Effect of binary conductive additive mixtures on electrochemical performance of polyoxomolybdate as cathode material of lithium ion battery
来源期刊:中南大学学报(英文版)2016年第10期
论文作者:倪尔福 李文良 李新海 郭华军
文章页码:2506 - 2512
Key words:lithium ion battery; cathode; Na3[AlMo6O24H6] (NAM); conductive additive
Abstract: Binary carbon mixtures, carbon black ECP 600JD (ECP) combined with vapor grown carbon fiber (VGCF) or carbon nanotube (CNT), or graphene (Gr) in different mass ratios, are investigated as the conductive additives for the cathode material polyoxomolybadate Na3[AlMo6O24H6] (NAM). Field emission scanning electron microscopy and energy dispersive X-ray spectroscopy show that the surfaces of NAM particles are covered homogeneously with the binary conductive additive mixtures except the combination of ECP and CNT. The optimum combination is the mixture of ECP and VGCF, which shows higher discharge capacity than the combinations of ECP and CNT or Gr. Initial discharge capacities of 364, 339, and 291 mA·h/g are obtained by the combination of ECP and VGCF in the mass ratios of 2:1, 1:1, and 1:2, respectively. The results of electrochemical impedance spectra and 4-pin probe measurements demonstrate that the combination of ECP and VGCF exhibits the highest electrical conductivity for the electrode.
J. Cent. South Univ. (2016) 23: 2506-2512
DOI: 10.1007/s11771-016-3310-y
LI Wen-liang(李文良)1, 2, NI Er-fu(倪尔福)2, LI Xin-hai(李新海)1, GUO Hua-jun(郭华军)1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Highpower International Inc., Xinxia Road, Pinghu Town, Longgang District, Shenzhen 518111, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Binary carbon mixtures, carbon black ECP 600JD (ECP) combined with vapor grown carbon fiber (VGCF) or carbon nanotube (CNT), or graphene (Gr) in different mass ratios, are investigated as the conductive additives for the cathode material polyoxomolybadate Na3[AlMo6O24H6] (NAM). Field emission scanning electron microscopy and energy dispersive X-ray spectroscopy show that the surfaces of NAM particles are covered homogeneously with the binary conductive additive mixtures except the combination of ECP and CNT. The optimum combination is the mixture of ECP and VGCF, which shows higher discharge capacity than the combinations of ECP and CNT or Gr. Initial discharge capacities of 364, 339, and 291 mA·h/g are obtained by the combination of ECP and VGCF in the mass ratios of 2:1, 1:1, and 1:2, respectively. The results of electrochemical impedance spectra and 4-pin probe measurements demonstrate that the combination of ECP and VGCF exhibits the highest electrical conductivity for the electrode.
Key words: lithium ion battery; cathode; Na3[AlMo6O24H6] (NAM); conductive additive
1 Introduction
Among the many available electrochemical energy storage devices, lithium ion batteries (LIBs) have gained intense attention because they have a much higher energy density than current lead-acid batteries and nickel-metal hydride battery [1-3]. LIBs have been widely used as power sources for consumer electronics such as mobile phones and laptop computers. In current LIB technology, it is generally acknowledged that the cell voltages and capacities are mainly determined by the cathode materials [4-12]. The layered compound LiCoO2 is the most widely used cathode material in LIB. Although the theoretical capacity of LiCoO2 is 274 mA·h/g, the practically available capacity is only 140 mA·h/g because of the intrinsic structural instability of the material when more than half of the Li ions are extracted [13-16]. Thereafter, the cathode materials moved from LiCoO2 to its derivatives in which Co ions are partially or fully substituted by others metal ions, such as Ni, Mn, and Al [17-22]. Furthermore, the spinel compound LiMn2O4 with lower cost and the olivine compound LiFePO4 with more stable structure are explored as the cathode materials of LIB [23-31]. However, the achievable capacities of these materials are still lower than 200 mA·h/g. Therefore, the wider application of LIB is restricted due to the limited capacity of the cathode materials. For the development of advanced electric vehicles that can provide ~300 km driving distance range, the battery should provide a cell-level energy density of 350-400 W·h/kg. This would require almost double the energy density (~200 W·h/kg) of current LIB [32].
