电流密度对Al/导电涂层/α-PbO2-CeO2-TiO2/β-PbO2- MnO2-WC-ZrO2复合电极材料制备和性能的影响
来源期刊:中国有色金属学报(英文版)2014年第10期
论文作者:杨海涛 陈步明 郭忠诚 刘焕荣 张永春 黄惠 徐瑞东 付仁春
文章页码:3394 - 3404
Key words:composite electrode material; A1 substrate; β-PbO2-MnO2-WC-ZrO2; electrochemical co-deposition; current density
摘 要:通过电化学氧化共沉积技术在A1/导电涂层/α-PbO2-CeO2-TiO2 基体上,制备了A1/导电涂层/α-PbO2- CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2复合阳极材料。通过能量色散X射线光谱(EDXS)、阳极极化曲线、暂稳态极化曲线(Tafel)、交流阻抗谱(EIS)、扫描电子显微镜(SEM)以及X射线衍射(XRD)等方法研究电流密度对复合阳极材料的化学组分、电化学活性和稳定性的影响。研究结果表明:在电流密度为1 A/dm2条件下制备的A1/导电涂层/α-PbO2-CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 复合材料具有最低的析氧过电位(0.610 V,条件:500 A/m2),最好的电化学活性,最长的使用寿命(360 h,条件:150 g/L H2SO4, 2 A/cm2, 40 °C)以及最低的槽电压(2.75 V,条件:500 A/m2)。而且,随着电流密度的增加,涂层晶粒逐渐增大,MnO2含量也逐渐降低,晶体结构几乎没有变化。
Abstract: Al/conductive coating/α-PbO2-CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 composite electrode material was prepared on Al/conductive coating/α-PbO2-CeO2-TiO2 substrate by electrochemical oxidation co-deposition technique. The effects of current density on the chemical composition, electrocatalytic activity, and stability of the composite anode material were investigated by energy dispersive X-ray spectroscopy (EDXS), anode polarization curves, quasi-stationary polarization (Tafel) curves, electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and X-ray diffraction (XRD). Results reveal that the composite electrode obtained at 1 A/dm2 possesses the lowest overpotential (0.610 V at 500 A/m2) for oxygen evolution, the best electrocatalytic activity, the longest service life (360 h at 40 °C in 150 g/L H2SO4 solution under 2 A/cm2), and the lowest cell voltage (2.75 V at 500 A/m2). Furthermore, with increasing current density, the coating exhibits grain growth and the decrease of content of MnO2. Only a slight effect on crystalline structure is observed.
Trans. Nonferrous Met. Soc. China 24(2014) 3394-3404
Hai-tao YANG1, Bu-ming CHEN1, Zhong-cheng GUO1, 2, Huan-rong LIU3, Yong-chun ZHANG1, Hui HUANG1, Rui-dong XU1, 4, Ren-chun FU5
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Kunming Hendera Science and Technology Co., Ltd., Kunming 650022, China;
3. Faculty of Land Resource Engineering, Kunming University of Science and Technology, Kunming 650093, China;
4. State Key Laboratory Breeding Base of Complex Nonferrous Metal Resources Cleaning Utilization, Kunming University of Science and Technology, Kunming 650093, China;
5. Faculty of Science, Kunming University of Science and Technology, Kunming 650093, China
Received 21 October 2013; accepted 10 February 2014
Abstract: Al/conductive coating/α-PbO2-CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 composite electrode material was prepared on Al/conductive coating/α-PbO2-CeO2-TiO2 substrate by electrochemical oxidation co-deposition technique. The effects of current density on the chemical composition, electrocatalytic activity, and stability of the composite anode material were investigated by energy dispersive X-ray spectroscopy (EDXS), anode polarization curves, quasi-stationary polarization (Tafel) curves, electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and X-ray diffraction (XRD). Results reveal that the composite electrode obtained at 1 A/dm2 possesses the lowest overpotential (0.610 V at 500 A/m2) for oxygen evolution, the best electrocatalytic activity, the longest service life (360 h at 40 °C in 150 g/L H2SO4 solution under 2 A/cm2), and the lowest cell voltage (2.75 V at 500 A/m2). Furthermore, with increasing current density, the coating exhibits grain growth and the decrease of content of MnO2. Only a slight effect on crystalline structure is observed.
