磷酸溶液中稀土元素的溶解行为
来源期刊:中国有色金属学报(英文版)2018年第11期
论文作者:巫圣喜 赵龙胜 王良士 黄小卫 董金诗 冯宗玉 崔大立 张立峰
文章页码:2375 - 2382
关键词:稀土元素;磷酸;溶解行为;磷酸稀土;温度
Key words:rare earth elements; phosphoric acid; dissolution behavior; rare earth phosphates; temperature
摘 要:为了建立磷酸溶液中稀土回收工艺性基础数据和更好地了解磷酸蒸发浓缩过程中的稀土行为,测定不同温度条件下不同磷浓度的磷酸溶液中的稀土溶解度。建立关于磷酸溶液中稀土溶解度与磷浓度之间简单线性模型,该模型与实验测定的磷酸溶液中稀土溶解度具有较高的契合度(R2>0.94)。磷酸溶液中稀土溶解度的控制性影响因素为溶液中氢离子浓度。此外,升温能使磷酸溶液中稀土溶解度迅速下降,这主要是因为升温导致磷酸稀土的溶解反应吉布斯自由能升高,同时,升温还抑制磷酸分子的电离。
Abstract: In order to provide practical fundamental data for rare-earth elements (REEs) recovery from phosphoric acid and to better understand REEs behavior during the phosphoric acid evaporation process, the solubilities of REEs in phosphoric acid with various concentrations of phosphorus at different temperatures were measured. A simple linear model between REEs solubility and phosphoric acid concentration is built and the experimental data are found to fit it very well (R2>0.94). Hydrogen-ion concentration is found to be the predominant factor controlling the solubility of REEs in phosphoric acid. In addition, the solubility of REEs in phosphoric acid is found to sharply decrease with increasing temperature, which can be attributed to the increase of the Gibbs energy of the REEPO4 dissolution reaction or the restraint of the disassociation of phosphoric acid molecules owing to the elevated temperature.
Trans. Nonferrous Met. Soc. China 28(2018) 2375-2382
Sheng-xi WU1,2, Long-sheng ZHAO1, Liang-shi WANG1, Xiao-wei HUANG1, Jin-shi DONG1, Zong-yu FENG1, Da-li CUI1, Li-feng ZHANG2
1. National Engineering Research Center for Rare Earth Materials, GRINM Group Co., Ltd., Beijing 100088, China;
2. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China
Received 31 May 2018; accepted 21 September 2018
Abstract: In order to provide practical fundamental data for rare-earth elements (REEs) recovery from phosphoric acid and to better understand REEs behavior during the phosphoric acid evaporation process, the solubilities of REEs in phosphoric acid with various concentrations of phosphorus at different temperatures were measured. A simple linear model between REEs solubility and phosphoric acid concentration is built and the experimental data are found to fit it very well (R2>0.94). Hydrogen-ion concentration is found to be the predominant factor controlling the solubility of REEs in phosphoric acid. In addition, the solubility of REEs in phosphoric acid is found to sharply decrease with increasing temperature, which can be attributed to the increase of the Gibbs energy of the REEPO4 dissolution reaction or the restraint of the disassociation of phosphoric acid molecules owing to the elevated temperature.
Key words: rare earth elements; phosphoric acid; dissolution behavior; rare earth phosphates; temperature
1 Introduction
To achieve the sustainable development of the rare- earth-elements (REEs) industry, numerous studies have been focused on the recovery of REEs from secondary resources such as end-of-life wastes, industrial scraps [1], residues [2,3], and flotation tailings [4,5], and from REEs associated minerals such as uranium ore, and phosphate rock [6-9]. Among these, phosphate rock is considered as one of the most promising new sources of REEs since there are about 125000 t of potential REEs produced per year from phosphate rock [5].
Recovery of REEs from phosphate rock based on H2SO4, HNO3, HCl and H3PO4 processes has been extensively studied [10]. In particular, REEs recovery in the H2SO4 process has attracted considerable attention, with the main challenges in this process being technical issues and the cost overage owing to the low content of REEs in phosphoric acid and phosphogypsum [11,12]. Previous studies have been tried to recover REEs from phosphoric acid by means of crystallization, precipitation, solvent extraction, ion exchange, etc [13-16], but the industrial scale application of these processes has rarely been reported. Furthermore, these previous studies are mainly focused on the development of new technologies, while ignoring the fundamental research of REEs in the hydrometallurgical processes of phosphate rock.
