高温水溶液中铁、锌、镁多元硫酸盐体系的结晶行为
来源期刊:中国有色金属学报2020年第7期
论文作者:刘慧杨 邓志敢 魏昶 李兴彬 李旻廷 王长印 曾涛
文章页码:1691 - 1703
关键词:硫酸亚铁;硫酸锌;硫酸镁;溶解度;结晶;温度
Key words:ferrous sulfate; zinc sulphate; magnesium sulfate; solubility; crystallization; temperature
摘 要:通过研究FeSO4-ZnSO4-MgSO4-H2SO4体系中锌、铁、镁的结晶行为,考察不同硫酸盐浓度下温度对硫酸亚铁、硫酸锌和硫酸镁结晶的影响。结果表明:随着温度的升高,硫酸亚铁、硫酸锌和硫酸镁的溶解度降低;改变初始溶液中亚铁离子的浓度,硫酸亚铁的急剧降低区间(160~180 ℃)保持不变;硫酸锌和硫酸亚铁的存在提高了硫酸镁的结晶率;硫酸镁和硫酸亚铁的存在提高硫酸锌的结晶率;低于160 ℃时,硫酸锌和硫酸镁的存在提高了硫酸亚铁结晶率,高于170 ℃时,硫酸锌和硫酸镁的存在降低硫酸亚铁结晶率。
Abstract: The effects of different sulfate concentrations on the crystallization of ferrous sulfate, zinc sulfate and magnesium sulfate were investigated through investigating the crystallization behavior of zinc, iron and magnesium in FeSO4-ZnSO4-MgSO4-H2SO4 system. The results show that the solubility of ferrous sulfate, zinc sulfate and magnesium sulfate decreases with the increase of temperature. When the concentration of ferrous ions in the initial solution is changed, the sharp decrease interval of ferrous sulfate (160-180 ℃) remains unchanged. The presence of zinc sulfate and ferrous sulfate increases the crystallization rate of magnesium sulfate, the presence of magnesium sulfate and ferrous sulfate increases the crystallization rate of zinc sulfate. When the temperature is lower than 160 ℃, the presence of zinc sulfate and magnesium sulfate increases the crystallization rate of ferrous sulfate, when the temperature is higher than 170 ℃, it decreases the crystallization rate of ferrous sulfate.
DOI: 10.11817/j.ysxb.1004.0609.2020-37596
刘慧杨,邓志敢,魏 昶,李兴彬,李旻廷,王长印,曾 涛
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:通过研究FeSO4-ZnSO4-MgSO4-H2SO4体系中锌、铁、镁的结晶行为,考察不同硫酸盐浓度下温度对硫酸亚铁、硫酸锌和硫酸镁结晶的影响。结果表明:随着温度的升高,硫酸亚铁、硫酸锌和硫酸镁的溶解度降低;改变初始溶液中亚铁离子的浓度,硫酸亚铁的急剧降低区间(160~180 ℃)保持不变;硫酸锌和硫酸亚铁的存在提高了硫酸镁的结晶率;硫酸镁和硫酸亚铁的存在提高硫酸锌的结晶率;低于160 ℃时,硫酸锌和硫酸镁的存在提高了硫酸亚铁结晶率,高于170 ℃时,硫酸锌和硫酸镁的存在降低硫酸亚铁结晶率。
关键词:硫酸亚铁;硫酸锌;硫酸镁;溶解度;结晶;温度
文章编号:1004-0609(2020)-07-1691-12 中图分类号:TF813 文献标志码:A
随着全球炼锌产业的快速发展,优质锌资源不断减少,为满足锌资源市场的需要,低品位、多金属、杂质含量高的复杂锌矿物资源的开发与利用受到锌冶炼行业的重视,特别高铁闪锌矿的开发与利用引起全球的关注,仅云南的高铁闪锌矿资源中锌的储量就高达700万t[1-4]。锌铁分离已经成为湿法炼锌过程中一个普遍存在的重要问题,随着国家环保法规越来越严格,湿法炼锌过程中除铁工艺的选择尤其重要[5-6]。如何在含锌高铁溶液中实现锌铁的高效分离以及铁和有价金属的资源化利用已经成为当今湿法炼锌行业的热点问题[3]。目前,湿法炼锌行业在工业上已经成功应用的除铁方法有黄钾铁矾法、针铁矿法和赤铁矿法[7]。
黄钾铁矾法产出的渣量较大、且渣含铁低难以利用,同时渣中含有锌、铜、铟、砷等杂质离子,铁矾渣不稳定和硫酸的消耗量大等问题[8-9]。V.M.法的运营成本高,E.Z.法的稀散金属的损失率高,针铁矿堆存时部分硫酸根和氯离子可能渗漏而造成环境污染,且存在有价金属回收率低等问题[10-11]。相比之下,虽然赤铁矿法存在投资及维护费用较高和操作要求较为严格,但是赤铁矿法产出的渣量少,含铁高等优点,赤铁矿渣能成为水泥行业和钢铁行业的原料或者添加 剂[12]。随着全球环保法规越来越严格,迫使锌行业开发铁渣稳固化的方法或者开发无废渣的除铁技术[13],在目前现有的除铁方法中赤铁矿法无疑是最有潜力成为无废渣的除铁技术[14]。由于赤铁矿法的技术要求高,目前世界上正在运营的公司仅有日本饭岛冶炼厂和中国云南云锡文山锌铟冶炼有限公司。
