GNP-SnO2纳米气敏元件的气敏性能
来源期刊:中国有色金属学报(英文版)2017年第8期
论文作者:A. BORHANINIA A. NIKFARJAM N. SALEHIFAR
文章页码:1777 - 1784
关键词:GNP-SnO2;SnO2;纳米颗粒;气敏元件;溶胶-凝胶法
Key words:GNP-SnO2; SnO2; nanoparticles; gas sensor; sol-gel
摘 要:合成了不同金属纳米颗粒含量的SnO2纳米颗粒,并研究了其CO气敏性能。采用溶胶-凝胶法制备初始溶液,通过SEM、TEM、XRD、DLS和分光光度法表征纳米颗粒。在340 °C操作温度下,纯SnO2纳米气敏元件对(20-80)×10-6 CO的响应值为4~12.8;在50×10-6 CO浓度下,其响应和恢复时间分别为10和14 s。当m(Au)/m(Sn)=3.7663×10-4时,GNP-SnO2气敏元件具有良好的性能,在260 °C优化操作温度下,对(20-80)×10-6 CO的响应值为8.3~29.5。
Abstract: SnO2 nanoparticles mixed with different amounts of gold nanoparticles (GNPs) were synthesized and their CO sensing properties were investigated. The sol-gel method was employed to prepare the initial solution. SEM, TEM, XRD, DLS and spectrophotometry were used to characterize the nanoparticles. The pure sensors showed a response of about 4 to 12.8 for (20-80)×10-6 CO at operating temperature of 340 °C. The response and recovery time at 50×10-6 Co is about 10 and 14 s, respectively. The amount of GNPs optimized was used to create high performance GNP-SnO2 sensors (m(Au)/m(Sn)=3.7663×10-4) and optimal operating temperature was about 260 °C and the response at concentrations of (20-80)×10-6 was 8.3 to 29.5, respectively.
Trans. Nonferrous Met. Soc. China 27(2017) 1777-1784
A. BORHANINIA, A. NIKFARJAM, N. SALEHIFAR
Faculty of New Sciences & Technologies, University of Tehran, P.O. Box 14395-1561, Tehran, Iran
Received 20 July 2016; accepted 6 February 2017
Abstract: SnO2 nanoparticles mixed with different amounts of gold nanoparticles (GNPs) were synthesized and their CO sensing properties were investigated. The sol-gel method was employed to prepare the initial solution. SEM, TEM, XRD, DLS and spectrophotometry were used to characterize the nanoparticles. The pure sensors showed a response of about 4 to 12.8 for (20-80)×10-6 CO at operating temperature of 340 °C. The response and recovery time at 50×10-6 Co is about 10 and 14 s, respectively. The amount of GNPs optimized was used to create high performance GNP-SnO2 sensors (m(Au)/m(Sn)=3.7663×10-4) and optimal operating temperature was about 260 °C and the response at concentrations of (20-80)×10-6 was 8.3 to 29.5, respectively.
Key words: GNP-SnO2; SnO2; nanoparticles; gas sensor; sol-gel
1 Introduction
Gas sensors based on metal oxide semiconductors are widely used to detect combustible, toxic, and pollutant gases which can be harmful to the environment. A change in gas combination around a semiconductor affects its electrical conductivity. Different metal oxide semiconductors have been suggested for gas detection. Metal oxide nanostructures such as tin oxide, zinc oxide, titanium oxide, indium oxide, and tungsten oxide [1-9] along with additive materials such as Ag [10], Pd [1], Cu, and In, are the most common resistive gas sensors which are used to reveal gases such as CO at various temperatures. In recent years, tin oxide gas sensors have been highly regarded for their use in environmental pollution monitoring [11-13]. SnO2 in the form of bulk or thin film as a wide band gap n-type semiconductor (Eg=3.6-3.8 eV) is widely used in gas sensors, optical electronics, and catalysts because of its special chemical and physical properties. These characteristics of SnO2 depend on its structural parameters. For instance, the gas sensing properties of SnO2 strongly depend on particle size and surface area. Decreasing the size of the nanoparticles increases the surface area and sensor efficiency [14].
