J. Cent. South Univ. Technol. (2011) 18: 63-67
DOI: 10.1007/s11771-011-0659-9
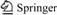
Optoelectronic properties of new functionalized heteroleptic iridium complex
LIANG Bo(梁波)1, 2, WANG Lei(王磊)1, ZHANG Yong(张勇)1, CAO Yong(曹镛)1
1. Institute of Polymer Optoelectronic Materials and Devices,
South China University of Technology, Guangzhou 510640, China;
2. School of Automobile and Mechanic Engineering,
Changsha University of Science and Technology, Changsha 410076, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: A new functionalized heteroleptic iridium complex coordinated with 1-phenylisoquinoline (1-piq) and a functionalized β-diketone (G1), Ir(1-piq)2G1, was synthesized and characterized by 1H-NMR, mass spectrometry and elemental analysis. The larger conjugation of the replacement of acetylacetone (acac) by a functionalized β-diketonate ligand led to a significant decrease in the HOMO level toward vacuum level, while Ir(1-piq)2G1 and Ir(1-piq)2(acac) showed red phosphorescent emissions of about 620 nm in dichloromethane solution. The phosphorescent polymer light-emitting devices were achieved, with the complexes incorporated with polyfluorene (PFO) as a host polymer doped with 30% of 5-(4-biphenylyl)-2-(4-tert-butylphenyl)-1,3,4-oxadiazole (PBD) as electron transport material. The energy transfer mechanism of the devices was also discussed. The lower EL performance of Ir(1-piq)2G1 is ascribed to the inter-ligand energy transfer, indicating that it is important to control the energy level of the cyclometalated and ancillary ligands.
Key words: iridium complex; phosphorescence; energy transfer; ancillary ligands
1 Introduction
Organic/polymer phosphorescent light emitting diodes (O/PPLEDs) have been hot research field because of their potential applications in flat panel displays [1-2]. Many progresses have been made in designing materials and optimizing device technology. Due to their extremely high efficiency (nearly 100% of internal quantum efficiency) and wavelength tunability over the entire visible spectrum, Ir(III) cyclometalated complexes are receiving a great deal of attention as efficient phosphor dopants for applications in the area of O/PPLEDs [3-4]. Different classes of homoleptic (Ir(C^N)3) and heteroleptic iridium complexes (Ir(C^N)2(LX)) have been developed, where C^N is a monoanionic cyclometalated ligand and LX is an ancillary ligand. By choosing appropriate C^N ligand and ancillary ligand, iridium complexes can be prepared, which emit in any color from blue to red [2, 5-8]. PPLEDs were prepared by spin-coating or printing of iridium complexes doped into a polymer host [9-10]. Solubility and charge-transporting and luminescence properties can be adjusted by designing the structure of the ligands [11-13].
In recent years, a new class of functional organic materials, dendrimers, has been developed [14-15]. The dendritic structure allows the independent modification of the core (light emission), branching groups (charge transport) and surface groups (processing properties), which combines high efficiency, good color tunability and solution processibility. The dendritic structure played a major role in the device performance and this was explained partially by a reduction in the intermolecular interactions between the emissive species [16].
Aryl-substituted quinolines are excellent cyclometallated ligands for high-efficiency red electrophosphorescent homoleptic and heteroleptic iridium complexes [17]. Iridium complexes containing 1-phenyisoquinoline type of cyclometalated ligands, such as Ir(piq)3, Ir(piq)2pt and Ir(tpaiq)2(acac), have been studied for the fabrication of saturated red OLEDs [18-20]. We have reported a detailed study of phosphorescent properties of new iridium dendrimers with charge-transporting dendrons in cyclometalated ligands which showed good performance [21]. Based on the previous reports, herein a new functionalized heteroleptic iridium complex was synthesized and characterized. 1-phenylisoquinoline was chosen as the cyclometalated ligand and a functionalized β-diketone (G1) as ancillary ligand. The energy transfer mechanism of the devices was also discussed.