Key words: composite electrode material; A1 substrate; β-PbO2-MnO2-WC-ZrO2; electrochemical co-deposition; current density
1 Introduction
Electrocatalytic oxidation co-deposition technology has been intensively studied [1,2] because of its many distinctive advantages [3,4], including environmental compatibility, versatility, energy efficiency, safety, selectivity, amenability to automation and cost effectiveness. PbO2 is usually used as an anode for the electrowinning of metals [5,6]. The traditional PbO2-coated metal anode used for electrowinning zinc possesses some advantages over conventional Pb-1% (mass fraction) Ag anode, such as the obviated need for preconditioning, higher quality of electrolytic zinc, good corrosion resistance toward chlorine or even removal of the chloride ion in electrolyte, less anode slime, higher output of precipitated zinc and longer lifetime of the anode at higher current density [7]. However, the PbO2-coated metal anode has high cell voltage in electrolytic process.
Manganese dioxide electrodes have been widely investigated as electrowinning zinc anodes [8,9]. Manganese dioxide anodes in acidic sulfate-based electrolytes possess excellent electrocatalytic activity and corrosion resistance. Manganese dioxide is not easily dissolved, does not pollute the cathode deposit during the electrolysis process, and produces a high-purity cathode product. However, this electrode material has poor conductivity and mechanical properties, which limits its wide application in various industries.
PbO2 and MnO2 composite electrodes have been widely investigated [10]. These composite electrodes are prepared by adding PbO2 to nano-MnO2 particles. The modifying effect of PbO2 on MnO2 is based on the introduction of Pb into the MnO2 lattice. PbO2 and MnO2 form the complexes Pb(X) and Mn(Y) (X=IV, II; Y=IV, III) in the MnO2 charge–discharge process. These compounds undergo co-oxidation and co-reduction, resulting in a complete MnO2/MnOOH homogeneous redox process. Furthermore, the discharge capacity of MnO2 can be greatly improved by doping nano-PbO2 powders to modify MnO2 electrodes [11]. Metal-based coated anodes with different substrates, such as Ti-based electrodes (Ti/PbO2, Ti/RuO2, Ti/IrO2, Ti/TiO2/PbO2, and Ti/SnO2-Sb2O3-MnO2/PbO2) [12–16], stainless steel-based electrodes (stainless steel/β-PbO2-TiO2- Co3O4 and stainless steel/PbO2-CeO2) [17] and Pb-based electrodes (Pb/Pb-MnO2) [8,9], have been widely studied. Whereas the cost of titanium is higher than that of other electrode materials, stainless steel has lower electroconductivity than copper, aluminum, lead, iron and so on. Lead has poor mechanical properties. In this work, an aluminum substrate is used because of its low cost, low specific gravity, and excellent electrical conductivity and mechanical properties.
To further improve the electrocatalytic activity for oxygen evolution and reduce the overpotential of oxygen evolution, a β-PbO2-MnO2-WC-ZrO2 composite coating with high catalytic activity was prepared on a previous Al/conductive coating/α-PbO2-CeO2-TiO2 substrate [18-20] through anodic electrochemical co- deposition of PbO2, MnO2, nano-ZrO2, and WC particles in lead nitrate and manganese nitrate acid baths. PbO2 with high electrical conductivity and MnO2 with high electrocatalytic activity were successfully compounded. Nano-ZrO2 and WC particles with high electrocatalytic activity were successfully incorporated [21-26]. This work was focused on the influences of current density on the chemical composition, electrocatalytic activity, and stability of the composite anode material.