为了保证良好的赤铁矿渣,赤铁矿法除铁温度不低于180 ℃,硫酸亚铁在180 ℃的纯水的溶解度为3.43 g/L[15]。在赤铁矿法除铁的操作条件下硫酸亚铁、硫酸锌和硫酸镁大量结晶[6]。盐类结晶理论指出[16]:晶型沉积物在持续的压力下,相互胶结在一起,其胶结强度随着晶体颗粒的增大和陈化时间的延长而不断增大,随着胶结强度的增加引起硫酸盐晶体沉淀造成釜内结垢,结垢的硫酸亚铁晶体溶解缓慢而引起除铁后液铁离子浓度较高和亚铁氧化缓慢等问题。针对以上问题,本文作者通过研究高温下FeSO4-ZnSO4- MgSO4-H2SO4体系中硫酸盐的结晶行为,探索硫酸亚铁、硫酸镁和硫酸锌之间的结晶规律,为解决硫酸亚铁晶体的溶解缓慢和亚铁氧化速率降低等问题提供理论基础。
1 实验
1.1 实验方法
用分析纯FeSO4·7H2O、MgSO4·7H2O和ZnSO4·7H2O配制Fe2+浓度为30 g/L、不同浓度的硫酸锌和硫酸镁溶液,用硫酸调溶液pH值为3(根据赤铁矿除铁工艺,第二段中和液(除铁前液)的pH维持在4左右,为了使研究的溶液接近实际溶液的酸度,并防止亚铁水解,同时为确保配置溶液的实验条件和结果对实际溶液的实验有指导意义,配置溶液的pH值维持在3左右)。采用2L-GSH型高压釜(材质为1Gr18Ni9Ti-TA20)作为反应容器。将1 L配制好的溶液加入高压釜内,连接供氮设备后检查高压釜的气密性,多次引入氮气(≥99.2%)并反复排出以获得完全的氮气氛围。调节釜内总压为1.6 MPa,随后加热并搅拌,搅拌转速为300 r/min,达到所设定的实验条件时,保持实验条件不变取样,取样液经玻璃砂芯过滤装置过滤后并保存,测量样液中各种离子的浓度。
1.2 分析与检测
采用EDTA滴定法和火焰原子吸收光谱法(WFX-110B)测量锌离子的浓度,采用重铬酸钾滴定法测量Fe2+离子的浓度,采用火焰原子吸收光谱法测量镁离子的浓度。部分试样送到昆明冶金研究院测量相关元素的含量,验证并保证实验数据的准确性。Fe2+、Mg2+、Zn2+的结晶率计算公式为
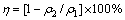 (1)
(1)
式中:η为FeSO4、ZnSO4、MgSO4的结晶率,%;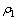 为初始溶液中Fe2+、Zn2+、Mg2+的浓度,g/L;
为初始溶液中Fe2+、Zn2+、Mg2+的浓度,g/L;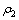 为取样液中Fe2+、Zn2+、Mg2+的浓度,g/L(每次取样小于5 mL,保证溶液体积无明显变化)。
为取样液中Fe2+、Zn2+、Mg2+的浓度,g/L(每次取样小于5 mL,保证溶液体积无明显变化)。
1.3 实验原理
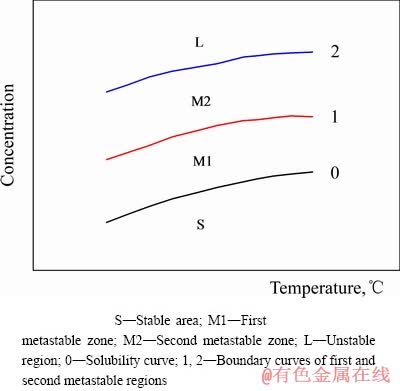
图1 溶液状态图[17]
Fig. 1 Solution state diagram[17]
哈姆斯基研究表明[17]:溶液通常可处于稳定态、介稳态和不稳态3种状态(见图1)。在稳定区,溶液溶液浓度小于或等于溶解度,结晶不能发生;在第一介稳定区,基本上不能均相成核,结晶过程不能发生;在第二介稳定区,有自发成核的可能,但成核不能同时马上发生,晶核的形成和晶体的长大需要时间间隔才能发生;在不稳定区,结晶马上开始。
BRUHN等[18]研究表明:硫酸亚铁在水中的溶解度随着温度的升高溶解度不断增加,并在55~65 ℃之间达到最大值,当温度继续升高,溶解度会不断降低。HASEGAWA等[15]研究不同温度和硫酸浓度下硫酸亚铁的溶解度与温度的关系(见图2)。硫酸镁和硫酸锌的溶解度与硫酸亚铁在水中的溶解度有相似的特性,硫酸镁在约70 ℃时溶解度达到最大值,当温度继续升高时,硫酸镁的溶解度将不断降低,硫酸镁的溶解度与温度的关系(见图3)[19]。硫酸锌的溶解度在55~65 ℃之间达到最大值,当温度继续升高,硫酸锌的溶解度会不断降低,硫酸锌的溶解度与温度的关系(见图4)[20]。由图5[18]和6[21]可知:随着温度的升高ZnSO4、FeSO4、MgSO4的溶解度降低,在高温下水溶液中ZnSO4、FeSO4、MgSO4的结晶沉淀物主要为一水硫酸盐。根据张宇婕等[22]和孙鸿等[23]作者研究表明,锌、铁、镁共存时,因类质同相等因素导致复混硫酸盐产生。在多元硫酸盐体系中加入硫酸不改变硫酸盐的结晶物相,只改变硫酸盐的溶解度。
HASEGAWA等[15]研究表明:在三元体系FeSO4-ZnSO4-H2SO4和FeSO4-MgSO4-H2SO4中,随着酸度的增加,硫酸亚铁的溶解度升高。在酸度一定的条件下,随着溶液中锌离子和镁离子浓度的升高,硫酸亚铁的溶解度随之降低;在四元系FeSO4-ZnSO4- MgSO4-H2SO4中,随着酸度的增加,硫酸亚铁的溶解度随之升高。在酸度一定的条件下,锌、镁离子的加入降低了硫酸亚铁的溶解度。
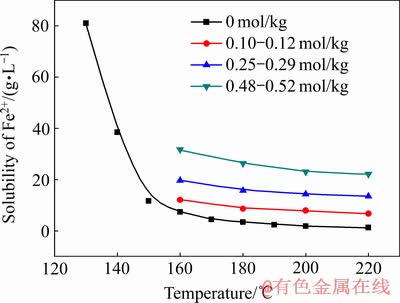
图2 硫酸亚铁在不同硫酸浓度下的溶解度与温度的关系[15]
Fig. 2 Relationship between solubility of ferrous sulfate and temperature at each sulfuric acid concentration[15]
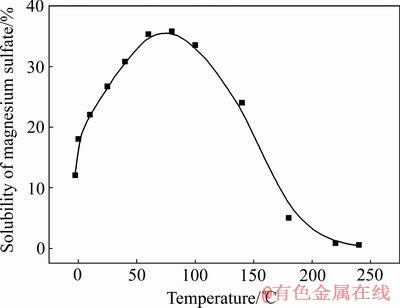
图3 硫酸镁的溶解度与温度的关系[19]
Fig. 3 Relationship between solubility and temperature of magnesium sulfate[19]