2 Experimental
2.1 Instrument and reagents
1HNMR spectra were collected on a Bruker DRX 400 spectrometer with tetramethylsilane as reference. EI-MS was recorded on a LCQ DECA XP liquid chromatography-mass spectrometer (Thremo Group) and ultraviolet (UV)-visible absorption spectra were recorded on a HP 8453 UV-vis spectrophotometer. Cyclic voltammetry was carried out on a CHI660A electro- chemical workstation.
All reagents and solvents were obtained from Aldrich, Acros, and TCI Chemical Co. and used as received.
2.2 Synthetic route
The cyclometalated ligand (1-piq) [18], ancillary ligand (G1) [22], and Ir(1-piq)2(acac)[4] were synthesized. The synthetic route of novel iridium complex, Ir(1-piq)2G1, is shown in Fig.1.
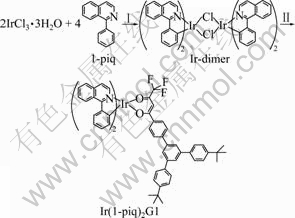
Fig.1 Synthetic route for iridium complex (Reagents and conditions: I—2-methoxylethanol/H2O (3/1), 120 °C, 24 h; II—G1, (n-Bu)4NOH, CH2Cl2, 50 °C, 5 h)
2.3 Synthesis of chloro-bridged dimer [Ir(1-piq)2Cl]2
Iridium trichloride hydrate (1.318 g, 3.8 mmol) was combined with 1-phenylisoquinoline (1.915 g, 9.4 mmol), dissolved in a mixture of 20 mL 2-ethoxyethanol and water (3:1, volume ratio), and refluxed for 24 h under Ar. The solution was cooled to room temperature, and a deep red precipitate was collected. The precipitate was washed with 95% ethanol (10 mL) and ethyl ether (10 mL) to give [Ir(1-piq)2Cl]2 (2.134 g, 90%), which was used directly in the next step without purification.
2.4 Synthesis of Ir(1-piq)2G1
G1 (0.278 g, 0.5 mmol) was mixed with (t-Bu)4NOH in degassed dichloromethane (15 mL) in a three-neck flask. The mixture was refluxed in an argon atmosphere for 20 min. A mixture of [Ir(1-piq)2Cl]2 (0.252 g, 0.2 mmol) in 10 mL dichloromethane was dropped into the reaction solution. The reacting mixture was refluxed for 8 h. After cooling down to room temperature, the dark red precipitate was filtered and washed with water and ethanol. It was purified by column chromatography (silica gel, dichloromethane) to get red powder (0.213 g, 46.1%). 1HNMR δ(CDCl3, 400 MHz): 9.06-9.04 (m, 2H), 8.52-8.50 (d, 1H, J= 6.9 Hz), 8.42-8.39 (t, 1H), 8.29-8.25 (t, 2H), 7.95-7.93 (m, 2H), 7.88-7.81 (t, 3H), 7.77-7.71 (m, 6H), 7.62-7.60 (d, 4H, J=8.6 Hz), 7.54-7.51 (m, 6H), 7.49- 7.46 (t, 2H), 7.02-6.96 (m, 2H), 6.76-6.72 (m, 2H), 6.52-6.50 (d, 1H, J=7.8 Hz), 6.45-6.43 (m, 1H), 6.38- 6.36 (d, 1H, J=8.3 Hz), 1.40 (s, 18H). Anal. Calcd for C66H54F3IrN2O2, C, 68.6; H, 4.7; N, 2.4; Found: C, 68.4; H, 4.6; N, 2.5. EI-MS: m/z: [M+1]+, 1158.4.
2.5 Device fabrication and characterization
Device configuration is ITO/PEDOT:PSS/PVK/ blend/Ba/Al (PEDOT:PSS poly(ethylendioxythiophene): poly(styrene sulfonic acid). A 40 nm PVK film was placed between the ITO/PEDOT and the emitting layer to facilitate hole injection. The fabrication of elelctro- phosphorescent devices was followed by a standard procedure below. A 70 nm-thick layer of PEDOT:PSS (Baytron P 4083, purchased from Bayer AG) was spin-cast onto pre-cleaned ITO-glass substrates. Then, a 40 nm-thick layer of PVK was spin cast on the top of PEDOT:PSS. A mixture of the iridium complex with PFO+PBD (30%, mass fraction) was spin cast from the solution in chlorobenzene with 70-80 nm. A thin layer of Ba with a 200 nm Al capping layer was used as the cathode.