However, the physico-chemical properties (practical fundamental data) of REEs in phoshoric acid are of vital importance for REEs recovery from phosphate rock, because all the solution systems involved in REEs recovery from phosphate rock are phosphoric acid extended solutions such as HCl-H3PO4, HNO3-H3PO4, H2SO4-H3PO4 and Ca(H2PO4)2-H3PO4. For instance, the solubility of REEs in phosphoric acid is a critical parameter for the prediction of REEs leachablity and precipitation efficiency [17] especially for the crystallization and precipitation of REEs from phosphoric acid (28%-40% P2O5), and for better understanding of REEs behaviors during the evaporation process of phosphoric acid (from 28%-32% to 54% P2O5) [18,19].
So far, the solubility of rare earth phosphates in non-phosphorus aqueous solution has been systemetically studied in geochemistry [20-23]. However, the solubility of rare earth phosphates in non- phosphorus aqueous solution may not be able to guide the exploitation of new technologies for REEs recovery from phosphate rock owing to the high phosphorus concentration and low pH value in the solutions involved in these processes. Generally, phosphorus concentration in leaching solution involved in phosphate rock processing ranges from 28% to 54% P2O5. The pH value of phosphoric acid solutions in practical hydrometallurgical processes ranges from 0.5 to 1.0 for WPA [24], and much less than 1.0 for HCl-H3PO4 and HNO3-H3PO4. These factors would significantly affect the precipitation-dissolution equilibrium of REEs in aqueous solutions.
To understand the regularity of REEs solubility in phosphoric acid and provide practical fundamental data for REEs recovery from phosphoric acid, we measured the solubility of La, Nd and Y in 15%-75% H3PO4 and investigated the effect of elevated temperature on the solubility of REEs in phosphoric acid. A linear model between the solubility of REEs and concentration of phosphoric acid was proposed on the basis of the precipitation-dissolution reactions of REEs in phosphoric acid and the ionization processes of phosphoric acid. The efficacy of the linear model was evaluated against the measured REEs solubility in phosphoric acid.
2 Experimental
The kinetic method [25] was introduced in this work to measure the solubility of rare earth elements since rare earth phosphates (REEPO4) particles are very difficult to dissolve in phosphoric acid even after immersion for 50 d. Rare earth carbonates (La2(CO3)3, Nd2(CO3)3, Y2(CO3)3) with purity of >99% rare earth oxides (REO) produced by Jiangsu Guosheng Rare Earth Co., Ltd. (China) were used as the raw materials for rare earth ions [26]. The phosphoric acid (>85% H3PO4) used was of analytical grade and produced by Beijing Chemical Works (China).
Prior to the experiments, 50 mL airtight glass tubes to be used for the REEs solubility measurements were soaked in an acid solution for more than 12 h and then rinsed thoroughly with deionized water. Phosphoric acid solutions of various concentrations were prepared by diluting concentrated acid (85% H3PO4) in an airtight tube. Rare earth (RE) carbonate particles were then added into the tubes in many batches, and the addition of solids in very small amounts for each batch was controlled to guarantee the complete dissolution of the RE carbonates until new particles were generated, which indicated that the RE ion concentration was supersaturated. The solid particles remaining in the centrifuge tube were collected, washed, dissolved (using diluted HNO3), and analyzed by ICP-OES (the particle samples of one group were collected). The results showed that in the newly generated precipitates more than 97% of the REEs presented as RE phosphates and a small amount of extra RE oxides were found that were likely to have originated from the undissolved RE carbonates (Table 1).
Table 1 Chemical analysis results of solid particle left in centrifuge tube after centrifugation