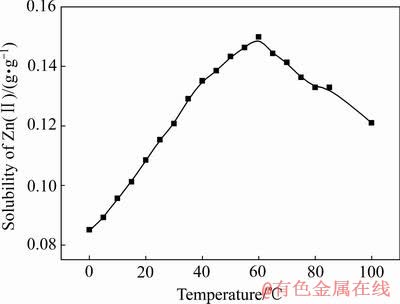
图4 硫酸锌的溶解度与温度的关系[20]
Fig. 4 Relationship between solubility of zinc sulfate and temperature[20]
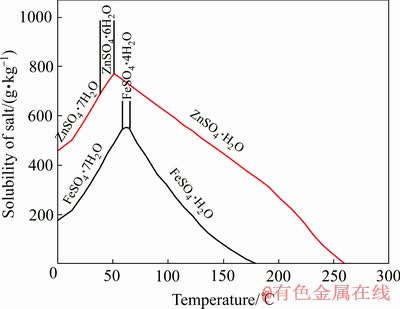
图5 ZnSO4/FeSO4-H2O体系溶解度图[18]
Fig. 5 Solubility diagram of ZnSO4/FeSO4-H2O system[18]
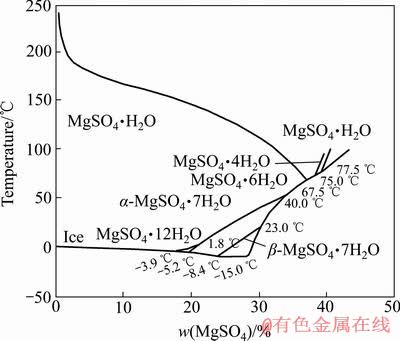
图6 MgSO4-H2O体系溶解度图[21]
Fig. 6 Solubility diagram of MgSO4-H2O system[21]
赤铁矿除铁前液富含硫酸锌、硫酸亚铁和硫酸镁,而赤铁矿除铁法的温度高达180~200 ℃,由图1、2、3和4可知,在此温度区间,硫酸锌、硫酸亚铁和硫酸镁的溶解度较低,除铁前液处于不稳定区或第二介稳定区,溶液中硫酸锌、硫酸亚铁和硫酸镁会发生如下结晶反应(见式(2)~(4))。为此,本文作者通过研究FeSO4-ZnSO4-MgSO4-H2SO4体系在高温水溶液中的结晶行为,探索硫酸亚铁、硫酸镁和硫酸锌之间的结晶规律,为解决除铁后液铁离子浓度较高、硫酸亚铁晶体的返溶缓慢和氧化速率降低等问题提供理论基础。
Zn2++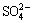 +xH2O=ZnSO4·xH2O (2)
+xH2O=ZnSO4·xH2O (2)
Fe2++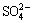 +xH2O=FeSO4·xH2O (3)
+xH2O=FeSO4·xH2O (3)
Mg2++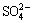 +xH2O=MgSO4·xH2O (4)
+xH2O=MgSO4·xH2O (4)
2 结果与讨论
2.1 FeSO4-H2SO4体系中硫酸亚铁的结晶行为
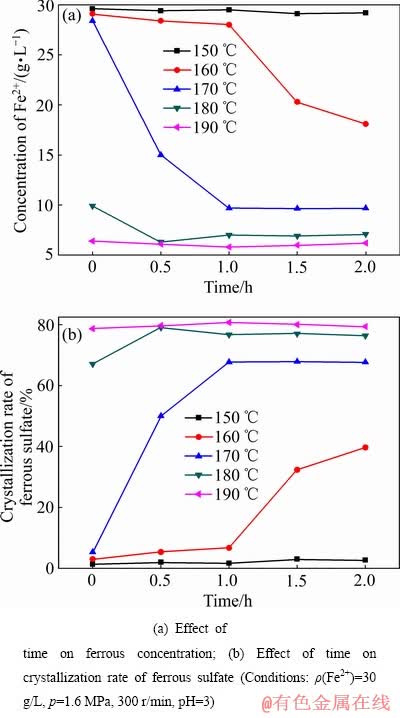
图7 高温体系下结晶时间对硫酸亚铁结晶的影响
Fig. 7 Effect of crystallization time on crystallization of ferrous sulfate under high temperature system
不同反应温度下结晶时间对硫酸亚铁的影响结果见图7。由图7(a)和(b)可知,在150 ℃时,随着结晶时间的延长,溶液中亚铁离子浓度和硫酸亚铁的结晶率保持恒定,此时,溶液处于稳定区或者第一介稳定区,溶液中硫酸亚铁不能结晶并沉淀下来。当溶液处于160~170 ℃时,随着结晶时间的延长,溶液中亚铁离子浓度降低和硫酸亚铁的结晶率明显上升,溶液处于第二介稳定区,溶液中的硫酸亚铁的成核与晶体长大需要较长的时间才能完成。随着温度的升高,硫酸亚铁的溶解度降低,溶液的极限过饱和度增加(极限饱和度是指过饱和溶液由不自发成核转向自发成核状态的界限,对应图1中的第一介稳定区界限的曲线,溶液中硫酸亚铁的浓度与该温度下极限饱和度之差为极限过饱和度,是结晶过程的推动力[24]),硫酸亚铁的结晶推动力增加,提高了硫酸亚铁的结晶率,在1 h、温度由160 ℃上升至170 ℃时,硫酸亚铁的结晶率由6.67%上升到67.67%。当温度为180~190 ℃时,溶液处于不稳定区,硫酸亚铁快速结晶,很快达到结晶-溶解平衡;在180 ℃和190 ℃时,结晶时间由0 h延长至2 h时,硫酸亚铁的结晶率分别提高了9.33%(由67%上升至76.33%)和0.66%(由78.67%上至79.33%)。
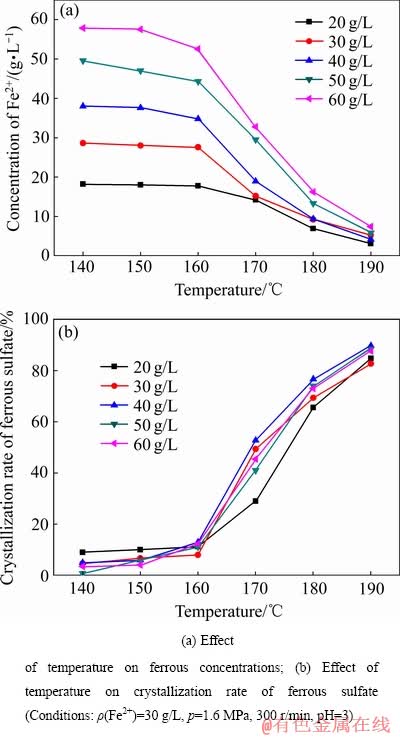
图8 不同硫酸亚铁浓度下温度对硫酸亚铁结晶的影响
Fig. 8 Effect of temperature on crystallization of ferrous sulfate under different ferrous sulfate concentrations
不同硫酸亚铁浓度下温度对硫酸亚铁结晶的影响结果见图8。由图8(a)和(b)可知,当温度低于160 ℃时,溶液中有少量的硫酸亚铁结晶。当硫酸亚铁溶液处于140~160 ℃时,溶液处于第二介稳定区,硫酸亚铁的极限过饱和度较小,硫酸亚铁缓慢成核且晶体颗粒较小,而结晶反应是一个动态过程,成核和晶体长大与硫酸亚铁晶体返溶是一个可逆的过程,较小的晶体和晶核易发生返溶,当温度160 ℃时,硫酸亚铁的结晶率最高仅有13%。当温度由160 ℃升高至180 ℃时,硫酸亚铁的溶解度急剧降低,溶液处于不稳区、硫酸亚铁的极限过饱度明显增加,硫酸亚铁爆发成核和硫酸亚铁晶体快速长大并沉淀下来,当初始铁离子浓度为40 g/L时,溶液温度由160 ℃升高至180 ℃时,硫酸亚铁的结晶率由13%上升到76.625%。HASEGAWA等[15]的研究表明,硫酸亚铁在纯水中溶解度的急剧减小区间为130~150 ℃。本实验研究结果为160~180℃,这是因为本实验所用溶液的pH约为3(用硫酸调pH),而HASEGAWA等[15]研究的硫酸亚铁在纯水溶液中。由于酸度的增加,溶液中自由氢离子的浓度增加,反应5向右进行,溶液中硫酸根的浓度降低,硫酸亚铁的溶解度增加,引起硫酸亚铁溶解度急剧降低区间由130~150 ℃上升至160~180 ℃,在20~60 g/L的范围内硫酸亚铁溶解度的急剧降低区间(160~180 ℃)保持不变。
H++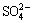 =