Profilometer (Tencor Alfa-Step 500) was used to determine the thickness of the films. Layer thickness during thermal deposition was monitored by using a crystal thickness monitor (Sycon). Device fabrication was carried out in a controlled dry-box (Vacuum Atmosphere Co.) in N2 circulation. Current density (J)- voltage (V)-luminance (L) data were collected using a Keithley 236 source measurement unit and a calibrated silicon photodiode. Absolute PL efficiencies were measured in integrating sphere (IS-080, Labsphere) under 325 nm line of HeCd laser. External EL quantum efficiencies (EQE) were obtained by measuring a total light output in all directions in an integrating sphere (IS-080, Labsphere). The luminance (cd/m2) was measured by Si photodiode, and calibrated by using a PR-705 spectra scan spectrophotometer (Photo Research). Photoluminescence (PL) spectra and electroluminescence (EL) spectra were recorded using CCD spectrophotometer (Instaspec 4, Oriel).
3 Results and discussion
3.1 Synthese of iridium complex
The target complex Ir(1-piq)G1 was prepared facilely by the reaction of the dimer with the excess of G1 in dichloromethane. The reaction was conducted under basic condition of (t-Bu)4NOH in degassed dichloromethane rather than conventional sodium carbonate solution in 2-ethoxyethanol. The advantage of the present synthetic route is that the reaction conditions are optimized (less reaction time and lower reaction temperature). The identity and high purity of the complex were then con?rmed by 1H NMR and ESI mass spectrometry. As a result, the yield reached 46.1%.
3.2 Absorption and photoluminescence
Fig.2 shows the normalized absorption spectrum of Ir(1-piq)2G1 and the normalized photoluminescence (PL) of PFO+PBD (30%) host on quartz substrates at room temperature. Here, the normalized absorption of Ir(1-piq)2(acac) is also shown for comparison. The intense absorption band at around 250- 350 nm can be assigned to a spin allowed π–π* transition on the cyclometalated ligands, and the broad absorption band with lower energy (450-500 nm) is typical of metal to ligand charge-transfer (MLCT), as described previously [23].
Table 1 summarizes the absorption bands of the complexes. The 466 nm peak for Ir(1-piq)2G1 is blue-shifted compared with 477 nm for Ir(1-piq)2(acac) in CH2Cl2 solution. This indicates that the introduction of fluoro-substituent and dendritic group into the ancillary ligand has influence on the absorption properties of the corresponding iridium complex. There is good overlap between the fluorescence spectrum of PFO+PBD (30%) and MLCT absorption band of the iridium complexes. This overlap enables efficient F?rester energy transfer from the singlet-excited state in the host to the MLCT band of the guest [24].
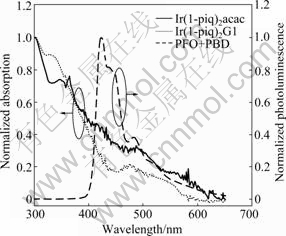
Fig.2 Absorption of iridium complexes (neat film) and photo- luminescence of PFO+PBD (30%)
3.3 Electrochemical analysis
The cyclic voltammetry (CV) curves were measured in a solution of tetrabutylammonium hexafluorophosphate (Bu4NPF6) (0.1 mol/L) in dichloromethane at a scan rate of 10 mV/s at room temperature under argon protection. A platinum electrode was used as the working electrode, a Pt wire was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode. The CV curve of Ir(1-piq)2G1 is shown in Fig.3. The reversible oxidation wave was recorded at 0.845 V for Ir(1-piq)2G1. This value is similar to that of the reported biscyclometalated iridium complexes, which is generally attributed to the Ir-aryl center [25]. The highest occupied molecular orbital (HOMO) and lower unoccupied molecular orbital (LUMO) levels were estimated from the oxidation potential and the optical band gap (Eopt) according to an empirical formula [21]. The electrochemical studies show that the oxidation of the dendritic complex occurs at more positive potentials than Ir(1-piq)2(acac) (summarized in Table 1). The optical band gap was estimated from the onset of the absorption edge of neat films. As a result of the larger conjugation in the structure, the replacement of acac by a functionalized β-diketonate ligand leads to a significant decrease in the HOMO level toward vacuum level.