2 Experimental
2.1 Preparation
Al/conductive coating/α-PbO2-CeO2-TiO2/β-PbO2- MnO2-WC-ZrO2 composite electrode materials were synthesized by applying a conductive undercoating to an A1 substrate, covering the undercoating with an intermediate coating consisting of the α-PbO2- CeO2-TiO2 deposit, and finally covering the intermediate coating with a top coating consisting of a mixture of β-PbO2-MnO2 deposit and WC and ZrO2 particles. The schematic diagram of the composite electrode materials is shown in Fig. 1.

Fig. 1 Schematic diagram of Al/conductive coating/α-PbO2- CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 composite electrode materials
The substrates were 25 mm×50 mm×2 mm aluminum (1060) plates, both sides of which were roughened by sand-blasting, degreased and chemically etched, and then coated by a conductive material. The conductive coating consisted of waterborne resins (40%, mass fraction), conductive fillers (25%), and solvents (35%). The waterborne resins were either acrylic or polyurethane. The conductive fillers consisted of silver powder (5%) and copper powder (95%). The solvent was a mixture of ethanol (60%) and ethyl acetate (40%). The procedure was performed as follows: first, the conductive coating solution was applied to the substrate by brushing; second, the substrate was surface dried under infrared lamp; and finally, the substrate was dried in an electric drying oven at 423 K for 2 h. The conductive undercoating produced in this work was approximately 20 μm to 30 μm thick. The details were presented in Ref. [27]. The composition and process conditions of the α-PbO2 plating bath are as follows: 4 mol/L NaOH with litharge PbO(s) (the soluble PbO species were HPbO2- anions), pH≥14, anode current density of 1.0 A/dm2, mild stirring using a magnetic stirrer, bath temperature of 40 °C, 15 g/L TiO2 grain (rutile, average particle size of 30 nm), 100 g/L CeO2 grain (average particle size of 50 nm), and electroplating time of 4 h. The composition and process conditions of the β-PbO2-MnO2 plating bath are as follows: 30% Pb(NO3)2 (pH=1.5), 80 g/L Mn(NO3)2, 50 g/L ZrO2 particles (average particle size of 20 nm), 40 g/L WC (average particle size of 3 μm), anode current density of 0.5-2.5 A/dm2, mild stirring using a magnetic stirrer, bath temperature of 60 °C, and electroplating time of 2 h. The granularities of the TiO2, CeO2, ZrO2, and WC particles were analyzed by LS900 laser grain size analyzer (OMEC Technology Co., Ltd., Zhuhai, China). The plating bath was dispersed for 30 min by an ultrasonic device before electrodeposition to assure particle dispersion in the oxide substrate.
2.2 Characterization
An electrochemical workstation (CHI660C) with three electrode systems was used in this experiment. The schematic diagram of the electrochemical test is shown in Fig. 2. The working electrode was the A1/ conductivecoating/α-PbO2-CeO2-TiO2/β-PbO2-MnO2-WC-ZrO anode experimental sample with a working area of 1.0 cm2, and the reference electrode used was saturated calomel electrode (SCE; Hg, Hg2C12/saturated KCl). The potential of the mercurous chloride electrode (vs SCE) used in the anodic polarization curves and EIS measurements was 0.231 V (vs NHE) at 40 °C. The counter electrode used was a graphite plate (6 cm2). All potentials shown in the figures are against SCE.
The anodic polarization curves were obtained in a synthetic electrolyte of 50 g/L Zn2+ and 150 g/L H2SO4 at 40 °C. Anodic polarization experiments were carried out at a constant scan rate of 5 mV/s from an initial potential of 1.2 V (vs SCE) to a final potential of 2.1 V (vs SCE). Electrochemical impedance measurements were performed in a synthetic electrolyte consisting of 50 g/L Zn2+ and 150 g/L H2SO4 at 40 °C. The frequency interval was from 105 Hz to 0.1 Hz, and the AC amplitude was 5 mV (root mean square). The applied anodic potential was 1.4 V (vs SCE). The impedance data were converted into Nyquist data format and then fitted to appropriate simulative circuits. The surface morphology of the composite coating was characterized by SEM (XL30 SEM, Philips, Holland). The chemical compositions were obtained by EDXS (Phoenix, EDAI, USA). The phase composition of the films was studied by XRD using Cu Kα radiation (D8ADVANCE, Bruker, Germany).