The prepared supersaturated solutions were then kept in a shaker table in a water bath with 180 r/min rotation for 15 d. The reaction temperature was maintained at (25±2) °C set as room temperature and [(30±2)-(90±2)] °C set as elevated temperature via the water bath. During this period of time, several parallel samples were tested to examine the equilibrium time (See Fig. 1; La, Nd and Y concentrations in 15% and 75% H3PO4 were also tested and showed a similar decreasing trend). Approximately 8 d was proven sufficient to achieve the equilibrium of precipitation- dissolution reaction of REEPO4. To ensure reaching equilibrium, 15 d of shaking was adopted. After being shaken for 15 d, the equilibrium solutions were centrifuged to obtain clear solutions for the ICP-OES tests.

Fig. 1 c(La3+) vs time in 15% H3PO4 phosphoric acid
Chemical analysis of aqueous solutions was conducted by inductively coupled plasma optical emission spectroscopy (ICP-OES) (PerkinElmer- Optima 8300). A specific analysis method was built for REEs and P tests in phosphoric acid, with the wavelength and the recovery given in Table 2. Samples of equilibrium solution were diluted for element analysis (La, Nd, Y and P).
Table 2 Recovery of REEs and phosphorus in phosphate solution tested with ICP-OES

3 Results and discussion
3.1 Equilibrium concentration of REEs in phosphoric acid
The equilibrium concentrations of La, Nd and Y (g/L) in 15%-75% H3PO4 are plotted in Fig. 2 and listed in Table 3. It should be noted that the phosphorus concentrations measured by ICP-OES after equilibration are slightly less than the calculated values (error is less than 8%), but can be neglected. As the curves demonstrate, the solubility of La3+, Nd3+ and Y3+ increases with increasing phosphoric acid concentration. It is noted that increasing the concentration of phosphoric acid leads to the increased concentration of hydrogen ions, which facilitates the solubility of REEs, while the presence of phosphate-ion would increase and induce the precipitation of REEs. Therefore, a model for the effect of phosphoric acid concentration on the solubility of REEs in phosphoric acid solutions is needed.
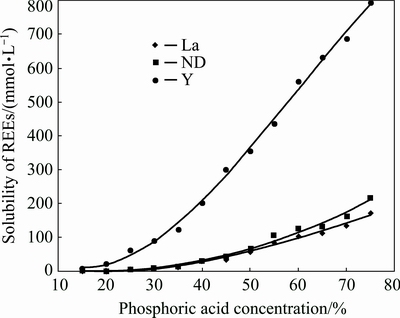
Fig. 2 Solubility of REEs in various concentrations of phosphoric acid
3.2 Theoretical analyses on solution chemistry
Table 3 Concentration of REEs and phosphorus in phosphoric acid after equilibrium

In phosphoric acid, phosphorus generally presents as  ,
,  ,
,  and
and  , and their concentrations depend on the ionization degree of phosphoric acid. However, in phosphoric acid, hydrolysis and phosphate complexation of the trivalent REEs could be negligible at low pH (less than 1.0), at which strongly protonated forms of
, and their concentrations depend on the ionization degree of phosphoric acid. However, in phosphoric acid, hydrolysis and phosphate complexation of the trivalent REEs could be negligible at low pH (less than 1.0), at which strongly protonated forms of  and
and  are predominant [27,28]. Therefore, the dissolution of RE carbonates by H3PO4 and the precipitation of RE phosphates can be expressed by the following reactions:
are predominant [27,28]. Therefore, the dissolution of RE carbonates by H3PO4 and the precipitation of RE phosphates can be expressed by the following reactions:

 2REE3++
2REE3++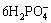 +3H2O+3CO2↑ (1)
+3H2O+3CO2↑ (1)
 =REEPO4↓+nH+ (2)
=REEPO4↓+nH+ (2)
where n=0, 1, 2, 3, and REE3+ represents RE ions.
The equilibrium constants of this reaction can then be written as
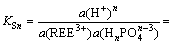
 (3)
(3)
where a represents the activity of ions, γ the activity coefficient of ions in phosphoric acid, and c the concentration of ions in phosphoric acid.
The average activity coefficient of these ions can be calculated via the extended Debye-Huckel expression [29], as follows:
 (4)
(4)
where z is the charge on the ion, I is the ionic strength, A and B are constants, and a′ is an adjustable ion size parameter.
Generally, in hydrometallurgy, the solubility constant calculated by ionic concentrations is used to replace the solubility constant calculated by ion activities. From the perspective of engineering practice and to simplify the deduction of equations between REEs solubility and phosphorus concentration, we define QSn as follows:

 (5)
(5)
The total concentration of phosphorus in phosphoric acid solution is calculated as the accumulated concentration of 

 and
and  , as
, as
 (6)
(6)
where c(P) represents total phosphorus concentration.
Then, combining Eqs. (5) and (6) gives the following equation, in which the relationship between REEs solubility and total phosphorus concentration is built:

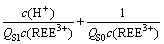 (7)
(7)