3.4 Electroluminescent properties
Fig.4 shows the normalized photoluminescence (PL)
Table 1 Optical and electrochemical properties of complexes
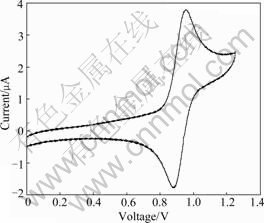
Fig.3 Cyclic voltammogram of Ir(1-piq)2G1( in CH2Cl2)
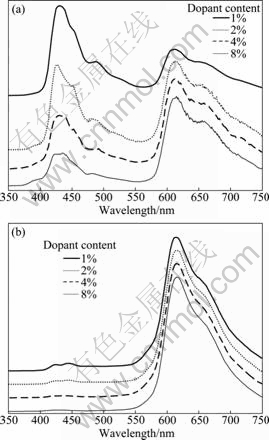
Fig.4 PL (a) and EL (b) spectra of Ir(1-piq)2G1 doped in PFO+ PBD (30%)
and electroluminescence (EL) spectra of Ir(1-piq)2G1- doped PFO+PBD (30%) devices with different dopant contents (mass fraction). PL spectra of Ir(1-piq)2G1 show a significant contribution from the host at low doping contents. EL emission is dominated by Ir(1-piq)2G1 emission peak at a wavelength of 618 nm. The EL emission profiles of Ir(1-piq)2G1 devices show no host emission at low doping content (1%) (Fig.4(b)). The PL profiles of 618 nm peak in position and band width are almost identical with EL emission of the same region, and result from the triplet emission due to Ir(1-piq)2G1. This indicates that the energy transfer from host to iridium complex is efficient [9].
Fig.5 shows the external quantum efficiency (EQE) vs current density characteristics of devices fabricated with Ir(1-piq)2G1 doped into PFO+PBD(30%) host. In the low current density region, the efficiency increases with current density increasing because better charge balance is achieved. After reaching the maximum value, EQE goes down with current density increasing, indicating that the saturation of dopant emission sites begins to occur. For comparison, the devices of Ir(1-piq)2(acac) were also fabricated under the same conditions. Ir(1-piq)2G1 shows less external quantum efficiency compared with Ir(1-piq)2(acac). For example, An external quantum efficiency of 1.46% and a luminous efficiency of 1.06 cd/A with luminance of 1.0 cd/m2 was achieved at 0.1 mA/cm2 for Ir(1-piq)2G1 devices. The highest efficiency of 7.8% at 1.2 mA/cm2 is reached from the device with Ir(1-piq)2(acac) doped.
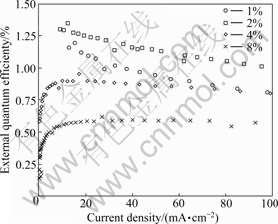
Fig.5 Quantum efficiency vs current density curves of Ir(1-piq)2G1 at different doping contents (Device structure: TO/PEDOT(40 nm)/PVK(40 nm)/blends(70-80 nm)/Ba(5 nm)/ Al(200 nm))
Obviously, the increase of π-system in functionalized β-diketone decreases its triplet energy level. The triplet level of the LX ligand (acac) lies well above the energy of the C^N ligand and MLCT-excited states. Thus, the luminescence is dominated by C^N ligand and MLCT transitions, leading to efficient phosphorescence. Ligand G1 shows lower triplet level than Ir(1-piq)2 fragment, so the phosphorescence is inefficient [23, 26]. Ancillary ligands with different band gap energies are designed by changing the fused ring conjugation of the β-diketone-based ligand structure.
The absolute PL efficiency data of neat films are 0.2% for Ir(1-piq)2G1 and 1.8% for Ir(1-piq)2(acac), further indicating that the emitting states of Ir(1-piq)2G1 comprise an intramolecular charge transfer (ICT) state.
4 Conclusions
(1) Optoelectronic properties based on a new functionalized iridium complex were demonstrated and compared with those of Ir(1-piq)2(acac). The lower EL performance of Ir(1-piq)2G1 is ascribed to the inter-ligand energy transfer, indicating that it is important to control the energy level of the cyclometalated and ancillary ligands.