=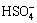 (5)
(5)
2.2 FeSO4-ZnSO4-H2SO4体系中硫酸盐的结晶行为
高温体系下FeSO4与ZnSO4间的相互作用对硫酸盐结晶率的影响结果如图9所示。由图9可知,随着温度的升高,硫酸亚铁和硫酸锌的结晶率随之升高,BRUHN等[18]和周甫立[20]的研究表明:当温度高于65 ℃时,随着温度的升高,硫酸亚铁和硫酸锌的溶解度不断降低。硫酸亚铁的存在提高了硫酸锌结晶率,当温度低于160 ℃时,硫酸锌的存在提高了硫酸亚铁的结晶率;当温度高于170 ℃时,硫酸锌的存在降低了硫酸亚铁结晶率;当温度低于160 ℃时,硫酸锌和硫酸亚铁共存时,溶液中硫酸根浓度增加,溶液中锌离子和亚铁离子的总浓度也增加,因同离子效应,锌离子和亚铁离子降低H+的活度[11],H+的活度降低,不利于反应式(5) 向右进行,溶液中硫酸根的总浓度增加,促进反应(2)和(3)向右进行,提高了硫酸锌和硫酸亚铁的结晶率。由图9中曲线a可知,当温度为高于170 ℃时,硫酸亚铁的溶解度急剧降低,溶液中存在大量的硫酸亚铁晶体,有利于硫酸锌非均相成核,促进硫酸锌结晶。BRUHN等[18]的研究可知,从室温到250 ℃之间,硫酸亚铁的溶解度低于硫酸锌的溶解度,溶液含有大量自由锌离子,锌离子以类质同相[22, 25]取代FeSO4·xH2O中的部分铁原子,引起硫酸亚铁的结晶率降低而硫酸锌的结晶率提高(见图9中的曲线b和d)。随着温度的升高,硫酸亚铁和硫酸锌的溶解度降低,溶液中硫酸根和锌离子的浓度降低、同离子效应和类质同相的作用减弱,降低了硫酸亚铁和硫酸锌结晶间的相互影响。
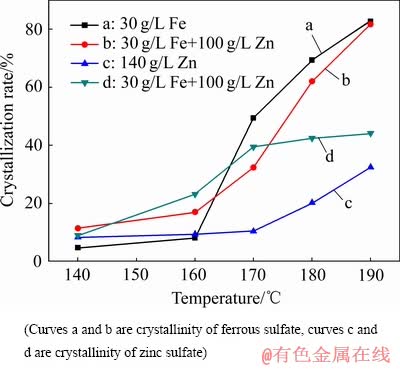
图9 高温体系下FeSO4与ZnSO4间的相互作用对硫酸盐结晶率的影响
Fig. 9 Effect of interaction between FeSO4 and ZnSO4 on sulfate crystallization rate under high temperature system
不同硫酸锌浓度下温度对硫酸锌和硫酸亚铁结晶的影响结果见图10和图11。由图10(a),(b)和图11(a)、(b)可知,当温度低于160 ℃时,随着锌离子的浓度增加硫酸锌和硫酸亚铁的结晶率提高,当锌离子达到120 g/L时,硫酸锌的结晶率最高,溶液中的锌离子继续增加硫酸锌的结晶率降低。初始锌离子浓度越高,在相同的温度下,溶液中硫酸锌的极限过饱和度升高,硫酸锌的结晶率提高。由于硫酸锌的溶解度高于硫酸亚铁的溶解度,随着硫酸锌浓度的增加,溶液中自由硫酸根的浓度增加,更有利于式(3)向右进行,同时因同离子效应,锌离子浓度增加降低了H+的活度[11],不利于反应(5)向右进行,溶液中硫酸根增加,促进反应(3)向右进行,提高硫酸亚铁的结晶率而降低了硫酸锌的结晶率。
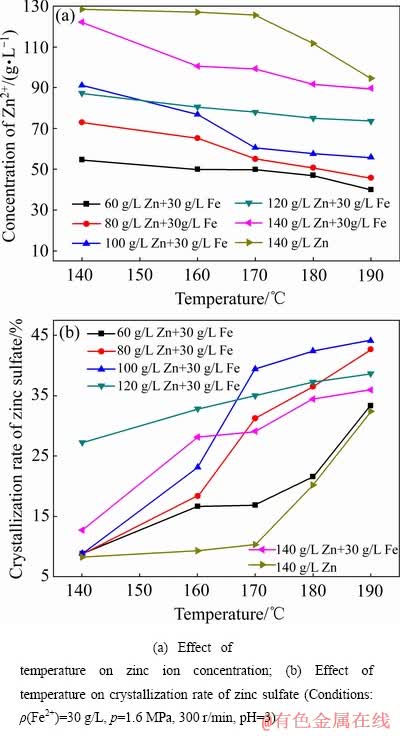
图10 不同硫酸锌浓度下温度对硫酸锌结晶的影响
Fig. 10 Effect of temperature on crystallization of zinc sulfate under different zinc sulphate concentration
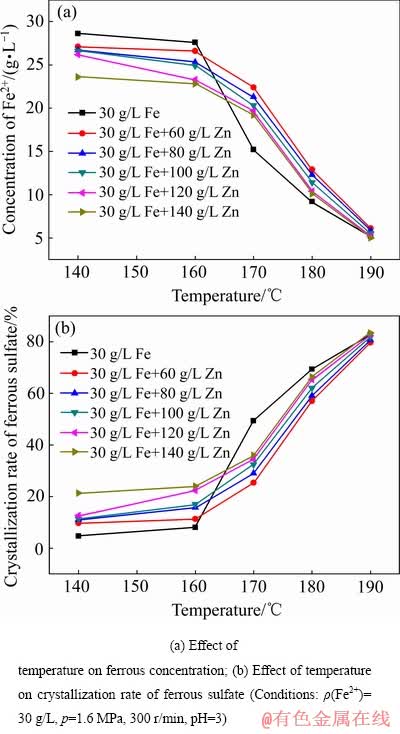
图11 不同硫酸锌浓度下温度对硫酸亚铁结晶的影响
Fig. 11 Effect of temperature on crystallization of ferrous sulfate under different zinc sulphate concentration
当温度高于160 ℃时,溶液中的硫酸亚铁提高了硫酸锌的结晶率而溶液中的硫酸锌降低了硫酸亚铁的结晶率,随着温的升高硫酸亚铁和硫酸锌的相互影响减弱(见图10(b)和图11(b))。随着温度的升高,硫酸亚铁的溶解度急剧降低,溶液中硫酸亚铁晶体增加,溶液中的硫酸亚铁晶体作为晶核,促进硫酸锌的结晶,提高硫酸锌的结晶率,同时,溶液中自由的锌离子以类质同相取代FeSO4·xH2O中的部分铁原子,提高了硫酸锌的结晶率而降低了硫酸亚铁的结晶率。随着温度的增加,硫酸锌和硫酸亚铁的溶解度降低,硫酸锌和硫酸亚铁的大量结晶,溶液中自由移动的硫酸根和锌离子浓度降低,类质同相的作用减弱,降低了硫酸锌和硫酸亚铁结晶间的相互作用。当温度为170 ℃时,初始锌离子浓度为140 g/L时,加入81.43 g/L的硫酸亚铁时(Fe2+浓度为30 g/L),硫酸锌的结晶率提高了25.7%(由10.3%上升至36%);而当温度为190 ℃时,硫酸锌的结晶率仅增加了3.55%(由32.41%上升至35.96%);当温度为170 ℃时,初始亚铁离子浓度为30 g/L时,加入247.7 g/L (Zn2+为100 g/L)硫酸锌时,硫酸亚铁的结晶率降低了17%(由49.33%降低至32.33%);而当温度为190 ℃时,硫酸亚铁的结晶率降低了1%(由82.67%降至81.67%)。
FeSO4-ZnSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L、温度为140~190 ℃之间、锌离子浓度为60~140 g/L的范围内,硫酸亚铁的存在提高了硫酸锌的结晶率,当温度低于160 ℃时,硫酸锌的存在提高了硫酸亚铁的结晶率;当温度高于170 ℃时,硫酸锌的存在降低了硫酸亚铁的结晶率。
2.3 FeSO4-MgSO4-H2SO4体系中硫酸盐的结晶行为