Semiconductor gas sensors work based on electrical change of semiconductor surface. Sensor specifications vary at different temperatures. Because the sensitivity of semiconductor sensors at room temperature decreases, researchers have increased the operating temperature of the sensors to allow them to work properly [1,15-19]. Gas sensors produced by thin or thick film technology operate at 100 to 500 °C [1,14,20-22]. SnO2 film at high temperatures due to the high electron affinity of oxygen atoms eliminate or trap electrons when there is a lack of oxygen atoms or in the presence of extra oxygen atoms. The absorption of reducing gas molecules like CO, through reacting with oxygen ion species to form CO2, tends to release trapped electron and SnO2 nanoparticle conductivity [12]. The working mechanism of the gas sensors based on semiconductor metal oxides such as SnO2 is
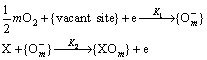
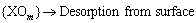
Some methods of preparing SnO2 nanoparticles include the sol-gel [2,20-22] and hydrothermal methods [3]. The sol-gel process is a chemical method of producing metal oxide nanoparticles. Sols are particles of 1 to 100 nm in diameter which are immersed into the solution. Because they show slight Brownian movement, they remain suspended in the solution. Gel is a solid adherent network with sub-micrometer pores and polymeric chains. In the present study we produced SnO2 nanoparticles having high dispersion and low diameter size using the sol-gel method [23,34]. Besides, GNPs synthesized and mixed with SnO2 Nanoparticles. Then, we optimized the amount of mixing agent for the GNP-SnO2 sensors. The best operating temperature for sensors was achieved and the response, response time of the GNP-SnO2 and pure SnO2 sensors were analyzed under different conditions.
2 Experimental
The current study fabricated and investigated pure SnO2 and GNP-SnO2 gas sensors. Different experiments were then carried out to compare their performances.
2.1 SnO2-nano particle preparation
For preparation of SnO2 sol, 6.2 mL SnCl4 and 23.865 mL 1-propan (C3H7-OH) were poured into a closed container and mixed rigorously. In another container, 2.865 mL H2O and 11.425 mL 1-propan were mixed. The two solutions were then combined and mixed thoroughly. Next, 5.715 mL H2O and 24.435 mL 2-propan (2-C3H7-OH) were mixed and added to the existing solution and the resulting solution was blended for 2 h.
2.2 Preparation of GNP-SnO2
For preparation of GNP-SnO2, the GNP solution was prepared by sodium citrate reduction of gold chloride in an aqueous solution. The GNPs were encapsulated in 4-methylbenzenthiol synthesized as follows. Initially, 10 mL of 0.0288 mol HAuCl4·3H2O aqueous solution was poured into a container. Next, 20.6 mL of 0.0358 mol toluene [N(C8H17)4Br] was added to the container and stirred for 15 min. Then, 23.8 mL of 0.0139 mol 4-methylbenzenthiol (HS-C6H4-CH3) and 8.25 mL of 0.3836 mol NaBH4 in the form of an aqueous solution were stirred into the solution in the container and mixed for 3 h.
The solution formed separate black/brown phases. To separate the GNPs from the solution, ethanol was added to increase the volume of the solution, which was then frozen for 12 h. The black sediment was filtered using a 0.2 μm PTFE filter [35]. The sizes of the GNPs were 2.4 to 4.4 nm with Gaussian size distribution. Different amounts of GNP solution were added to the SnO2 sol and were mixed by magnetic stirring for about 3 h to prepare a solution containing varying amounts of GNPs.
Samples 1-5 were produced by adding 0.0125, 0.0083, 0.00625, 0.0041 and 0.00312 mL GNP solution to 1 mL pure SnO2 sol, respectively. The mass ratio of Au/Sn was 1.1482×10-3 for sample 1 to 2.8477×10-4 for sample 5.
2.3 Test set-up
Alumina ceramics with 1 mm thickness were sliced into 1.6 cm × 1 cm pieces and platinum interdigitated contacts having 1 mm gaps and 10 fingers with 1 cm in length were DC sputtered onto the ceramic substrate. The ceramics pieces were dipped into the solution for 30 min. After dipping, the prepared sample was sintered at 600 °C for 30 min.
The experimental set-up consisted of a glass tube reactor, a heater with 400 W electrical power, and a data acquisition system for continuous monitoring of sensor response. The set-up had gas inlet, gas outlet and mass flow controller. The temperature of the sensors was varied from 100 to 400 °C.
XRD analysis was performed using a Philips X’Pert MPD instrument with Cu Kα radiation (λ=1.54178 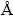 ) at 40 kV/30 mA. Hitachi S4160 model field emission scanning electron microscope was used for FESEM imaging. The response was defined as the ratio of sensor resistance in dry air to sensor resistance in the detected gas (Rair/Rgas). The response time was the length of time required for sensor resistance to arrive at 90% modification. Recovery time was defined as the time required for 60% variation to gain the initial content in the air.
) at 40 kV/30 mA. Hitachi S4160 model field emission scanning electron microscope was used for FESEM imaging. The response was defined as the ratio of sensor resistance in dry air to sensor resistance in the detected gas (Rair/Rgas). The response time was the length of time required for sensor resistance to arrive at 90% modification. Recovery time was defined as the time required for 60% variation to gain the initial content in the air.