Polyoxometalate(POM) is a promising candidate for the next generation LIB that can provide higher energy density because of the high theoretical capacity over 400 mA·h/g [33-43]. However, the main challenging issue of POM is the very low electrical conductivity. The electrochemical performance of POM can be improved by decreasing the particle size or mixing with the carbon additive with higher electrical conductivity. The polyoxovanadates K7[NiV13O38] and K7[MnV13O38] have been prepared by re-crystallization in the solution containing organic solvent acetone and the nanosize particles showed improved electrochemical performance [35, 41]. The Anderson type polyoxomolybadate Na3[AlMo6O24H6] (NAM) shows improved electrochemical performance by mechanical ball milling with the conductive additive Ketjen black (KB) [40]. Although the KB can effectively improve the electrochemical performance of the cell, KB with very high specific surface area would result in the decrease in the practically available specific volumetric capacity per the unit mass of cathode composite, and would further decrease the specific volumetric energy density of cell, particularly for the pouch cell. Therefore, the optimal choice of conductive additives and the combination of conductive additives are required. In this work, in order to improve the specific volumetric capacity of NAM, the use of the conductive carbon black ECP600JD (ECP) with high specific surface area was mixed with vapor grown carbon fiber (VGCF) or graphene (Gr), or carbon nanotube (CNT). The VGCF, Gr and CNT not only show high electrical conductivity, but also possess lower specific surface area than the ECP. Therefore, the combinations of ECP with VGCF or Gr, or CNT are expected to improve the specific volumetric capacity of cell, meanwhile improve the electrochemical performance of NAM.
2 Experimental
The NAM was synthesized by the reported method [40]. The water in the as-prepared samples was removed by drying at 120 °C for 1 h before electrode preparation. ECP (battery grade) was purchased from Lion Co., Ltd., Japan. The VGCF (battery grade), carbon nanotube (battery grade), and Gr (battery grade) were obtained from Showa Denko, Japan, Zhenjiang Cnano Technology Ltd., China, and Chengdu Organic Chemicals Co. Ltd., China, respectively. Before the electrode preparation, the binary conductive additive mixtures ECP/VGCF, ECP/CNT, and ECP/Gr were mixed respectively together with the NAM powders by dry mechanical ball milling in planetary ball mill under air. The detail conditions of the mechanical ball milling are as follows. The mass ratios of the binary conductive additive mixtures for ECP: VECF, ECP:CNT, and ECP:Gr were 2:1, 1:1, and 1:2, respectively. Milling media included six balls with 10 mm in diameter each and 12 smaller balls with 5 mm in diameter each; volume of milling vessel was 100 mL; milling media and vessel material were zirconium dioxide; milling speed was 500 r/min; milling time was 1 h. After ball milling, the cathode was prepared by mixing the above ball-milled samples and polyvinylidene fluoride (PVDF) in N- methyl-pyrrolidon (NMP) as the solvent to form a homogeneous slurry. The slurry was then cast on an aluminum foil and dried at 80 °C for 20 h under vacuum. All of the cathodes consisted of active material NAM, the binary conductive additive mixtures, and PVDF in a mass ratio of 30:60:10. The morphologies were observed with field emission scanning electron microscope (FESEM, Nova NanoSEM 450) equipped with energy dispersive X-ray spectroscope (EDXs).
The electrochemical performance of cathodes was tested at 25 °C by using CR-2032 coin cells, which were assembled in an argon filled glovebox using metallic lithium as anode, and 1 mol/L LiPF6 in a mixed solvent of ethylene carbonate and diethyl carbonate at a volume ratio of 3:7 as electrolyte. Cycle performance was tested on a NEWARE CT-4008 equipment. Unless otherwise noted, the voltage range for the discharge and charge was in 1.5-4.2 V (vs. Li/Li+) at 0.04C (1C=455 mA/g). Electrochemical impedance spectra (EIS) were performed with a CHI660C (Shanghai Chenhua) impedance analyzer in the frequency range of 105- 10-2 Hz with an amplitude of 10 mV. The electrical conductivities were measured by the 4-pin probe method on a powder resistivity test equipment (Mitsubishi, MCP-PD51).