高温体系下FeSO4与MgSO4间的相互作用对硫酸盐结晶率的影响结果详见图12。由图12可知,随着温度的升高,硫酸亚铁和硫酸镁的结晶率升高,硫酸亚铁的存在提高了硫酸锌的结晶率;当温度低于160 ℃时,硫酸镁的存在提高了硫酸亚铁的结晶率;当温度高于170 ℃时,硫酸镁的存在降低了硫酸亚铁的结晶率;当温度低于160 ℃时,硫酸镁和硫酸亚铁共存时,溶液中硫酸根浓度增加,且溶液中镁离子和亚铁离子的总浓度增加,根据同离子效应,镁离子和亚铁离子降低H+的活度[11],H+的活度降低,不利于反应式(5)向右进行,溶液中硫酸根的总浓度增加,促进反应(3)和(4)向右进行,提高了硫酸亚铁和硫酸镁的结晶率。当温度高于170 ℃时,溶液中存在大量的硫酸亚铁晶体,有利于硫酸镁碰撞成核,提高硫酸镁的结晶率。梁英教等[19]研究表明,温度为180 ℃时,硫酸镁的溶解度为250 g/kg(溶液中镁离子的浓度为50 g/kg),硫酸镁的溶解度高于硫酸亚铁的溶解度。溶液中的自由移动的镁离子以类质同相[24]取代FeSO4·xH2O中的部分铁原子,引起硫酸亚铁的结晶率降低而硫酸镁的结晶率提高(如图12中的曲线b和c)。随着温度的升高,硫酸亚铁和硫酸镁溶解度降低,溶液中硫酸根和镁离子的浓度降低、同离子效应和类质同相的作用减弱,硫酸亚铁和硫酸镁结晶间的相互作用降低。
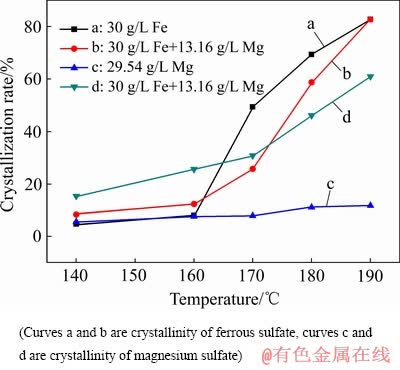
图12 高温体系下FeSO4与MgSO4间的相互作用对硫酸盐结晶率的影响
Fig. 12 Effect of interaction between FeSO4 and MgSO4 on sulfate crystallization rate under high temperature system
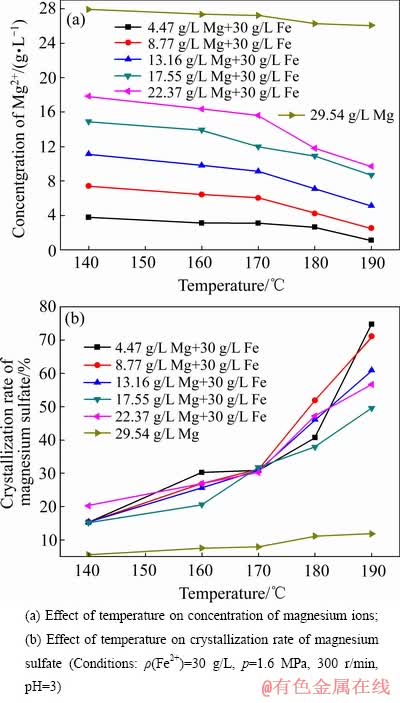
图13 不同硫酸镁浓度下温度对硫酸镁结晶的影响
Fig. 13 Effect of temperature on crystallization of magnesium sulfate under different concentrations of magnesium sulfate
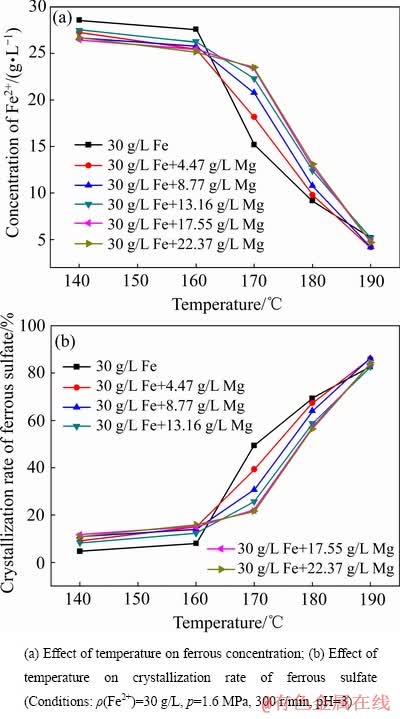
图14 不同硫酸镁浓度下温度对硫酸亚铁结晶的影响
Fig. 14 Effect of temperature on crystallization of ferrous sulfate at different concentrations of magnesium sulfate
不同镁离子浓度下温度对硫酸镁和硫酸亚铁结晶的影响如图13和14所示。由图13(a)、(b)和图14(a)、 (b)可知,当溶液中Zn2+浓度为29.54 g/L、温度由170 ℃上升到190 ℃时,硫酸镁的结晶率由7.85%上升到11.78%。梁英教等[19]研究表明,温度为180 ℃时,硫酸镁的溶解度为250 g/kg(溶液中镁离子的浓度为50 g/kg)。故在140~190 ℃温度区间,硫酸镁溶液处于第二介稳定区或不稳定区,但硫酸镁溶液的极限过饱和度较小,硫酸镁的结晶推动力小,结晶持续时间短,溶液很快达到结晶-溶解平衡[22]。由图13和14可知,硫酸亚铁的存在提高了硫酸镁的结晶率,当低于160 ℃时,硫酸镁的存在提高了硫酸亚铁的结晶率;当高于170 ℃时,硫酸镁的存在降低了硫酸亚铁的结晶率。随着硫酸镁浓度的增加,溶液中硫酸根的浓度增加,同时,溶液中亚铁离子和镁离子的总浓度增加,亚铁离子和镁离子的同离子效应降低了H+的活度[11],H+的活度降低,不利于反应式(5)向右进行,溶液中硫酸根的总浓度增加,促进反应(3)和(4)向右进行,提高了硫酸亚铁和硫酸镁的结晶率。当温度高于170 ℃时,硫酸亚铁的溶解度急剧降低,溶液中大量的硫酸亚铁晶体而有利于硫酸镁碰撞成核,提高硫酸镁的结晶率。同时,溶液中自由移动的镁离子因类质同相的作用取代硫酸亚铁晶体中部分铁原子,提高了硫酸镁的结晶率而降低了硫酸亚铁的结晶率。随着温度的增加硫酸镁和硫酸亚铁的溶解度降低,硫酸镁和硫酸亚铁的大量结晶,溶液中自由移动的镁离子、亚铁离子和硫酸根的浓度降低,类质同相的作用减弱,硫酸亚铁和硫酸镁结晶间的相互影响减弱。
FeSO4-MgSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L、温度为140~190 ℃之间、镁离子浓度为4.47~22.37 g/L的范围内,硫酸亚铁的存在提高了硫酸镁的结晶率。当温度低于160 ℃时,硫酸镁的存在提高了硫酸亚铁的结晶率;当温度高于170 ℃时,硫酸镁的存在降低了硫酸亚铁的结晶率。
2.4 FeSO4-ZnSO4-MgSO4-H2SO4体系中硫酸盐的结晶行为
高温体系下FeSO4、ZnSO4和MgSO4间的相互作用对硫酸盐结晶率的影响结果如图15所示。对比图15中的曲线a、b、c和d可知,在140~190 ℃之间,由FeSO4-ZnSO4-H2SO4体系转为FeSO4-ZnSO4- MgSO4-H2SO4时,硫酸亚铁和硫酸锌的结晶率降低。溶液中硫酸镁的浓度增加,溶液中镁离子的浓度增加,而镁离子更易取代硫酸亚铁晶体中的铁原子,引起硫酸亚铁和硫酸镁的结晶率降低;对比图15中的曲线b、c、f、g可知,在140~170 ℃之间时,由FeSO4-ZnSO4- H2SO4体系转为FeSO4-ZnSO4-MgSO4-H2SO4时,硫酸亚铁和硫酸镁的结晶率提高;在170~190 ℃之间时,硫酸亚铁和硫酸镁的结晶率降低;当温度低于170 ℃时,加入硫酸锌,溶液中硫酸根的浓度增加,溶液中硫酸根的浓度增加促进反应(3)和(4)向右进行,提高了硫酸亚铁和硫酸镁的结晶率;当温度高于170 ℃,加入硫酸锌,溶液中锌离子的浓度增加,锌离子因类质同相的作用,取代了硫酸亚铁晶体中部分有铁原子而降低了镁离子的类质同相用,引起硫酸亚铁和硫酸镁的结晶率降低。
不同锌离子和镁离子浓度下温度对硫酸锌、硫酸镁和硫酸亚铁结晶的影响结果见图16所示。
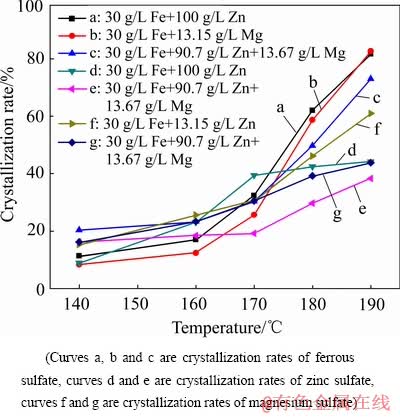