3 Results and discussion
The GNPs used as catalyst with specific size and shape have additional photo catalysis effect under visible light [36]. The GNPs supported on the SnO2 nanoparticles exhibited higher catalytic activity when illuminated with visible light. So, CO molecules could be oxidized more easily by lowering the activation energy of the following reactions which tend to release the trapped electrons to the body and increase conductivity.
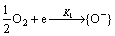
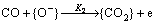
Visible light can enhance local electromagnetic fields, heat, and activate GNPs by local surface plasmon resonance (LSPR) effect. This lowers the activation energy of the reactions. Figure 1(a) shows the absorption spectra of the GNP solution. As shown, GNPs have high absorption in UV spectrum and have a local maximum at 550 nm (visible light).
The size distribution for the GNPs was obtained using DLS technique as shown in Fig. 1(b). The size of the GNPs was 2 to 3 nm. The shape of the nanoparticles was investigated by TEM and XRD as shown in Figs. 2(a) and (b), respectively. A TEM core size of 3-5 nm and a spherical shape for GNPs are evident.
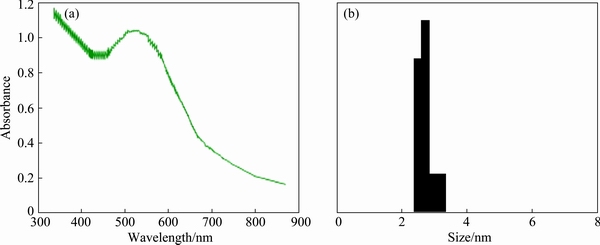
Fig. 1 Absorption spectra of GNP solution (a) and GNP size distribution (b) through DLS
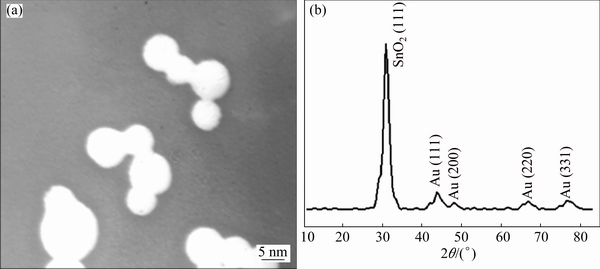
Fig. 2 TEM image (a) and XRD pattern (b) of GNPs
The SnO2 solution was mixed with GNP solution at different ratios and the results of the sensors were compared for different parameters. The amount of additive agent in the GNP-SnO2 sensor was then optimized. Figure 3 shows the XRD patterns for SnO2 and GNP-SnO2 nanoparticles calcined at 600 °C for 2 h. When compared with the standard pattern of JCPDS (File No. 41-1445), the peaks agreed with the tetragonal structure of SnO2 crystal. The XRD pattern of the GNP-SnO2 showed the peak of GNP at about 2θ=42° (111), 2θ=51° (200), 2θ=67° (220) and 2θ=75° (331). The diffraction peaks of SnO2 in the GNP-SnO2 sample became sharper, indicating an increase in the size of the SnO2 particles.
Figure 4 shows the SEM images of pure-SnO2 and GNP-SnO2 samples. The mean diameters of pure SnO2 and GNP-doped SnO2 were about 20 and 35 nm, respectively, which indicates that the average grain size increases with the addition of GNP. So, the addition of GNPs increases the grain boundaries and the electronic transmission channels, which are important for the current passing through the sensor.

Fig. 3 XRD patterns of pure SnO2 and GNP-SnO2 calcined at 600 °C
To improve the selectivity and sensor response, catalyst materials such as noble metals can be added to SnO2 solution. Au is one of the most interesting catalysts for CO gas sensing. In the present study, we produced five sample solutions containing different amounts of GNP solution (m(Au)/m(Sn)=1.143×10-3-6.889×10-4) and related sensors were tested under identical conditions to obtain the best Au/Sn mass ratio. Figure 5 shows the response of the GNP-SnO2 sensors at five GNP ratios and different temperatures. All samples were produced under the same conditions. The best performance for GNP-SnO2 sensors was observed at 3.28×10-5 g GNP in 1 mL of SnO2 solution (m(Au)/m(Sn)=3.7663×10-4) at a sintering temperature of 500 °C (Fig. 6). This ratio was then used for all further tests (m(Au)/m(Sn)= 3.7663×10-4).