3 Results and discussion
Figure 1 shows the XRD pattern of the as-prepared NAM, which is consistent with the NAM in ICSD No. 281185. However, some intensity ratios of the as- prepared NAM do not match well the ICSD pattern, which should be due to the difference in orientation of powder. The morphology of the as-prepared NAM is shown in the inset of Fig. 1. It can be seen that the NAM exhibits large particle in tens of microns. As reported previously, the large size particles show low electrical conductivity and serious polarization in discharge- charge process, resulting in the low capacity of NAM [35, 40-41, 43]. In this work, to alleviate the polarization and improve the electrochemical performance of the NAM as the cathode material of LIB, the NAM was ball milled with the binary conductive additive mixtures firstly. The specific surface area, tap density and pore volume are shown in Table 1. The morphologies of ECP, VGCF, Gr, and CNT used as the conductive additives in this work are shown in Fig. 2. The four conductive additives possess different morphologies, and it should be noted that the ECP, which is similar to the KB, has the high specific surface area of 1400 m2/g due to its mesopore and micropore structures [43]. However, the ECP shows better dispersion performance than the KB, because the KB is easy to aggregate in the powder state. Figure 3 shows the morphologies of NAM after mechanical ball milling with the conductive additives. It can be seen that the NAM was pulverized to smaller particles and covered with the conductive additives. Figure 3(a) shows that the mixture of ECP/VGCF covers intimately on the surface of NAM particle, whereas the surface of NAM particle was not fully covered by the ECP/CNT, as shown in Fig. 3(c). The elemental distribution maps by EDXs are shown in Fig. 4. It can be seen that the carbon elements in the ECP/VGCF and ECP/Gr were mixed homogeneously with the NAM, while that of the ECP/CNT shows inhomogeneous dispersion. This indicates that the covering effect of ECP/VGCF and ECP/Gr should be much better than the ECP/CNT.
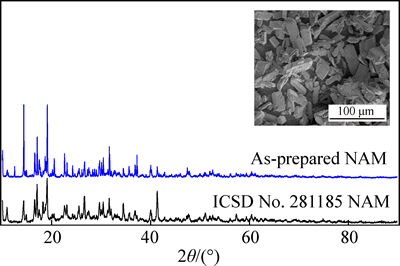
Fig. 1 X-ray diffraction patterns of as-prepared NAM and NAM in ICSD No.281185 (inset: FESEM images of as-prepared NAM)
Table 1 Comparison of conductive additives in specific surface area, tap density and pore volume

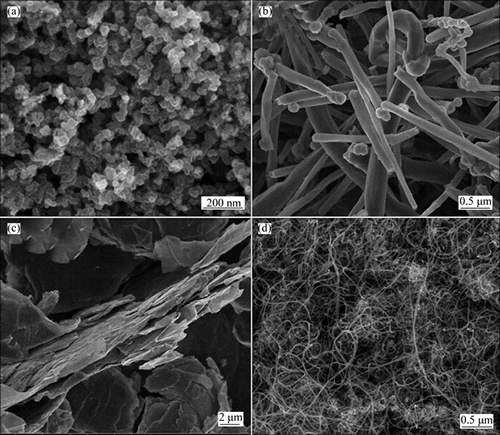
Fig. 2 Morphologies of ECP (a), VGCF (b), Gr (c) and CNT (d)
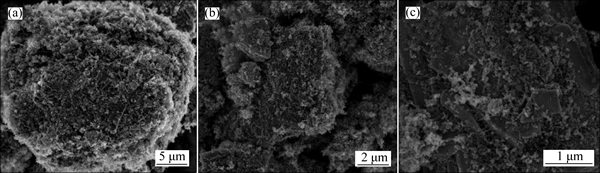
Fig. 3 Morphologies of NAM ball milled with ECP/VGCF (a), ECP/Gr (b), ECP/CNT (c) (mass ratio of ECP:VGCF or Gr, or CNT is 2:1)
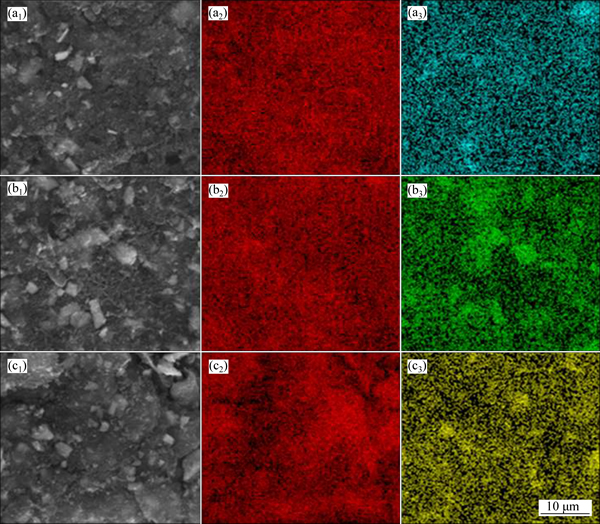
Fig. 4 FESEM images (a1, b1, c1) and corresponding EDXs mappings of C (a2, b2, c2) and Mo (a3, b3, c3) for NAM ball milled with ECP/VGCF (a1, a2, a3), ECP/Gr (b1, b2, b3), ECP/CNT (c1, c2, c3) (mass ratio of ECP:VGCF or Gr, or CNT is 2:1)