图15 高温体系下FeSO4、ZnSO4和MgSO4间的相互作用对硫酸盐结晶率的影响
Fig. 15 Effect of interaction between FeSO4, ZnSO4 and MgSO4 on sulfate crystallization rate under high temperature system
由图16(a)和(a′)可知,升高温度、加入硫酸镁和硫酸亚铁都促进硫酸锌结晶,随着温度的升高,硫酸锌的溶解度降低,硫酸锌的结晶率提高。加入硫酸镁和硫酸亚铁的同时也加入了大量的硫酸根,溶液中硫酸根的浓度增加。根据同离子效应,镁离子和铁离子的加入降低了H+的活度,不利于反应(5)向右进行,引起溶液中硫酸根浓度的增加,硫酸根的增加促进反应(2)向右进行,提高了硫酸锌的结晶率。当温度高于170 ℃时,硫酸亚铁的大量结晶,溶液中的硫酸亚铁晶体有利于硫酸锌碰撞成核,提高硫酸锌的结晶率,同时溶液中自由移动的锌离子因类质同相的作用取代硫酸亚铁结晶中部分铁离子而引起硫酸锌的结晶率提高。当温度为190 ℃时,硫酸根的浓度2.14 mol/L(140 g/L Zn)上升至2.56 mol/L(122.5 g/L Zn+3.63 g/L Mg+ 30 g/L Fe)时,硫酸锌的结晶率由32.41%上升至45.19%。
由图16(b)和(b′)可知,升高温度、硫酸锌和硫酸亚铁的加入有利于硫酸镁的结晶,随着温度的升高硫酸镁的溶解度降低,提高了硫酸镁的结晶率。加入硫酸锌和硫酸亚铁的同时也加入了大量的硫酸根,溶液中硫酸根的浓度增加。根据同离子效应,锌离子和铁离子的加入降低了H+的活度,不利于反应(5)向右进行,引起溶液中硫酸根浓度增加,硫酸根的浓度增加促进反应(4)向右进行,硫酸镁的结晶率增加。当温度为190 ℃时,硫酸亚铁的结晶率高于70%,溶液中大量的硫酸亚铁晶体,溶液中硫酸亚铁晶体有利于硫酸镁碰撞成核,提高硫酸镁的结晶率,且溶液中自由镁离子因类质同相的作用取代硫酸亚铁晶体中部分铁离子而引起硫酸镁的结晶率提高。当温度为190 ℃时,硫酸根的浓度1.234 mol/L(30 g/L Mg)上升至2.56 mol/L (122.5 g/L Zn+3.63 g/L Mg+30 g/L Fe)时,硫酸镁的结晶率由11.78%上升至44.35%。
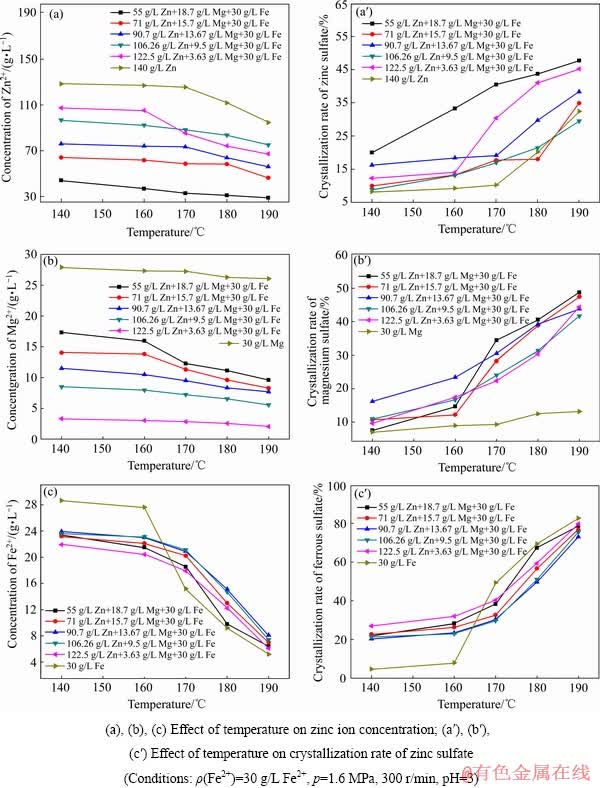
图16 不同硫酸锌和硫酸镁浓度下温度对硫酸锌、硫酸镁和硫酸亚铁结晶的影响
Fig. 16 Effect of temperature on crystallization of zinc sulfate((a), (a′)), magnesium sulfate((b), (b′)) and ferrous sulfate((c), (c′)) at different concentrations of zinc sulfate and magnesium sulfate
由图16(c)和(c′)可知,当温度小于或等于160 ℃时,加入硫酸锌和硫酸镁提高了硫酸亚铁的结晶率。当温度小于160 ℃时,硫酸亚铁、硫酸锌和硫酸镁的溶解度较大且硫酸亚铁的溶解度最大,加入硫酸锌和硫酸镁使溶液中的硫根的浓度增加,根据同离子效应,锌离子和镁离子的加入降低了H+的活度,不利于反应(5)向右进行,溶液中硫酸根增加,促进反应(3)向右进行,提高了硫酸亚铁的结晶率;反应温度为170~190 ℃时,加入硫酸锌和硫酸镁降低了硫酸亚铁的结晶率。随着温度的增加硫酸锌和硫酸镁对硫酸亚铁结晶的影响减弱;当温度高于170 ℃时,硫酸亚铁的大量结晶,而硫酸锌和硫酸镁的溶解度高于硫酸亚铁的溶解度,溶液中自由移动的锌离子和镁离子因类质同相取代硫酸亚铁晶体中的部分亚铁离子,引起硫酸亚铁的结晶率降低;随着温度的增加硫酸锌和硫酸镁的溶解度降低和硫酸亚铁晶体作为晶核,促进硫酸锌和硫酸镁结晶,溶液中自由移动的锌离子和镁离子减少,类质同相的作用减弱,硫酸锌和硫酸镁对硫酸亚铁结晶的影响减弱;当温度为170 ℃时,加入122.5 g/L的锌离子和3.63 g/L的镁离子时,硫酸亚铁的结晶率由49.33%降至40.33%;当温度为190 ℃时,硫酸亚铁的结晶率由82.67%降至79.67%。
FeSO4-ZnSO4-MgSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L、温度为140~190 ℃之间,当溶液由三元体系FeSO4-ZnSO4-H2SO4或FeSO4-MgSO4-H2SO4转为FeSO4-ZnSO4-MgSO4-H2SO4体系时,提高了硫酸锌和硫酸镁的的结晶率;当温度低于160 ℃时,提高了硫酸亚铁的的结晶率;当温度高于170 ℃时,降低了硫酸亚铁的的结晶率。
3 结论
1) FeSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L,温度为150 ℃时,溶液处于第一介稳定区;在160~170 ℃时硫酸亚铁溶液处于第二介稳定区;在180~190 ℃时,溶液处于不稳定区。改变初始硫酸亚铁的浓度硫酸亚铁溶解度的急剧降低区间(160~180 ℃)保持不变。
2) FeSO4-ZnSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L、温度为140~190 ℃之间、锌离子浓度为60~140 g/L的范围内,硫酸亚铁的存在提高了硫酸锌的结晶率;当温度低于160 ℃时,硫酸锌的存在提高了硫酸亚铁的结晶率;当温度高于170 ℃时,硫酸锌的存在降低了硫酸亚铁的结晶率。
3) FeSO4-MgSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L、温度为140~190 ℃、镁离子浓度为4.47~22.37 g/L的范围内,硫酸亚铁的存在提高了硫酸镁的结晶率;当温度低于160 ℃时,硫酸镁的存在提高了硫酸亚铁的结晶率;当温度高于170 ℃时,硫酸镁的存在降低了硫酸亚铁的结晶率。
