analysis with flow velocity
4 Conclusions
1) Potential from -0.8 to -1.2 V is the concentration polarization range of dissolved oxygen reduction reaction which exhibits a clean surface with almost no corrosion. As a result of the cavitation experiment, the mass loss shows an overall increasing tendency over time.
2) The damage rate shows that there are incubation period, acceleration period, attenuation period and steady state period. In the static state, the passive potential is -0.851 to -0.675 V and the pitting potential is -0.675 V. The passive characteristics also appear when flow velocity is applied, and its range is wider than that in the static state because the passive film forms smoothly due to sufficient supply of oxygen.
3) As a result of the Tafel analysis, the corrosion potential shows the most valuable value in static state and turns to the active direction as the flow velocity increases. Furthermore, the corrosion current density is relatively higher when flow velocity is applied compared with the static state.
References
[1] TALKS M G, MORETON G. Cavitation erosion of fire-resistant hydraulic fluids [C]//Proceeding of Cavitation Erosion in Fluid Systems. Colorado, US, 1981: 139-152.
[2] JANG S K, KO S C, HAN M S, KIM S J. Characteristics evaluation with coating thickness in al thermal spray coating for 304 stainless steel [C]//Interfinish 2008 Conference Proceeding. 2008: 533.
[3] DELTOMBE E, POURBAIX M. Comportement electrochimique de l'aluminum. Diagramme d'equilibre tension-pH du system Al-H2O, at 25 °C [R]. Rapport Technique, 1956: 42. (in German)
[4] de SANCHEZ, SCHIFFRIN D J. The use of high speed rotating disc electrodes for the study of erosion-corrosion of copper base alloys in sea water [J]. Corrosion Science, 1988, 28(2): 141-151.
[5] CLARKE R J. Corrosion—Metal/Environment reactions [M]. London: Butterworths Publishers, 1976.
[6] MORGAN J. Cathodic Protection [M]. 2nd ed. NACE, 1987: 251.
[7] PARKINS R N, MARK WORTH A J, NOLBROOK J H. Hydrogen gas evolution from cathodically protected pipeline steel surfaces exposed to chloride-sulfate solutions [J]. Corrosion, 1988, 44(8): 572-580.
[8] JOHNSEN R, BARDAL E. Cathodic properties of different stainless steels in natural sea water [J]. Corrosion, 1985, 41(5): 296-302.
[9] KIM S J, OKIDO M, MOON K M. An electrochemical study of cathodic protection of steel used for marine structures [J]. The Korean Journal of Chemical Engineering, 2003, 20(3): 560-565.
[10] WON D S, HWANG C H, PARK Y S, KIM J C. Effects of velocity, turbidity, galvanic coupling and cathodic protection on the erosion-corrosion resistances of Cu alloys, Ti, cast iron, stainless in synthetic [J]. Corrosion Science and Technology, 1990, 19(1): 24-32.
[11] LEE H R. Corrosion of Metals [M]. Yeon Gyeong Publisher, 1995: 75-80.
Seung-Jun LEE, Seong-Jong KIM
Division of Marine Engineering, Mokpo Maritime University, Mokpo, 530729, Korea
摘 要:研究流速对铝制船舶的海底阀箱材料5083-H116铝合金电化学行为的影响。为检测电化学特性和流速对其性能的影响,实验在静态和通过搅拌仪产生的4个不同流速下进行,并使用超声波振荡器利用压电效应进行空化实验。结果表明,合金在施加流速后其腐蚀电流密度和损害程度较在静态环境下增加,更易于发生腐蚀。
关键词:铝制船舶;过度保护;船舶附生物预防系统;海底阀箱;流速
(Edited by FANG Jing-hua)
Foundation item: Project supported by the Cooperative Promotion Center of Science & Technology of JEONNAM TECHNOPARK and Ministry of Education, Science and Technology (MEST) through the Research & Development support project of JEONNAM southwest science park, Korea
Corresponding author: Seung-Jun LEE; Tel: +82-61-2407471; E-mail: corr-pro@mmu.ac.kr
DOI: 10.1016/S1003-6326(11)60918-7