Figure 5(a) shows the first discharge-charge curves of NAM mixed with the different binary conductive additive mixtures in the mass ratio of 2:1 at 0.04C. The initial discharge capacities of 364, 355, and 328 mA·h/g are obtained for the ECP/VGCF, ECP/Gr, and ECP/CNT, respectively. In the following charge process, coulombic efficiencies of 98.1%, 96.3%, and 94.8% are obtained for the ECP/VGCF, ECP/CNT, and ECP/Gr, respectively. The cycle performance is shown in Fig. 5(b). The three electrodes show gradual capacity loss in the first five cycles, and tend to be stable in the following cycles. It can be seen that the ECP/VGCF maintains higher discharge capacity than the ECP/Gr and ECP/CNT during cycling. After 30 cycles, discharge capacities of 288, 258, and 238 mA·h/g are obtained for the ECP/VGCF, ECP/Gr , and ECP/CNT, respectively.
To further decrease the loading weight of ECP in the electrode, because carbon additive with the high specific surface area would decrease the specific volumetric energy density of the cell, the weight ratios of ECP to VGCF or Gr, or CNT were decreased, and the discharge-charge performance was investigated. Figures 5(d) and (d) show the cycle performance of the NAM mixed with the different binary conductive additive mixtures in the mass ratios of ECP to VGCF (Gr, or CNT)=1:1 and 1:2, respectively. The discharge capacities deceased with decreasing the loading mass of ECP in the cathode. However, the ECP/VGCF still shows higher discharge capacity than ECP/Gr and ECP/CNT. Discharge capacity higher than 200 mA·h/g can be obtained for the ECP/VGCF after 30 cycles, even at the lowest loading mass of ECP in the ratio of ECP to VGCF=1:2. This demonstrates that the combination of ECP and VGCF is more favorable for achieving the high capacity of NAM. The high capacity of NAM mixed with the ECP/VGCF should be not only ascribed to homogeneous dispersion of the conductive additives in the electrode, but also the VGCF can act as the conductive bridge among the ECP particles, which would improve the electrical conductivity of the electrode. Moreover, NAM as the cathode material of LIB shows stable cycle performance with long-term cycling. This should be ascribed the stable molecular cluster ion which reacts with lithium ion reversibly [40].
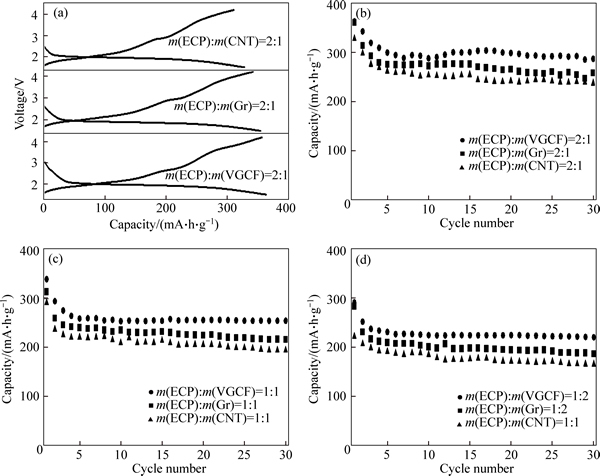
Fig. 5 First discharge-charge curves (a), cycle performance (b) of binary conductive additive mixtures for ECP/VGCF, ECP/Gr, and ECP/CNT in mass ratio of 2:1, and cycle performance of binary conductive additive mixtures for ECP/VGCF, ECP/Gr, and ECP/CNT in mass ratio of 1:1 (c) and 1:2 (d)
The rate capabilities of the binary conductive additive mixtures for ECP/VGCF, ECP/Gr, ECP/CNT in the mass ratio of 2:1 were investigated, as shown in Fig. 6. It can be seen that the ECP/VGCF shows better rate capability than ECP/Gr and ECP/CNT. Discharge capacity higher than 130 mA·h/g can be obtained for the ECP/VGCF at the rate of 1C.