3 Results and discussion
As shown in Fig. 3, cyclic voltammetric curves for the Al/conductive coating/α-PbO2-CeO2-TiO2 electrodes were measured at 60 °C in the potential range from 0 to 2.1 V (vs SCE) at a constant scan rate of 5 mV/s in different baths: 1) 30% Pb(NO3)2 (pH 1.5); 2) 100 g/L Mn(NO3)2 + 15 g/L HNO3 + 20 g/L KNO3; 3) 30% Pb(NO3)2 + 80 g/L Mn(NO3)2 (pH 1.5). Before recording CV curve, the Al/conductive coating/α-PbO2-CeO2-TiO2 electrode was kept in each bath until achieving steady open-circuit-potential. And the potential of mercurous chloride electrode (vs SCE) used in cyclic voltammetric curves is 0.215 V (vs NHE) at 60 °C. The potential of the beginning deposition of MnO2 is approximately 1.0 V (vs SCE). The potential of the beginning deposition of β-PbO2-MnO2 is nearly 1.0 V (vs SCE), and that of β-PbO2 is approximately 1.25 V (vs SCE). The beginning deposition potentials of MnO2 and β-PbO2-MnO2 are almost the same and lower than those of β-PbO2. Based on the standard redox potentials [28], the anodic deposition of MnO2 should proceed more easily than the deposition of PbO2.
Mn2++2H2O=MnO2+4H++2e
φΘ(V)=1.228-0.1182pH-lnα[Mn2+] (1)
Pb2++2H2O=PbO2+4H++2e
φΘ(V)=1.449-0.1182pH-lnα[Pb2+] (2)
where φΘ is the standard redox potential against NHE, and α[Mn2+] and α[Pb2+] are the activities of the [Mn2+] and [Pb2+] ion, respectively.
Equilibrium reactions (1) and (2) show that the standard equilibrium potential of MnO2 is 0.221 V lower than that of PbO2. When E<1.5 V (vs NHE), MnO2 electrodeposition predominates. Thus, lower potentials are required for the deposition of pure MnO2. However, upon the decrease of Mn2+ in solution, the amount of deposited PbO2 increases during the electrodepositing process. This phenomenon is more obvious as Mn2+ is further reduced. The presence of Pb2+ and/or formation of PbO2 may increase the overpotential of MnO2 deposition.
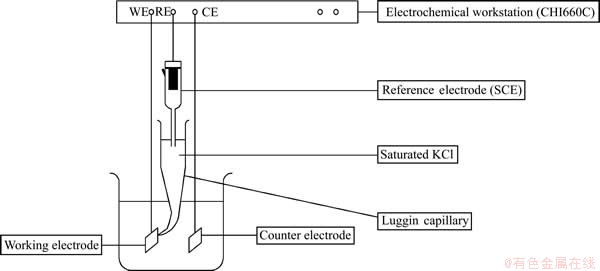
Fig. 2 Schematic diagram of electrochemical test

Fig. 3 Cyclic voltammograms on A1/conductive coating/ α-PbO2-CeO2-TiO2 at 60 °C in different baths
3.1 Chemical composition
Figure 4 shows the effects of current density on the chemical compositions of β-PbO2-MnO2-WC-ZrO2 composite coatings. Figure 4(a) shows the effect on WC and nano-ZrO2 contents, and Fig. 4(b) shows the effect on PbO2 and MnO2 contents.