4) FeSO4-ZnSO4-MgSO4-H2SO4体系中:初始亚铁离子浓度为30 g/L、温度为140~190 ℃之间,溶液由三元体系FeSO4-ZnSO4-H2SO4或FeSO4-MgSO4-H2SO4转为FeSO4-ZnSO4-MgSO4-H2SO4四元体系时,提高了硫酸锌和硫酸镁的的结晶率;当温度低于160 ℃时,提高了硫酸亚铁的的结晶率;当温度高于170 ℃时,降低了硫酸亚铁的的结晶率。
5) 采用赤铁矿法处理高铁溶液(30 g/L)时,防止因硫酸亚铁大量结晶导致硫酸亚铁晶体结实而引起硫酸亚铁晶体返溶缓慢和亚铁氧化缓慢等问题的发生,技术参数:温度为170~180 ℃、锌离子浓度为80~100 g/L、镁离子浓度小于15.7 g/L为宜。
REFERENCES
[1] PARKER R H. An introduction to chemical metallurgy [M].Oxford: Pergamon Press Ltd, 1978: 263-318.
[2] 田 磊, 刘 燕, 吕国志, 唐俊杰, 赵秋月, 张廷安. 加压搅拌浸出体系下氧气的气含率[J]. 中国有色金属学报, 2017, 27(3): 655-665.
TIAN Lei, LIU Yan, Lü Guo-zhi, TANG Jun-jie, ZHAO Qiu-yue, ZHANG Ting-an. Gas holdup in pressure agitation leaching system[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(3): 655-665.
[3] 王益昭, 李存兄, 魏 昶, 邓志敢, 李兴彬, 樊 刚, 易烁文. 湿法炼锌过程中赤铁矿生成及硫的吸附转化[J]. 中国有色金属学报, 2017, 27(10): 2145-2153.
WANG Yi-zhao, LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Xing-bin, FAN Gang, YI Shuo-wen. Production of hematite and conversion of adsorption S in zinc hydrometallurgy process[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(10): 2145-2153.
[4] 王吉坤, 冯桂林, 徐晓军. 有色金属矿产资源的开发及加工技术[M]. 昆明: 云南省科技出版社, 2000: 247-252.
WANG Ji-kun, FENG Gui-lin, XU Xiao-jun. Development and processing technology of nonferrous metal mineral resources[M]. Kunming: Yunnan science and Technology Press, 2000: 247-252.
[5] ZHANG Fan, WEI Chang, DENG Zhi-gan, LI Xing-bin, LI Cun-xiong. Reductive leaching of indium-bearing zinc residue in sulfuric acid using sphalerite concentrate as reductant[J]. Hydrometallurgy, 2016, 161: 102-106.
[6] 胡智润, 罗 佳, 邓志敢, 魏 昶, 朱北平, 牛 辉. 锌铁离子浓度对赤铁矿法除铁的影响[J]. 有色金属工程, 2017, 7(6): 54-57.
HU Zhi-run, LUO Jia, DENG Zhi-gan, WEI Chang, ZHU Bei-ping, NIU Hui. Effects of zinc and iron concentration on iron-removing by hematite precipitation[J]. Nonferrous Metals Engineering, 2017, 7(6): 54-57.
[7] 杨 凡, 邓志敢, 魏 昶, 李存兄, 李兴彬. 采用赤铁矿去除高铁闪锌矿浸出液中的铁[J]. 中国有色金属学报, 2014, 24(9): 2387-2392.
YANG Fan, DENG Zhi-gan, WEI Chang, LI Cun-xiong, LI Xing-bin. Iron-removal by hematite from leaching liquor of high iron sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2387-2392.
[8] 袁芳沁, 吴克明, 丁倩倩. 湿法炼锌除铁工艺的现状与展望[J]. 矿产综合利用, 2011(2): 30-32.
YUAN Fang-qin, WU Ke-ming, DING Qian-qian, KE Xing. Status and prospect of technology for iron removal in zinc hydrometallurgy[J]. Multipurpose Utilization of Mineral Resources, 2011(2): 30-32.
[9] 陈家镛, 于淑秋, 伍志春. 湿法冶金中铁的分离与应用[M]. 北京: 冶金工业出版社, 1991: 93-103.