To investigate the effect of the different binary the respective Nyquist plots are shown in Fig. 7. It can be seen that all of the Nyquist plots consist of one semicircle in the high- frequency region and a linear part in the low-frequency region. The semicircles in the high-frequency region would be attributed to the resistances of the charge- transfer in the cathode. The slope lines in the low- frequency region are related to the solid-state diffusion of lithium ions in the cathode. It is clearly shown that the charge-transfer resistance of the ECP/VGCF is much smaller than that of the ECP/Gr and ECP/CNT after the first cycle. After 30 cycles, the charge-transfer resistance of ECP/VGCF does not change too much, while that of ECP/Gr and ECP/CNT increases significantly. This indicates that the combination of ECP and VGCF can improve the electrical conductivity of the electrode, which is beneficial for the charge transfer and lithium ion diffusion in the electrode. To further clarify that the difference in the electrochemical performance of the electrodes consisting of different combinations of conductive additives, the pressure dependence of electrical conductivities for the NAM ball milled with the ECP/VGCF, ECP/Gr and ECP/CNT, respectively, is shown in Fig. 8. It is clearly shown that the electrical conductivity of ECP/VGCF is much higher than that of ECP/Gr and ECP/CNT, which should be responsible for the better electrochemical performance of cell by combination of ECP and VGCF.
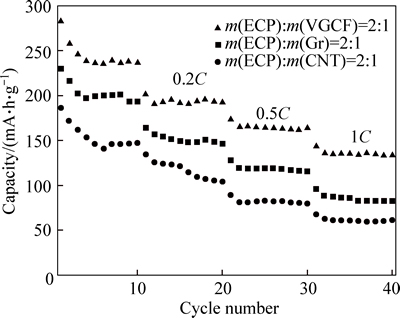
Fig. 6 Rate capability of binary conductive additive mixtures for ECP/VGCF, ECP/Gr, and ECP/CNT in mass ratio of 2:1
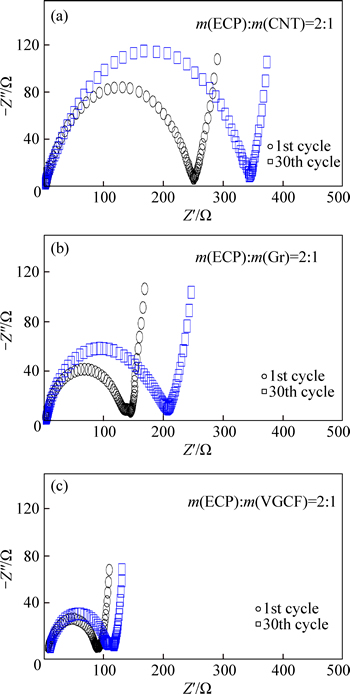
Fig. 7 Nyquist plots of binary conductive additive mixtures for ECP/VGCF (a) ECP/Gr (b) and ECP/CNT (c) in mass ratio of 2:1 after 1 cycle and after 30 cycles

Fig. 8 Pressure dependence of electrical conductivities of binary conductive additive mixtures for ECP/VGCF, ECP/Gr, and ECP/CNT in mass ratio of 2:1
4 Conclusions
The electrochemical performance of polyoxomolybdate NAM was investigated by mixing with the binary conductive additive mixtures. The mixture of conductive additives ECP and VGCF can be covered well on the surface of the NAM particles by the mechanical ball milling. The combination of ECP and VGCF shows better effect in achieving the high capacity of NAM. In comparison of the previously reported work that NAM mixed solely with the carbon black (KB) possessed high specific surface area [40], the use of binary conductive additive mixtures should be a feasible way that can improve the specific volumetric energy density of cell. This study provided valuable clues to develop the polyoxometalates with high capacity and energy density as the alternative cathode materials of lithium ion battery.
References
[1] LINDEN D, REDDY T B. Handbook of batteries: Third edition [M]. New York: McGraw-Hill, 2001.
[2] AIFANTIS K E, HACKNEY S A , KUMAR R V. High energy density lithium batteries: Materials, engineering, applications [M]. Germany: Wiley-VCH, 2010.