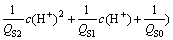 (8)
(8)

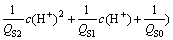 (9)
(9)
In fact, reaction represented by Eq. (1) is one-step reaction in the entire precipitation process. Previously,  was generated through ionization of
was generated through ionization of  . For the three-stage ionization processes of H3PO4, as shown in Eqs. (10)-(12), their ionization constants at 25 °C are pK1=2.14 [Eq. (10)], pK2=7.20 [Eq. (11)], and pK3=12.34 [Eq. (12)], respectively. Hence, phosphorus is dominated by
. For the three-stage ionization processes of H3PO4, as shown in Eqs. (10)-(12), their ionization constants at 25 °C are pK1=2.14 [Eq. (10)], pK2=7.20 [Eq. (11)], and pK3=12.34 [Eq. (12)], respectively. Hence, phosphorus is dominated by  under this experimental condition:
under this experimental condition:

 H++
H++ (10)
(10)

 H++
H++ (11)
(11)

 H++
H++ (12)
(12)
Based on the above-mentioned two facts, assuming that the total concentration of phosphorus is equal to the concentration of  (Assumption 1), Eq. (9) can be simplified as follows:
(Assumption 1), Eq. (9) can be simplified as follows:
 (13)
(13)
However, the concentration of hydrogen ions in phosphoric acid can be calculated using the reaction equilibrium constant of Eq. (10) via the following equation:
 (14)
(14)
 (15)
(15)
For the ionization of phosphoric acid, the generation of each mole H+ would also produce one mole of  , which means that c(H+)=
, which means that c(H+)=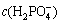 .
.
 (16)
(16)
According to Assumption 1, the total phosphorus concentration can be expressed as
 (17)
(17)
By combining Eqs. (15) and (17), the equation expressing the relationship between total phosphorus concentration and hydrogen concentration is obtained:
 (18)
(18)
With an inverse function, the equation between c(H+) and c(P) is obtained:
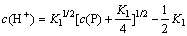 (19)
(19)
where K1=1×10-2.14 and c(P) ranges from 1.07 to 11.35 mol/L, indicating that c(P)>>(K1/4); therefore, K1/4 can be neglected during the calculation of c(H+). Then, Eq. (19) is obtained:
 (20)
(20)
Combining Eqs. (20) and (13), one obtains

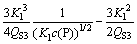 (21)
(21)
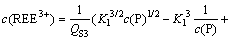
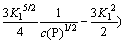 (22)
(22)
In Eq. (22), K1=1×10-2.14 and c(P) ranges from 1.07 to 11.35 mol/L, indicating that the values of 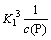 and
and  are much less than those of
are much less than those of  and
and  ; therefore,
; therefore, 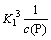 and
and  can be neglected in the calculation of c(REE3+):
can be neglected in the calculation of c(REE3+):