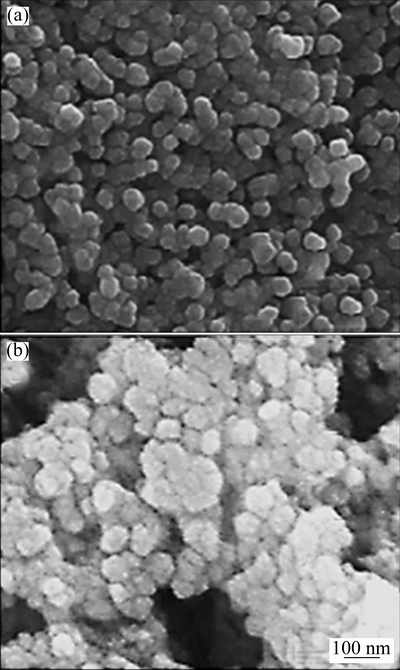
Fig. 4 SEM images of pure-SnO2 (a) and GNP-SnO2(b)
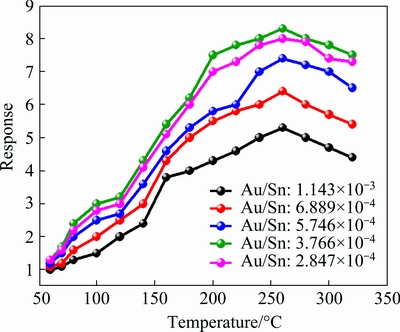
Fig. 5 Response of GNP-SnO2-based sensor to 20×10-6 CO gas with different amounts of GNP as function of temperature
Testing was carried out at different temperatures to obtain the best operating temperature. The response values for the pure SnO2 sensor at different concentrations of CO gas are shown in Fig. 6(a). As the temperature increased, the response value increased. It continued up to about 340 °C, then the response gradually decreased. The optimal working temperature for the sensor was determined to be 340 °C for the pure SnO2 CO gas sensor. The response (Rair/Rgas) for (20-80)×10-6 CO gas was about 4 to 12.8. For GNP-doped samples, the operating temperature decreased to about 260 °C, and the response at this temperature for (20-80)×10-6 CO was about 8.3 to 29.5 (Fig. 6(b)).
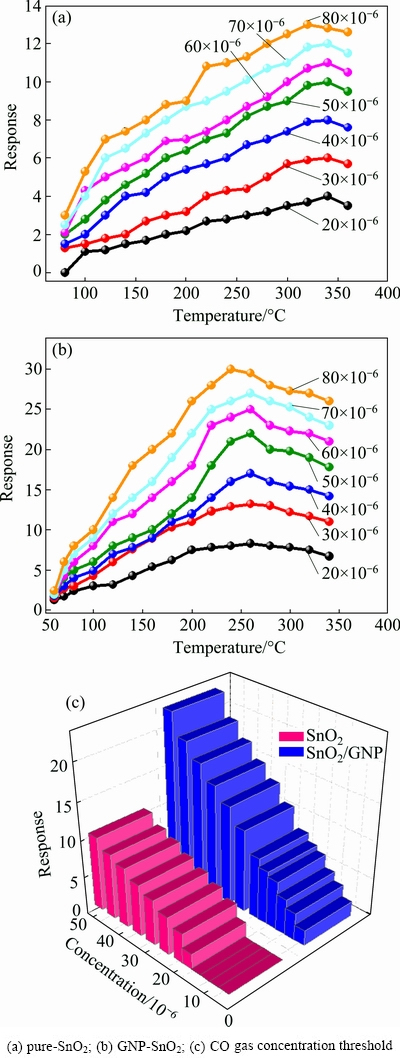
Fig. 6 Response of sensor at different CO concentrations as function of temperature
For comparison, the response of GNP-SnO2 samples produced at Au/Sn=3.7663×10-4 is improved by 2-fold at different CO concentrations. Besides, the operating temperature for the doped sensor dropped to 260 °C, which was 80 °C lower than the pure sensor. This was the result of using gold catalyst to lower the activation energy required for the reactions to detect CO gas. Besides, since all tests were performed at a constant illumination of sunlight, the photo catalytic effect of the GNPs played an important role in lowering the operating temperature. UV radiation can reduce the operating temperature too. The photocatalytic effect of GNPs which tends to absorb UV-visible light illumination and gas sensing activation is due to their LSPR effect.
Figure 6(c) demonstrates and compares the outcomes for pure and optimized GNP-SnO2 samples as a function of CO concentration. The CO gas concentration thresholds for pure SnO2 and SnO2/GNP samples were 5×10-6 and 20×10-6, respectively. The pure SnO2 sample showed no noticeable response at less than 18×10-6 CO.