[3] ABRAHAM K M, SCHALKWIJK W A V, HASSOUN J. Lithium batteries: Advanced technologies and applications [M]. New Jersey: John Wiley & Sons, Inc, 2013.
[4] AURBACH D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries [J]. J Power Sources, 2000, 89: 206-218.
[5] TARASCON J M, ARMAND M. Review article Issues and challenges facing rechargeable lithium batteries [J]. Nature, 2001, 414: 359-367.
[6] WHITTINGHAM M S. Lithium batteries and cathode materials [J]. Chem Rev, 2004, 104: 4271-4301.
[7] CABANA J, MONCONDUIT L, LARCHER D,  M R. Beyond intercalation-based Li-Ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions [J]. Adv Mater, 2010, 22: E170-E192.
M R. Beyond intercalation-based Li-Ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions [J]. Adv Mater, 2010, 22: E170-E192.
[8] ELLIS B L, LEE K T, NAZAR L F. Positive electrode materials for Li-ion and Li-batteries [J]. Chem Mater, 2010, 22: 691-714.
[9] GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries [J]. Chem Mater, 2010, 22: 587-603.
[10] XU B, QIAN D N, WANG Z Y, MENG S Y. Recent progress in cathode materials research for advanced lithium ion batteries [J]. Mater Sci Eng, 2012, R73: 51-65.
[11] GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: A perspective [J]. J Am Chem Soc, 2013, 135: 1167-1176.
[12] MELOT B C, TARASCON J M. Design and preparation of materials for advanced electrochemical storage [J]. Acc Chem Res, 2013, 46: 1226-1238.
[13] MIZUSHIMA K, JONES P C, WISEMAN P J, GOODENOUGH J B. LixCoO2 (0 [14] REIMERS J N, DAHN J R. Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2 [J]. J Electrochem Soc, 1992, 139: 2091-2097. [15] OHZUKU T, UEDA A. Solid-state redox reactions of LiCoO2(R3m) for 4 volt secondary lithium cells [J]. J Electrochem Soc, 1994, 141: 2972-2977. [16] LEVI M D, SALITRA G, MARKOVSKY B, TELLER H, AURBACH D, HEIDER U, HEIDERB L. Solid-state electrochemical kinetics of Li-ion intercalation into Li1-xCoO2: Simultaneous application of electroanalytical techniques SSCV, PITT, and EIS [J]. J Electrochem Soc, 1999, 164(4): 1279-1289. [17] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiNi1/2Mn1/2O2: A possible alternative to LiCoO2 for advanced lithium-ion batteries [J]. Chem Lett, 2001, 30(8): 744-745. [18] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries [J]. Chem Lett, 2001, 30(7): 642-643. [19] LU Z H, BEAULIEU L Y, DONABERGER R A, THOMAS C L, DAHN J R. Synthesis, structure, and electrochemical behavior of Li[NixLi1/3-2x/3Mn2/3-x/3]O2 [J]. J Electrochem Soc, 2002, 149(6): A778-A791. [20] CHO J, KIM H, PARK B. Comparison of overcharge behavior of AlPO4-coated LiCoO2 and LiNi0.8Co0.1Mn0.1O2 cathode materials in Li-ion cells [J]. J Electrochem Soc, 2004, 151(10): A1707-1711. [21] MENG X L, DOU S M, WANG W L. High power and high capacity cathode material LiNi0.5Mn0.5O2 for advanced lithium-ion batteries [J]. J Power Sources, 2008, 184(2): 489-493. [22] LI Z, DU F, BIE X, ZHANG D, CAI Y, CUI X, WANG C. Electrochemical kinetics of the Li[Li0.23Co0.3Mn0.47]O2 cathode material studied by GITT and EIS [J]. J Phys Chem C, 2010, 114: 22751-22757. [23] TARASCON J M, GUYOMARD D. The Li1+xMn2O4/C rocking- chair system: A review [J]. Electrochimica Acta, 1993, 39(9): 1221-1231. [24] GUMMOW R J, KOCK A D, THACKERAY M M. Improved capacity retention in rechargeable 4 V lithium/lithium-manganese oxide (spinel) cells [J]. Solid State Ionics, 1994, 69(1): 59-67. [25] JANG D H, SHIN Y J, OH S M. Dissolution of spinel oxides and capacity losses in 4 V Li/LixMn2O4 cells [J]. J Electrochem Soc, 1996, 143: 2204-2211. [26] XIA Y Y, ZHOU Y H, YOSHIO M. Capacity fading on cycling of 4 V Li/LiMn2O4 cells [J]. J Electrochem Soc, 1997, 144(8): 2593-2600. [27] THACKERAY M M, SHAO-HORN Y, KAHAIAN A J, KEPLER K D, SKINNER E, VAUGHEY J T, HACKNEY S A. Structural fatigue in spinel electrodes in high voltage (4 V) Li/LixMn2O4 cells [J]. Electrochem Solid-State Lett, 1998, 1: 7-9. [28] PADHI A K. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144(4): 1188-1194. [29] PADHI A K, NANJUNDASWAMY K S, MASQUELIER C, OKADA S, GOODENOUGH J B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates [J]. J Electrochem Soc, 1997, 144(5): 1609-1613. [30] HUANG H, YIN S C, NAZAR L F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates [J]. Electrochem Solid-State Lett, 2001, 4(10): A170-A172. [31] DELACOURT C, POIZOT P, TARASCON J M, MASQUELIER C. The existence of a temperature-driven solid solution in LixFePO4 for 0 [32] SONG M K, ZHANG Y G, CAIRNS E J. A long-life, high-rate lithium/sulfur cell: A multifaceted approach to enhancing cell performance [J]. Nano Lett, 2013, 13: 5891-5899. [33] SONOYAMA N, SUGANUMA Y, KUME T, QUAN Z. Lithium intercalation reaction into the Keggin type polyoxomolybdates [J]. J Power Sources, 2011, 196(16): 6822-6827. [34] UEMATSU S, QUAN Z, SUGANUMA Y, SONOYAMA N. Reversible lithium charge–discharge property of bi-capped Keggin- type polyoxovanadates [J]. J Power Sources, 2012, 217: 13-20. [35] NI E F, UEMATSU S, QUAN Z, SONOYAMA N. Improved electrochemical property of nanoparticle polyoxovanadate K7NiV13O38 as cathode material for lithium battery [J]. J Nanopart Res, 2013, 15: 1732-1741. [36] CHEN W, HUANG L J, HU J, LI T F, JIA F F, SONG Y F. Connecting carbon nanotubes to polyoxometalate clusters for engineering high-performance anode materials [J]. Phys Chem Chem Phys, 2014, 16: 19668-19673. [37] KUME K, KAWASAKI N, WANG H, YAMADA T, YOSHIKAWA H, AWAGA K. Enhanced capacitor effects in polyoxometalate/ graphene nanohybrid materials: A synergetic approach to high performance energy storage [J]. J Mater Chem A, 2014, 2: 3801-3807. [38] NAUMAAN R, KHAN N, MAHMOOD N, LV C, SIMA G, ZHANG J, HAO J, HOU Y, WEI Y. Pristine organo-imido polyoxometalates as an anode for lithium ion batteries [J]. RSC Adv, 2014, 4: 7374-7379. [39] NI E F, KUME T, UEMATSU S, QUAN Z, SONOYAMA N. Effect of annealing treatment on the electrochemical properties of polyoxomolybdate K4[SiMo12O40] as cathode material of lithium battery [J]. Electrochemistry, 2014, 82: 14-18. [40] NI E F, UEMATSU S, SONOYAMA N. Anderson type polyoxomolybdate as cathode material of lithium ion battery and its reaction mechanism [J]. J Power Sources, 2014, 267: 673-681. [41] NI E F, UEMATSU S, SONOYAMA N. Lithium intercalation into the polyoxovanadate K7MnV13O38 as cathode material of lithium ion battery [J]. Solid State Ionics, 2014, 268: 222-225. [42] WANG H, YAMADA T, HAMANAKA S, YOSHIKAWA H, AWAGA K. Cathode composition dependence of battery performance of polyoxometalate (POM) molecular cluster batteries [J]. Chem Lett, 2014, 43: 1067-1069. [43] NI E F, UEMATSU S, TSUKADA T, SONOYAMA N. Lithium intercalation into polyoxomolybdate (NH4)6[NiMo9O32] as the cathode material of lithium battery [J]. Solid State Ionics, 2016, 285: 83-90. (Edited by YANG Hua) Received date: 2016-06-03; Accepted date: 2016-08-22 Corresponding author: NI Er-fu, Senior Engineer; Tel/Fax: +86-755-89686522; E-mail: nierfu0733@163.com