(2) The control of the structure of the ancillary ligands is very important for the light-emitting device performance, which instructs further improvements on the design of these materials.
References
[1] BALDO M A, LAMANSKY S, BURROWS P E, THOMPSON M E, FORREST S R. Very high-efficiency green organic light-emitting devices based on electrophosphorescence [J]. Appl Phys Lett, 1999, 75(1): 4-6.
[2] HOLDER E, LANGEVELD B M W, SCHUBERT U S. New trends in the use of transition metal-ligand complexes for applications in electroluminescent devices [J]. Adv Mater, 2005, 17(9): 1109-1121.
[3] ADACHI C, BALDO M A, THOMPSON M E, FORREST S R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device [J]. J App1 Phys, 2001, 90(10): 5048-5051.
[4] YANG C H, TAI C C, SUN I W. Synthesis of a high-efficiency red phosphorescent emitter for organic light-emitting diodes [J]. J Mater Chem, 2004, 14(6): 947-950.
[5] LU H P, LIU Q D, DING J F, TAO Y. New triscyclometalated iridium complexes for applications in phosphorescent light-emitting diodes [J]. Synthetic Met, 2008, 158(3/4): 95-103.
[6] PARK G Y, HA Y. Red phosphorescent iridium(III) complexes containing 2,3-diphenylquinoline derivatives for OLEDs [J]. Synthetic Met, 2008, 158(3/4): 120-124.
[7] SONG Y H, YEH S J, CHEN C T, CHI Y, LIU C S, YU J K, HU Y H, CHOU P T, PENG S M, LEE G H. Bright and efficient, non-doped, phosphorescent organic red-light-emitting diodes [J]. Adv Funct Mater, 2004, 14(12): 1221-1226.
[8] WEN Z L, HU Z Y , LIU Y, XIAO F L , MA X Y , ZHU M X, ZHU W G. Synthesis and optophysical properties of blue-emitting iridium (III) complex bearing oxadiazole-based picolinic acid derivative [J]. J Cent South Univ Technol, 2009, 16(3): 380-384.
[9] JIANG C Y, YANG W, PENG J B, XIAO S, CAO Y. High-efficiency, saturated red-phosphorescent polymer light-emitting diodes based on conjugated and non-conjugated polymers doped with an Ir complex [J]. Adv Mater, 2004, 16(5): 537-541.
[10] ZHANG X, CHEN Z, YANG C, LI Z, ZHANG K, YAO H, QIN J, CHEN J, CAO Y. Highly efficient polymer light-emitting diodes using color-tunable carbazole-based iridium complexes [J]. Chem Phys Lett, 2006, 422(4/5/6): 386-390.
[11] ORSELLI E, KOTTAS G S, KONRADSSON A E, COPPO P, FR?HLICH R, DE COLA L, VAN DIJKEN A, B?CHEL M, B?RNER H. Blue-emitting iridium complexes with substituted 1,2,4-triazole ligands: Synthesis, photophysics, and devices [J]. Inorg Chem, 2007, 46(26): 11082-11093.
[12] HWANG F M, CHEN H Y, CHEN P S, LIU C S, CHI Y, SHU C F, WU F L, CHOU P T, PENG S M, LEE G H. Iridium(III) complexes with orthometalated quinoxaline ligands: Subtle tuning of emission to the saturated red color [J]. Inorg Chem, 2005, 44(5): 1344-1353.
[13] COPPO P, PLUMMER EA, DE COLA L. Tuning iridium(III) phenylpyridine complexes in the “almost blue” region [J]. Chem Commun, 2004, 15: 1774-1775.
[14] LO S C, MALE NAH, MARKHAM J P J, MAGENNIS S W, BURN P L, SALATA O V, SAMUEL I D W. Green phosphorescent dendrimer for light-emitting diodes [J]. Adv Mater, 2002, 14(13/14): 975-979.
[15] BURN P L, LO S C, SAMUEL I D W. The development of light-emitting dendrimers for displays [J]. Adv Mater, 2007, 19 (13): 1675-1688.