Figure 7 shows the response and recovery time of pure-SnO2 and GNP-SnO2 sensors at 50×10-6 CO as a function of temperature. The response and recovery time for the pure SnO2 sensor was about 10 and 14 s at 340 °C, respectively. These values for the GNP-SnO2 sensor decreased to about 4 and 6 s at 260 °C, respectively.
YANAGIMOTO et al [37] made Au/SnO2 core-shell nanoparticle CO sensors using the precipitation (A) and microwave hydrothermal synthesis (B) methods. The sensors were tested in the presence of 1000×10-6 CO gas at 300 °C. This sensor recorded responses of about 0.18 and 0.965 for A and B, respectively. The response time for sensors A and B was about 1.13 and 0.57 min, respectively. The recovery time was also about 6.7 and 15 min for sensors A and B, respectively. In all sensing specifications, our sensors showed higher performances which include lower operating temperature by 40 °C and higher responses at lower gas concentrations (at 50×10-6 CO gas, the response was about 22). Besides, the response and recovery time of our sensor was about 4 and 6 s, respectively, which is tremendously lower than that obtained by YANAGIMOTO et al [37].
Figures 8(a) and (b) show the transient response of pure-SnO2 and GNP-SnO2 sensors for 50×10-6 CO gas. Figures 8(c) and (d) show the transient response for 20×10-6 to 60×10-6 CO gas as a function of time at their optimal operating temperatures.
Figure 9 shows the response of the SnO2 and GNP-SnO2 sensors to several 50×10-6 gases at their optimal operation temperatures. The GNP-SnO2 sensor showed the lowest response to NH3 gas of 12 and the highest response for CO of about 22. The pure SnO2 sensor showed the lowest response of 5 to NH3 and the highest response of about 12 for H2. The GNP-SnO2 sensor responded well to CO, suggesting that it can be employed to detect this gas for different usages.
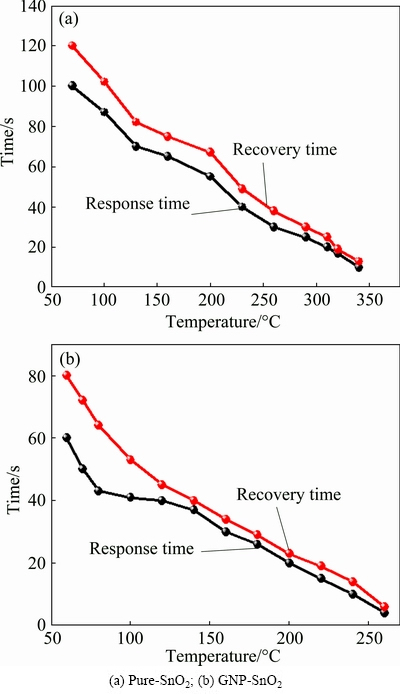
Fig. 7 Response and recovery time of sensor for 50×10-6 CO as function of temperature
4 Gas-sensing mechanism
The detection mechanism of our sensors in the presence of a reducing gas such as CO involves the partial chemical reduction of the sensitive layer surface [13]. At higher temperatures, the O2 molecules ionized on the SnO2 nanoparticles to form active ionic oxygen species like [O-]. Due to the high electron affinity of oxygen and because metal oxide semiconductors are n-type, they extract electrons from the semiconductor conduction band and create a depletion area on the surface of the grains, decreasing the effective radius of the grains for electron transport. By introducing CO gas, a reaction between the ionic oxygen atoms [O-] and CO molecules occurs [CO+O-→CO2+e]. This interaction releases electrons and re-injects them into the depletion region created in SnO2 nanoparticles surfaces. This significantly reduces the height of barriers created between neighboring particles, resulting in an increase in sensor conductance. GNPs also cover SnO2 nanoparticles to some extent but CO molecules can penetrate through the free spaces between the particles and react with ionic oxygen species (O2-, O2-, O- ) [14,21,25].