 (23)
(23)
3.3 Effect of phosphoric acid concentration on solubility of REEs
It is known from Eqs. (2) and (3) that two major factors controlling the solubility of REEs in phosphoric acid are the concentration of hydrogen ions and that of phosphorus. Furthermore, the equation between c(P) and c(H+) has been deduced (Eq. (20)). Therefore, to study the effect of phosphoric acid concentration on the solubility of REEs, a linear model that defines [K1c(P)]0.5 as the independent variable and the solubility of REEs as the dependent variable was proposed. The experimental data were then processed using this linear model.
As is known from Fig. 2, the increasing trend of REEs solubility in phosphoric acid is quite mild at 15%-35% H3PO4, while the trend becomes fierce at 35%-75% H3PO4, which can be attributed to the variation of hydrogen-ion concentration caused by the dissolution of RE carbonates. The dissolution of RE carbonates introduces c(H2PO4-) by 3 times the amount of c(REE3+) and restrains the ionization process of H3PO4. It is known from Eq. (14) that the concentration of hydrogen ions in phosphoric acid depends on c( )/c(
)/c( ), while the value of c(
), while the value of c( )/ c(
)/ c( ) at 15%-35% of H3PO4 is much smaller than that at 35%-75% of H3PO4. Therefore, the experimental data were fitted using a linear model (Eq. (23)).
) at 15%-35% of H3PO4 is much smaller than that at 35%-75% of H3PO4. Therefore, the experimental data were fitted using a linear model (Eq. (23)).
As shown in Figs. 3 and 4, the experimental data fit well with the linear model (the correlation coefficient R2 ranges from 0.94 to 0.99). Furthermore, the solubility of REEs in phosphoric acid increases with increasing phosphoric acid concentration. In addition, the slopes and intercepts of simulated straight lines are obtained from Figs. 3 and 4. The equilibrium constants of the precipitation-dissolution reaction (QS3) of REEs in two phosphoric acid concentration ranges are obtained according to Eq. (23). For example, the equilibrium constants of the precipitation-dissolution reaction of La3+ in phosphoric acid can be calculated using the following equations:
 (24)
(24)
 (25)
(25)
where QS3 is the equilibrium constant of the precipitation-dissolution reaction of La3+, Nd3+ and Y3+ in phosphoric acid, K1 the first-stage ionization constant of phosphoric acid, and kn and bn the slope and intercept, respectively, of the simulated straight line in Fig. 3 (k1 and b1 for La, k2 and b2 for Nd, and k3 and b3 for Y, with c(P) ranging from 15% to 35% H3PO4) and Fig. 4 (k4 and b4 for La, k5 and b5 for Nd, and k6 and b6 for Y, with c(P) ranging from 35% to 75% H3PO4). The QS3 value calculated by Eq. (24) is much less sensitive to the error of the REEs solubility measurement than that calculated by Eq. (25) due to different order-of-magnitude values between K1 and K12. Therefore, the QS3 value calculated by Eq. (24) is considered to represent the equilibrium constant of the precipitation-dissolution reaction of REE3+ in phosphoric acid. The equilibrium constants obtained from Figs. 3 and 4 using Eq. (24) are given in Table 4. The deviation between the two slopes for the same element (La, Nd and Y) with different H3PO4 concentration ranges in Figs. 3 and 4 is primarily caused by the effect of the dissolution of RE carbonates. The dissolution of RE carbonates consumes hydrogen ions in three folds of REEs in phosphoric acid and generates  , leading to the retardation of the ionization of
, leading to the retardation of the ionization of  . Moreover, in the low range of 15%-35% H3PO4, the reduction concentration of hydrogen ions is less sensitive because of the relatively low solubility of REEs. At 15%-35% H3PO4, the sharply increased REE solubility leading to a large consumption of hydrogen ions and the retardation of
. Moreover, in the low range of 15%-35% H3PO4, the reduction concentration of hydrogen ions is less sensitive because of the relatively low solubility of REEs. At 15%-35% H3PO4, the sharply increased REE solubility leading to a large consumption of hydrogen ions and the retardation of  ionization becomes significant. In addition, QS3 is very sensitive to the variation of hydrogen concentration according to Eq. (5) (n=3).
ionization becomes significant. In addition, QS3 is very sensitive to the variation of hydrogen concentration according to Eq. (5) (n=3).
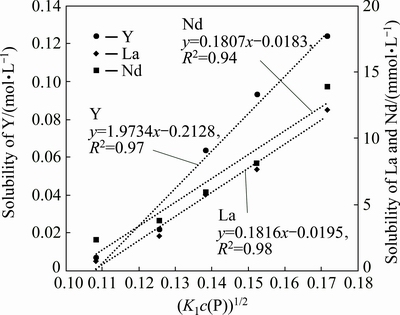
Fig. 3 Solubility of La, Nd and Y vs (K1c(P))1/2 (15%-35% H3PO4)

Fig. 4 Solubility of La, Nd and Y vs (K1c(P))1/2 (35%-75% H3PO4)
Table 4 Values of slope and intercept of simulated curves and corresponding QS3