[16] ZHOU G, WONG W Y, YAO B, XIE Z, WANG L. Triphenylamine- dendronized pure red iridium phosphors with superior OLED efficiency/color purity trade-offs [J]. Angew Chem Int Ed Engl, 2007, 46(7): 1149-1151.
[17] LIANG B, WANG L, ZHU X H, PENG J B, CAO Y. Application of heteroleptic iridium complexes with fluorenyl-modified 1-phenylisoquinoline ligand for high-efficiency red polymer light-emitting devices [J]. J Organomet Chem, 2009, 694(19): 3172-3178.
[18] LIANG B, JIANG C Y, CHEN Z, ZHANG X J, SHI H H, CAO Y. New iridium complex as high-efficiency red phosphorescent emitter in polymer light-emitting devices [J]. J Mater Chem, 2006, 16(3): 1281-1286.
[19] HU Z Y, LUO C P, WANG L, HUANG F L, ZHU K M, WANG Y F, ZHU M X, ZHU W G, CAO Y. Highly efficient saturated red electrophosphorescence from isoquinoline-based iridium complex containing triphenylamino units in polymer light-emitting devices [J]. Chem Phys Lett, 2007, 441(4/5/6): 277-281.
[20] TSUBOYAMA A, IWAWAKI H, FURUGORI M, MUKAIDE T, KAMATANI J, IGAWA S, MORIYAMA T, MIURA S, TAKIGUCHI T, OKADA S, HOSHINO M, UENO K. Homoleptic cyclometalated iridium complexes with highly efficient red phosphorescence and application to organic light-emitting diode [J]. J Am Chem Soc, 2003, 125(42): 12971-12979.
[21] LIANG B, WANG L, XU Y H, SHI H H, CAO Y. High-efficiency red phosphorescent iridium dendrimers with charge-transporting dendrons: Synthesis and electroluminescent properties [J]. Adv Funct Mater, 2007, 17(17): 3580-3589.
[22] ZHANG Y, SHI H H, CAO Y. Synthesis of conjugated polyphenylene dendritic beta-diketones [J]. Chin J Chem, 2006, 24(11): 1631-1638.
[23] LAMANSKY S, DJUROVICH P, MURPHY D, ABDEL-RAZZAQ F, LEE H E, ADACHI C, BURROWS P E, FORREST S R, THOMPSON M E. Highly phosphorescent bis-cyclometalated iridium complexes: Synthesis, photophysical characterization, and use in organic light emitting diodes [J]. J Am Chem Soc, 2001, 123(18): 4304-4312.
[24] NAZEERUDDIN M K, HUMPHRY-BAKER R, BERNER D, RIVIER S, ZUPPIROLI L, GRAETZEL M. Highly phosphorescence iridium complexes and their application in organic light-emitting devices [J]. J Am Chem Soc, 2003, 125(29): 8790-8797.
[25] TAMAYO A B, ALLEYNE B D, DJUROVICH P I, LAMANSKY S, TSYBA I, HO N N, BAU R, THOMPSON M E. Synthesis and characterization of facial and meridional tris-cyclometalated iridium(III) complexes [J]. J Am Chem Soc, 2003, 125(24): 7377-7387.
[26] YOU Y M, PARK S Y. Inter-ligand energy transfer and related emission change in the cyclometalated heteroleptic iridium complex: Facile and efficient color tuning over the whole visible range by the ancillary ligand structure [J]. J Am Chem Soc, 2005, 127(36): 12438-12439.
(Edited by YANG Bing)
Foundation item: Project(50803008) supported by the National Natural Science Foundation of China; Project(2002CB613403) supported by the Ministry of Science and Technology (MOST) of China; Project(09JJ6085) supported by the Natural Science Foundation of Hunan Province, China; Project(08hjyh02) supported by the Open Project Program of Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, China
Received date: 2010-06-12; Accepted date: 2010-11-18
Corresponding authors: LIANG Bo, Associate Professor; Tel/Fax: +86-731-85258638; E-mail: liangbo26@126.com; CAO Yong, Professor; Tel: +86- 20-87114346; Fax: +86-20-87110606; E-mail: poycao@scut.edu.cn