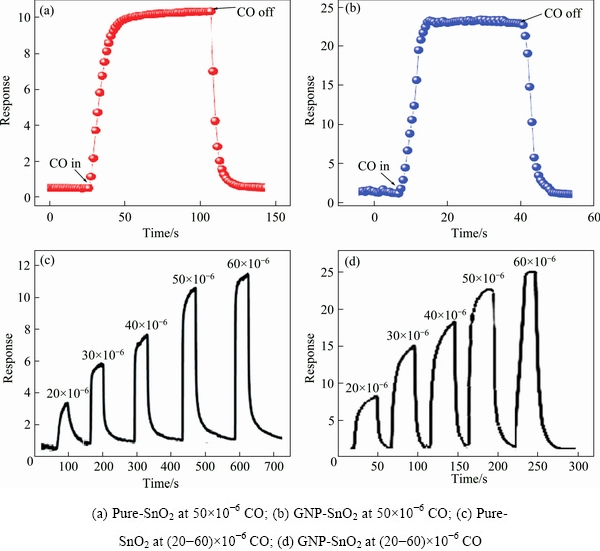
Fig. 8 Transient response of sensors at optimal temperature
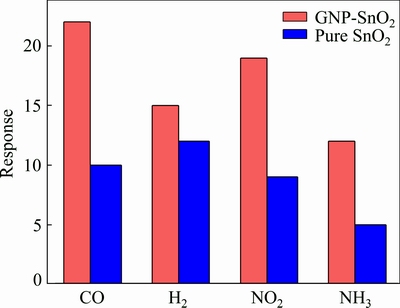
Fig. 9 Response of sensors at different 50×10-6gases
Through reducing the activation energy required for interactions as mentioned, GNPs increase the sensor response. Besides, as GNPs contact with SnO2 nanoparticles, a Schottky barrier forms between them and electrons flow from the SnO2 nanoparticles to the GNPS. This occurs because of the work function difference between the gold and SnO2 nanoparticles. This widens the depletion region into the SnO2 nanoparticles and tends to further reduce the sensor conductivity. So, when CO molecules interact with pre-adsorbed oxygen ionic atoms to release and re-inject electrons into the depletion region created in SnO2 nanoparticles, the changes of the sensor conductivity will be improved [14,21,25]. The GNPs affect the rate of attraction and repulsion of the gas molecules and improves the response and recovery time. These improvements are dependent on the interaction of the CO molecules and ionic oxygen species (O2-, O2-, O-) under the catalytic effect of the GNPs [21,38,39].
5 Conclusions
The amount of GNPs optimized was used to create high performance GNP-SnO2 sensors (Au/Sn= 3.7663×10-4). The optimal operating temperature is about 260 °C and the responses at concentrations of (20-80)×10-6 are 8.3-29.5. This means that the operating temperature decreases by about 80 °C and the response improved by more than 2-fold compared with the pure samples. At 50×10-6 CO gas, the response and recovery time for the pure SnO2 sensor were approximately 10 and 14 s, and for the GNP-SnO2 sensor, these values were approximately 4 and 6 s, respectively. This means that the sensor speed increases by more than 2-fold. The selectivity of the sensor response was evaluated for several gases. The GNP-SnO2 sensor shows the best response for CO, suggesting that it can be employed for different related usages.
References
[1] ADNAN ROHANA, RAZANA NUR ARIESMA, RAHMAN ISMAIL ABDUL, FARRUKH MUHAMMAD AKHYAR. Synthesis and characterization of high surface area tin oxide nanoparticles via the sol-gel method as a catalyst for the hydrogenation of styrene [J]. Journal of the Chinese Chemical Society, 2010, 57: 222-229.
[2] HAMOON H Z R, DEVI G S, BEIGI H, RAO J V R, REDDY K R, NANAJI A. Synthesis and characterization of Co-doped SnO2/TiO2 semiconductor nano crystallites via sol-gel method [J]. J Nano-Electron Phys, 2011, 1: 53-58.
[3] GUO Xian-zhi, KANG Yan-fei, YANG Tai-li, WANG Shu-rong. Low-temperature NO2 sensors based on polythiophene/WO3 organic-inorganic hybrids [J]. Transactions of Nonferrous Metals Society of China,2012, 22: 380-385.
[4] LI Chao, YU Zhi-shuo, FANG Shao-ming, WU Shi-de, GUI Yang-hai, CHEN Rong-feng. Synthesis and gas-sensing properties of Ce-doped SnO2 materials [J]. Journal of Physics, 2009, 152: 1-5.
[5] HAENG Yu-ji, CHOI Man-gyeong. Selective CO gas detection of CuO- and ZnO-doped SnO2 gas sensor [J]. Sensor and Actuators B, 2001,75: 56-61.
[6] ZHANG Tong, LIU Li, QI Qi, LI Shou-chun, LU Ge-yu. Development of microstructure In/Pd-doped SnO2 sensor for low-level CO Detection [J]. Sensors and Actuators B, 2009, 139: 287-291.
[7] BAI Hua, SHI Gao-quan. Gas sensors based on conducting polymers [J]. Sensors, 2007, 7: 267-307.
[8] MADLER L, SAHM T, GURLO A, GRUNWALDT J D, BARSAN N, WEIMAR U, PRATSINIS S E. Sensing low concentrations of CO using flame-spray-made Pt/SnO2 nanoparticles [J]. Journal of Nanoparticle Research, 2006, 8: 783-796.