CHEN Jia-yong, YU Shu-qiu, WU Zhi-chun. Separation and application of iron in hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1991: 93-103.
[10] 彭秋荣. 铅锌冶金学[M]. 北京: 科学出版社, 2003: 328-349.
PENG Qiu-rong. Metallurgy of lead and zinc[M]. Beijing: Science Press, 2003: 328-349.
[11] 杨 帆, 邓志敢, 魏 昶, 李兴彬, 邱伟佳, 朱如龙. 高铁闪锌矿赤铁矿法除铁中锌离子和镁离子的影响及存在机理[J]. 中国有色金属学报, 2014, 24(11): 2906-2913.
YANG Fan, DENG Zhi-gan, WEI Chang, LI Cun-xiong, QIU Wei-jia, ZHU Ru-long. Effect and existence mechanism of zinc ion and magnesium ion in iron removal from high iron sphalerite by hematite process[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(11): 2906-2913.
[12] 岳 明, 孙宁磊, 邹 兴, 邵建春, 刘金山, 王魁珽, 陆业大. 锌浸出液三价铁直接水解赤铁矿法除铁的探讨[J].中国有色冶金, 2012, 41(4): 80-85.
YUE Ming, SUN Ning-lei, ZOU Xing, SHAO Jian-chun, LIU Jin-shan, WANG Kui-ting, LU Ye-da. The discussion on hydrolysis precipitation of ferric oxide directly from ferric-ion rich zinc leachate[J]. China Nonferrous Metallurgy, 2012, 41(4): 80-85.
[13] Von ROPENACK A. Hematite-the solution to a disposal problem-an example from the zinc industry[J]. Iron Control in Hydrometallurgy, 1986, 11(12): 730-741.
[14] 张成松, 赵 婷. 赤铁矿除铁法在湿法炼锌工艺中的应用[J]. 湖南有色金属, 2014, 30(2): 39-41.
ZHANG Cheng-song, ZHAO Ting. Application of the hematite process for removing iron in the zinc hydrometallurgical production[J]. Hunan Nonferrous Metals, 2014, 30(2): 39-41.
[15] HASEGAWA F, TOZAWA K, NISHIMURA T. Solubility of ferrous sulfate in aqueous solutions at high temperatures[J]. Journal of the Mining and Materials Processing Institute of Japan, 1996, 112(12): 879-884. (in Japanese)
[16] 王文磊. 重金属湿法冶金过程钙镁结晶的基础相关化学研究[D]. 长沙: 湖南大学, 2010: 1-3.
WANG Wen-lei. Removing calcium and magnesium form heavy metal hydrometallurgical process based on phase chemistry[D]. Changsha: Hunan University, 2010: 1-3.
[17] E. B.哈姆斯基. 化学工业中的结晶[M]. 古涛, 叶铁林, 译. 北京: 化学工业出版社, 1984: 3-97.
HAMSKY E B. Crystallization in chemical industry[M]. GU Tao, YE Tie-lin, transl. Beijing: Chemical Industry Press, 1984: 3-97.
[18] BRUHN B, GERLACH J, PAWLEK F. Solubilities of salts and gasses in water[J]. Journal of Inorganic and General Chemistry, 1965, 337: 68-79. (in German)
[19] 梁英教, 车荫昌. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社, 1993: 573-574.
LIANG Ying-jiao, CHE Yin-chang. Handbook for thermodynamic data of inorganic substances[M]. Shenyang: Northeastern University Press, 1993: 573-574.
[20] 周甫立. 七水硫酸锌结晶过程研究[D]. 天津: 天津大学, 2017: 7-18.
ZHOU Fu-li. Study on the crystallization process of zinc sulfate heptahydrate[D]. Tianjin: Tianjin University, 2017: 7-18.
[21] M.E.波任. 无机盐工艺学[M]. 北京: 化学工业出版社, 1982: 153-155.
ВОЛНОВАТЬ M E. Inorganic salt processing technology[M]. Beijing: Chemical Industry Press, 1982: 153-155.
[22] 张宇婕, 朱家文, 陈 葵, 范先国, 周晓葵. 三元体系 MgSO4-FeSO4-H2O 的相平衡研究[J]. 化学工程, 2017, 45(1): 36-39.
ZHANG Yu-jie, ZHU Jia-wen, CHEN Kui, FAN Xian-guo, ZHOU Xiao-kui. Metastable phase equilibrium in the aqueous ternary system MgSO4-FeSO4-H2O[J]. Chemical Engineering, 2017, 45(1): 36-39.
[23] 孙 鸿, 景永峰. 植物补铁剂 —— 硫酸亚铁镁复混盐的探讨[J]. 山西化工, 2002, 22(4): 10-12.
SUN Hong, JING Yong-feng. Plants iron supplies—Mixture of iron and magnesium protosulfate[J]. Shanxi Chemical Industry, 2002, 22(4): 10-12.
[24] 张丽清. 硫酸镁溶液高温结晶规律的研究[D]. 沈阳: 东北大学, 1999: 11-16.
ZHANG Li-qing. Study on high temperature crystallization of magnesium sulfate solution[D]. Shenyang: Northeastern University, 1999: 11-16.
[25] 秦 善. 晶体学基础[M]. 北京: 北京大学出版社, 2004: 97-100.
QIN Shan. Basis fundamentals of crystallography[M]. Beijing: Peking University Press, 2004:97-100.
LIU Hui-yang, DENG Zhi-gan, WEI Chang, LI Xing-bin, LI Min-ting, WANG Chang-yin, ZENG Tao
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: The effects of different sulfate concentrations on the crystallization of ferrous sulfate, zinc sulfate and magnesium sulfate were investigated through investigating the crystallization behavior of zinc, iron and magnesium in FeSO4-ZnSO4-MgSO4-H2SO4 system. The results show that the solubility of ferrous sulfate, zinc sulfate and magnesium sulfate decreases with the increase of temperature. When the concentration of ferrous ions in the initial solution is changed, the sharp decrease interval of ferrous sulfate (160-180 ℃) remains unchanged. The presence of zinc sulfate and ferrous sulfate increases the crystallization rate of magnesium sulfate, the presence of magnesium sulfate and ferrous sulfate increases the crystallization rate of zinc sulfate. When the temperature is lower than 160 ℃, the presence of zinc sulfate and magnesium sulfate increases the crystallization rate of ferrous sulfate, when the temperature is higher than 170 ℃, it decreases the crystallization rate of ferrous sulfate.
Key words: ferrous sulfate; zinc sulphate; magnesium sulfate; solubility; crystallization; temperature
Foundation item: Project(2018YFC1900402) supported by the National Key Research and Development Plan of China; Projects(51804146, 51964029, 51564030, 51664030, 51664029) supported by the National Natural Science Foundation of China
Received date: 2019-07-26; Accepted date: 2020-04-28
Corresponding author: DENG Zhi-gan; Tel: +86-15925127947; E-mail: dengzhigan83@163.com
(编辑 李艳红)
基金项目:国家重点研发计划资助项目(2018YFC1900402);国家自然科学基金资助项目(51804146,51964029,51564030,51664030,51664029)
收稿日期:2019-07-26;修订日期:2020-04-28
通信作者:邓志敢,讲师,博士;电话:15925127947;E-mail:dengzhigan83@163.com