It is found that the slope of the simulated straight lines in Figs. 3 and 4 increases with increasing atomic number of the REEs. This demonstrates that the increasing trend of REEs solubility in phosphoric acid increases with increasing REEs atomic number. In other words, the difference between light REEs (LREEs) and heavy REEs (HREEs) becomes more significant with increasing phosphoric acid concentration.
In fact, the linear model (Eq. (23)) can be converted to an equation expressing the relationship between the solubility of REEs and the concentration of hydrogen ions as follows:
 (26)
(26)
Equation (26) clearly demonstrates that in phosphoric acid (15%-75% H3PO4) the predominant factor affecting the solubility of REEs is the hydrogen-ion concentration [c(H+)].
3.4 Effect of temperature on solubility of REEs in phosphoric acid
It has been proven that elevated temperature reduces the solubility of REEs in phosphoric acid [20]. Based on this principle, KIJKOWSKA et al [14] recovered 98% of the REEs from phosphoric acid by heating the solution to 200 °C and maintaining at that temperature for 1 h. Since then, some improvements have been made, but no further reports on this method have been carried out, to the best of our knowledge, due to the high energy consumption and stringent apparatus requirements. In our previous study, we designed a stepwise method that achieved the recovery and grouping of LREEs and HREEs from phosphoric acid according to the difference in their solubilities at elevated temperature. However, fundamental data about the effect of temperature on REEs solubility in phosphoric acid have rarely been available.
The effect of temperature on the solubility of REEs in phosphoric acid was investigated by measuring REE solubility in phosphoric acid at various concentrations (10%, 20% and 30% P2O5) and different temperatures (20, 30, 40, 50, 60, 70, 80 and 90 °C) for a treatment time of 15 d. The curves in Figs. 5-7 show that the solubility of REEs in phosphoric acid decreases dramatically with increasing temperature. The decreasing trend of LREEs (represented by La and Nd) is consistent with that of HREEs (represented by Y), while the solubility of Y is one order of magnitude larger than that of La and Nd.