[9] LI Yan, LIU Jin-cheng, LIAN Xiao-xue, LU Tan, ZHAO Fang-xian. Morphology, photoluminescence and gas sensing of Ce-doped ZnO microspheres [J]. Transactions of Nonferrous Metals Society of China,2015, 25: 3657-3663.
[10] BAHRAMI B, KHODADADI A, KAZEMEINI M, MORTAZAVI Y. Enhanced CO sensitivity and selectivity of gold nanoparticles- doped SnO2 sensor in presence of propane and methane [J]. Sensor and Actuators B, 2008, 133: 352-356.
[11] SADEK A Z M. Investigation of nanostructured semiconducting metal oxide and conducting polymer thin films for gas sensing applications [M]. Melbourne, Australia: RMIT University, 2008.
[12] GILBERT M M, WENDELL P E. Introduction to environmental engineering and science [M]. 3rd ed. Prentice Hall: Pearson, 2008.
[13] BEACH E R. Picoiter drop deposition of oxide nanoparticles a route to high performance microsensor arrays [D]. Ohio: The Ohio State University, 2009.
[14] HASSANZADEH A, MOAZZEZ B, HAGHGOOIE H, NASSERI M, GOLZAN M M, SEDGHI H. Synthesis of SnO2 nano powders by a sol-gel process using propanol-isopropanol mixture [J]. Cent Eur J Chem, 2008, 6: 651-656.
[15] BAGHERI MOHAGHEGHI M M, SHAHTAHMASEBI N, ALINEJAD M, YOUSSWFI A, SHOKOOH S M. The effect of the post-annealing temperature on the nano-structure and energy band gap of SnO2 semiconducting oxide nano-particles synthesized by polymerizing-complexing sol–gel method [J]. Physica B, 2008, 403: 2431-2437.
[16] CHEN Huan, LIU Zhi-yu, FU Gang. Analysis of the aging characteristics of SnO2 gas sensors [J]. Sensors and Actuators B, 2011, 156: 1-6.
[17] OLIAEE S N, KHODADADI A, MORTAZAVI Y, ALIPOUR S. Highly selective Pt/SnO2 sensor to propane or methane in presence of CO and ethanol, using gold nanoparticles on Fe2O3 catalytic filter [J]. Sensors and Actuators B , 2010, 147: 400-405.
[18] DIEGUEZ A, VILA A, CABOT A, ROMANO R A, MORANTE J R, KAPPLER J, BARSAN N, WEIMAR U, GOPEL W. Influence on the gas sensor performances of the metal chemical states introduced by impregnation of calcinated SnO sol–gel nanocrystals [J]. Sensors and Actuators B, 2000, 68: 94-99.
[19] JITIANU A, ALTINDAG Y, ZAHARESCU M, WARK M. New SnO2 nano-clusters obtained by sol-gel route structural characterization and their gas sensing applications [J]. Journal of Sol-Gel Science and Technology, 2003, 26: 483-488.
[20] JIANG Lu-hua, SUN Gong-quan, ZHOU Zhen-hua, SUN Shi-guo, WANG Qi, YAN Shi-you, LI Huan-qiao, TIAN Juan, GUO Jun-song, ZHOU Bing, XIN Qin. Size-controllabl synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts [J]. Journal of Physical Chemistry B, 2005, 109: 8774-8778 .
[21] MENINI P, PARRET F, GUERRERO M, SOULANTICA K, ERADES L, MAISONNAT A, CHAUDRET B. CO response of a nanostructured SnO2 gas sensor doped with palladium and platinum [J]. Sensor and Actuators B, 2004, 103: 111-114.
[22] LU Gan-hua, L. HUEBNER K, LEONIDAS E O, JOSIFOVSKA-JOSIFOVSKA M, CHEN Jun-hong. Gas sensors based on tin oxide nanoparticles synthesized from a mini-arc plasma source [J]. Journal of Nanomaterials, 2006, 2006: 1-7.
[23] CHAMPION Y, FECHT H J. Nano-architectured and nanostructured materials fabrication, control and properties [M]. German: Wiley-VCH, 2005.
[24]  L, ROESSLER A, PRATSINIS S E, SAHM T, GURLO A, BARSAN N, WEIMAR U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles [J]. Sensors and Actuators B, 2006, 114: 283-295.
L, ROESSLER A, PRATSINIS S E, SAHM T, GURLO A, BARSAN N, WEIMAR U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles [J]. Sensors and Actuators B, 2006, 114: 283-295.
[25] LEE Duk-dong, LEE Dae-sik. Environmental gas sensor [J]. IEEE Sensors, 2001, 3: 214-224.
[26] DEVI G S, HAMOON H Z R, NANAJI A, REDDY K R, SREEDHAR B, RAMANA RAO J V. A Simple sol gel protocol towards synthesis of semiconducting oxide nanomaterial [J]. Nano- Electron Phys, 2011, 3: 53-58.
[27] SADEK A Z, TRINCHI A, WLODARSKI W, KALANTAR-ZADEH K, GALATSIS K, BAKER C, KANER R B. A room temperature polyaniline nanofiber hydrogen gas sensor [J]. IEEE Sensors, 2005, 207-210.
[28] GNANAM S, RAJENDRAN V. Synthesis of tin oxide nanoparticles by sol–gel process: Effect of solvents on the optical properties [J]. Sol-Gel Science and Technology, 2010, 53: 555-559.
[29] YUE Li, YANQUN Guo, RUIQIN Tan, PING Cui, YONG Li, WEIJIE Song. Selective synthesis of SnO2 hollow microspheres and nano-sheets via a hydrothermal route [J]. Materials Chemistry, 2010, 55: 581-587.
[30] MILLER T A, BAKRANIA S D, PEREZ C, WOOLDRIDGE M S. A new method for direct preparation of tin dioxide nanocomposite materials [J]. Materials Research Society, 2005, 20: 2977-2987.
[31] SIKHWIVHILU L, PILLAI S, HILLIE T. Influence of citric acid on SnO2 nanoparticles synthesized by wet chemical processes [J]. Nanoscience and Nanotechnology, 2011, 11: 4988-4994.
[32] ZHANG Peng. Design and fabrication of chemiresistor type micro/nano hydrogen gas sensors using interdigitated electrodes [D]. Florida: University of Central Florida, 2008.
[33] PARK C O, AKBAR S A, HWANG J. Selective gas detection with catalytic filter [J]. Materials Chemistry and Physics, 2002, 75: 56-60.
[34] KE Xue-bin, ZHANG Xing-guang, ZHAO Jian, SARINA Sarina, BARRY John, ZHU Huai-yong. Selective reductions using visible light photocatalysts of supported gold nanoparticles [J]. Green Chemistry, 2013, 15: 236-244.
[35] HANWELL M D. Structural properties of metal-organic nanosystems for sensing applications [D]. Sheffield: University of Sheffield, 2004.
[36] YAMAZOE N, MIURA N. Development of gas sensors for environmental protection [J]. IEEE Transactions on Components, Packaging, and Manufacturing Technology, 1995, 18: 252-256.
[37] YANAGIMOTO T, YU Y T, KANEKO K. Microstructure and CO gas sensing property of Au/SnO2 core–shell structure nanoparticles synthesized by precipitation method and microwave-assisted hydrothermal synthesis method [J]. Sensors and Actuators B, 2012, 166-167: 31-35.
[38] CHEN Guo-hui, JI Shao-zheng, LI Hai-dong, KANG Xue-liang, CHANG Su-jie, WANG Ya-na, YU Guang-wei, LU Jian-ren, CLAVERIE J, SANG Yuan-hua, LIU Hong. High-energy faceted SnO2-coated TiO2 nanobelt heterostructure for near-ambient temperature-responsive ethanol sensor [J]. ACS Applied Materials Interfaces, 2015, 44: 24950-24956.
[39] WANG Xiao-hang, SANG Yuan-hua, WANG Dong-zhou, JI Shao-zheng, LIU Hong. Enhanced gas sensing property of SnO2 nanoparticles by constructing the SnO2–TiO2 nanobelt heterostructure [J]. Journal of Alloys and Compounds, 2015, 639: 571-576.
A. BORHANINIA, A. NIKFARJAM, N. SALEHIFAR
Faculty of New Sciences & Technologies, University of Tehran, P.O. Box 14395-1561, Tehran, Iran
摘 要:合成了不同金属纳米颗粒含量的SnO2纳米颗粒,并研究了其CO气敏性能。采用溶胶-凝胶法制备初始溶液,通过SEM、TEM、XRD、DLS和分光光度法表征纳米颗粒。在340 °C操作温度下,纯SnO2纳米气敏元件对(20-80)×10-6 CO的响应值为4~12.8;在50×10-6 CO浓度下,其响应和恢复时间分别为10和14 s。当m(Au)/m(Sn)=3.7663×10-4时,GNP-SnO2气敏元件具有良好的性能,在260 °C优化操作温度下,对(20-80)×10-6 CO的响应值为8.3~29.5。
关键词:GNP-SnO2;SnO2;纳米颗粒;气敏元件;溶胶-凝胶法
(Edited by Xiang-qun LI)
Corresponding author: A. NIKFARJAM; E-mail: a.nikfarjam@ut.ac.ir
DOI: 10.1016/S1003-6326(17)60200-0