Fig. 5 Effect of temperature on solubility of REEs in 30% P2O5 phosphoric acid
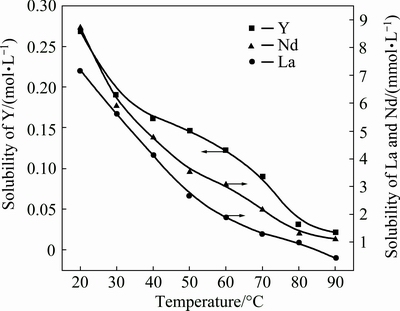
Fig. 6 Effect of temperature on solubility of REEs in 20% P2O5 phosphoric acid
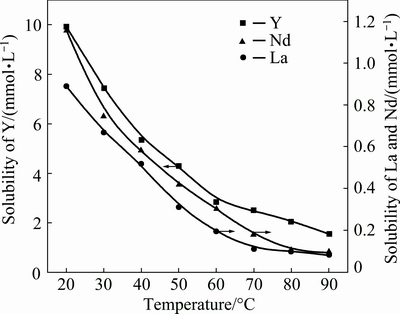
Fig. 7 Effect of temperature on solubility of REEs in 10% P2O5 phosphoric acid
The reduction of REE solubility in phosphoric acid caused by elevated temperature can be explained by two phenomena: weakening of the phosphoric acid ionization process and decrease of the Gibbs energy of the REEPO4 precipitation reaction. Regarding the former, when the temperature is elevated from 20 to 50 °C, a reduction of K1 from 10-2.14 to 10-2.23 occurs, indicating that the hydrogen-ion concentration decreases with increasing temperature [23,30]. In turn, the solubility of REEs in phosphoric acid decreases. Regarding the latter phenomenon, the Gibbs energy of the REEPO4 dissolution with nitric acid increases with increasing temperature [31]. Investigations carried out by CETINER [30], in fact, demonstrated that the solubility reactions of LREE phosphates are exothermic. Therefore, the elevated temperature would reduce the solubility of REEs in aqueous solutions, including phosphoric acid.
From the results above, we know that LREEs are easily precipitated, but HREEs are difficult to remove in the process of phosphoric acid evaporation (from 30% P2O5 to 54% P2O5).
4 Conclusions
1) Solubilities of La, Nd and Y in phosphoric acid with various phosphoric acid concentrations at different temperatures were measured. It is found that the solubility of REEs increases with increasing phosphoric acid concentration.
2) A linear model 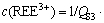
 is built that fits the measured REEs solubility very well (R2>0.94). The predominant factor controlling the solubility of REEs in phosphoric acid is, on the basis of the linear model of the experimental data, determined to be the hydrogen-ion concentration.
is built that fits the measured REEs solubility very well (R2>0.94). The predominant factor controlling the solubility of REEs in phosphoric acid is, on the basis of the linear model of the experimental data, determined to be the hydrogen-ion concentration.
3) In addition, the experimental data demonstrate that elevated temperature significantly decreases the solubility of REEs in phosphoric acid, because the elevated temperature leads to the retardation of the ionization process of phosphoric acid and the decrease of the Gibbs energy of the REEPO4 precipitation reaction.
References
[1] BINNEMANS K, JONES P T, BLANPAIN B, van GERVEN T, YANG Y X, WALTON A, BUCHERT M. Recycling of rare earths: A critical review [J]. Journal of Cleaner Production, 2013, 51: 1-22.
[2] LIU Zhao-bo, LI Hong-xu. Metallurgical process for valuable elements recovery from red mud—A review [J]. Hydrometallurgy, 2015, 155: 29-43.
[3] DAVRIS P, BALOMENOS E, PANIAS D, PASPALIARIS I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid [J]. Hydrometallurgy, 2016, 164: 125-135.
[4] APERGIS E, APERGIS N. The role of rare earth prices in renewable energy consumption: The actual driver for a renewable energy world [J]. Energy Economics, 2017, 62: 33-42.
[5] TSE P K. China’s rare earth industry [J]. China Nonferrous Metals Monthly, 1994, 12: 13-16.
[6] WANG Liang-shi, HUANG Xiao-wei, YU Ying, ZHAO Long-sheng, WANG Chun-mei, FENG Zong-yu, CUI Da-li, LONG Zhi-qi. Towards cleaner production of rare earth elements from bastnaesite in China [J]. Journal of Cleaner Production, 2017, 165: 231-242.
[7] WU Sheng-xi, WANG Liang-shi, ZHAO Long-sheng, ZHANG P, EL-SHALL H, MOUDGIL B, HUANG Xiao-wei, ZHANG Li-feng. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review [J]. Chemical Engineering Journal, 2017, 335: 774-800.
[8] AL-THYABAT S, ZHANG P. Extraction of rare earth elements from upgraded phosphate flotation tailings [J]. Minerals & Metallurgical Processing, 2016, 33: 23-30.
[9] TIAN Jun, YIN Jing-qun, CHI Ru-an, RAO Guo-hua, JIANG Min-tao, OUYANG K. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution [J]. Hydrometallurgy, 2010, 101: 166-170.
[10] ASSOCIATION E, FERTILIZER M. Booklet No. 4 of 8 production of phosphoric acid, best available techniques for pollution prevention and control in the European fertilizer industry [M]. Brussels, Belgium: European Fertilizer Manufacturers’ Association, 2000.
[11] WANG Liang-shi, YU Ying, LIU Ying. Centrifugal extraction of rare earths from wet-process phosphoric acid [J]. Rare Metals, 2011, 30: 211-215.
[12] WANG Liang-shi, LONG Zhi-qi, HUANG Xiao-wei, YU Ying, CUI Da-li, ZHANG Guo-cheng. Recovery of rare earths from wet-process phosphoric acid [J]. Hydrometallurgy, 2010, 101: 41-47.
[13] LOKSHIN E P, TAREEVA O A, ELIZAROVA I R. Deposition of rare earth elements from a wet-process phosphoric acid by fluorine compounds [J]. Russian Journal of Application Chemistry, 2011, 84: 773-781.
[14] KIJKOWSKA R. Recovery of lanthanides from phosphoric acid based on Kola apatite [C]// Inst Mondiale du Phosphate. Paris, 1981: 769-778.
[15] RADHIKA S, KUMAR B N, KANTAM M L, REDDY B R. Solvent extraction and separation of rare-earths from phosphoric acid solutions with TOPS 99 [J]. Hydrometallurgy, 2011, 110: 50-55.
[16] AL-THYABAT S, ZHANG P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum [J]. Mineral Processing & Extractive Metallurgy IMM Transactions, 2015, 124: 143-150.
[17] DIBROV I A, CHIRKST D E, CHALIYAN K N. Recovery of lanthanides in the sulphuric acid leaching process of kola apatites [J]. Mineral Process and Extractive Metallurgy: Reviews, 1995, 15: 142.
[18] WU Sheng-xi, WANG Liang-shi, ZHANG P, EL-SHALL H, MOUDGIL B, HUANG Xiao-wei, ZHAO Long-sheng, ZHANG Li-feng, FENG Zong-yu. Simultaneous recovery of rare earths and uranium from wet process phosphoric acid using solvent extraction with D2EHPA [J]. Hydrometallurgy, 2018, 175: 109-116.
[19] HABASHI F. The recovery of the lanthanides from phosphate rock [J]. Journal of Chemical Technology and Biotechnology, 1985, 35: 5-14.
[20] LIU Xue-wu, BYRNE R H. Rare earth and yttrium phosphate solubilities in aqueous solution [J]. Geochimica et Cosmochimica Acta, 1997, 61: 1625-1633.
[21] FIRSCHING F H, BRUNE S N. Solubility products of the trivalent rare-earth phosphates [J]. Journal of Chemical Engineering Data, 1991, 36: 93-95.
[22] WOOD S A. The aqueous geochemistry of the rare-earth elements and yttrium: 1. Review of available low-temperature data for inorganic complexes and the inorganic REE speciation of natural waters [J]. Chemical Geology, 1990, 88: 99-125.
[23] CETINER Z S, WOOD S A, GAMMONS C H. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150 °C [J]. Chemical Geology, 2005, 217: 147-169.
[24] KREA M, KHALAF H. Liquid–liquid extraction of uranium and lanthanides from phosphoric acid using a synergistic DOPPA–TOPO mixture [J]. Hydrometallurgy, 2000, 58: 215-225.
[25] LIPINSKI C A, LOMBARDO F, DOMINY B W, FEENEY P J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings [J]. Advanced Drug Delivery Reviews, 2001, 46: 3-26.
[26] SENANAYAKE G, JAYASEKERA S, BANDARA A M T S, KOENIGSBERGER E, KOENIGSBERGER L, KYLE J. Rare earth metal ion solubility in sulphate-phosphate solutions of pH range -0.5 to 5.0 relevant to processing fluorapatite rich concentrates: Effect of calcium, aluminium, iron and sodium ions and temperature up to 80 °C [J]. Minerals Engineering, 2016, 98: 169-176.
[27] SIMMONS S F, GRAHAM I. Volcanic geothermal and ore-forming fluids: Rulers and witnesses of processes within the earth [J]. Society of Economic Geologists Special Publication, 2003, 10: 133-158.
[28] BASE C F, MESMER R E. The hydrolysis of cation [M]. New York: Wiley, 1976: 2385-2387.
[29] MILLERO F J. Use of the ion pairing model to estimate activity coefficients of the ionic components of natural water [J]. American Journal of Science, 1982, 282: 1508-1540.
[30] CETINER Z S. Experimental investigation of the solubility of the REE phosphate minerals monazite/xinotime and chlorides complexation in hydrothermal solutions at 23 °C, 50 °C, 150 °C and water vapor pressure [D]. Moscow, Idaho: University of Idaho, 2003: 77-90.
[31] ABISHEVA Z S, KARSHIGINA Z B, BOCHEVSKAYA Y G, AKCIL A, SARGELOVA E A, NIKOLAYEVNA K M, SILACHYOV I Y. Recovery of rare earth metals as critical raw materials from phosphorus slag of long-term storage [J]. Hydrometallurgy, 2017, 173: 1-40.
巫圣喜1,2,赵龙胜1,王良士1,黄小卫 1,董金诗1,冯宗玉1,崔大立1,张立峰2
1. 有研科技集团有限公司 稀土材料国家工程研究中心,北京 100088;
2. 北京科技大学 冶金与生态工程学院,北京 100083
摘 要:为了建立磷酸溶液中稀土回收工艺性基础数据和更好地了解磷酸蒸发浓缩过程中的稀土行为,测定不同温度条件下不同磷浓度的磷酸溶液中的稀土溶解度。建立关于磷酸溶液中稀土溶解度与磷浓度之间简单线性模型,该模型与实验测定的磷酸溶液中稀土溶解度具有较高的契合度(R2>0.94)。磷酸溶液中稀土溶解度的控制性影响因素为溶液中氢离子浓度。此外,升温能使磷酸溶液中稀土溶解度迅速下降,这主要是因为升温导致磷酸稀土的溶解反应吉布斯自由能升高,同时,升温还抑制磷酸分子的电离。
关键词:稀土元素;磷酸;溶解行为;磷酸稀土;温度
(Edited by Bing YANG)
Foundation item: Project (51674036) supported by the National Natural Science Foundation of China; Project (Z161100004916108) supported by the Beijing Nova Program, China
Corresponding author: Liang-shi WANG: Tel: +86-10-82241180; E-mail: wls1657@163.com
DOI: 10.1016/S1003-6326(18)64883-6