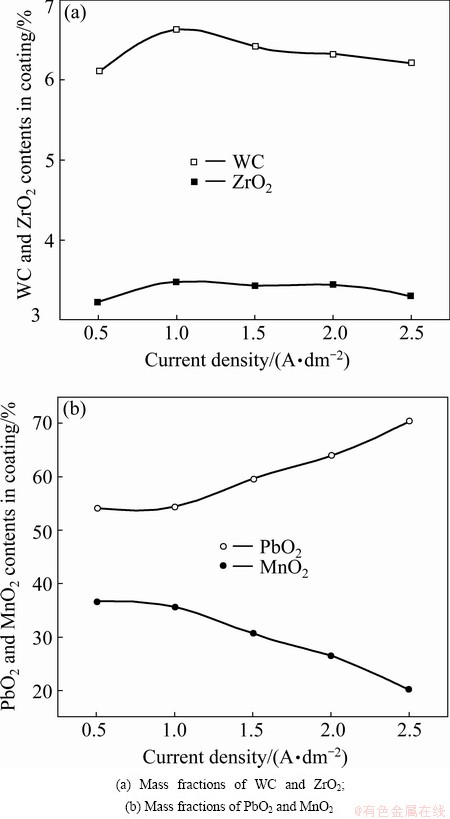
Fig. 4 Influence of current density on chemical compositions of composite coatings
The contents of WC and nano-ZrO2 in the composite coatings increase with the increase in current density and reach the highest value (6.61% and 3.51%, respectively) when the current density is 1 A/dm2. When the current density is higher than 1 A/dm2, the contents of WC and nano-ZrO2 in the composite coatings slowly decrease with increasing current density. PbO2 content monotonously increases and MnO2 content monotonously decreases with the increase in current density.
This phenomenon may be explained as follows. In the first case (J≤1 A/dm2), as the deposition rates of PbO2 and MnO2 increase, the capture probability of the particles becomes higher. In the second case (J>1 A/dm2), the content of particles in the coating remains nearly constant or decreases when the deposition rate reaches its limiting value [29]. The high current density causes the simultaneous reactions of Pb2++Mn2+ oxidation and oxygen evolution. In this current density region, oxygen evolution significantly contributes to the partial current, thus decreasing the current efficiency of PbO2 and MnO2 formation. In other words, oxygen has sufficient time to hinder the co-deposition of particles with PbO2 and MnO2. Ultimately, the content of particles in the composite decreases with increasing current density in the higher-current density region. The content of WC particles in the composite coatings is higher than that of nano-ZrO2 particles. WC is thermodynamically unstable and is easily oxidized in the presence of water or oxygen at room temperature. WC powder is easily oxidized on the surface, and the oxidized surface layer mainly consists of WO3. The zeta-potential of the WC powder is highly negative in the pH range of 3 to 11. This behavior is typical of an acidic oxide with a very low isoelectric point. The surface of the WO3 particle can adsorb hydrates and —OH groups. The presence of these surface groups can result in a negative surface charge according to the following reaction [30]:
≡W—OH W—O-+H+ (3)
W—O-+H+ (3)
In this case, the increase in WC and MnO2 contents in PbO2 is due to the specific adsorption of Pb2+ and Mn2+ on the WC particles. The zero-charge pH value (pH0) of ZrO2 is approximately 6.82 [31]. Thus, the migration possibility of nano-ZrO2 particles to the anode surface is weak. The higher current density results in a higher deposition overpotential, which is favorable to PbO2 deposition and adverse to MnO2 deposition. This process ultimately results in the increased PbO2 content and the decreased MnO2 content with increase in current density [28].
3.2 Anode polarization curves and EIS
Figure 5(a) shows the anodic polarization curves of the Al/conductive coating/α-PbO2-CeO2-TiO2/β-PbO2- MnO2-WC-ZrO2 composite electrode materials obtained by anodic deposition at different current densities in 50 g/L Zn2+ and 150 g/L H2SO4 solution at 40 °C. A change in the oxygen evolution potential of the composite electrodes obtained at different current densities was clearly observed. As shown in Fig. 5(a), over the studied polarization range, the polarization curve of composite electrode obtained at 2.5 A/dm2 presented the highest oxygen evolution potential whereas that of composite electrode obtained at 1.0 A/dm2 presented the lowest oxygen evolution potential at a current density of 500 A/m2(0.05 A/cm2).
The relationship curve of the JRs-corrected overpotential for oxygen evolution, η, and the logarithm for current density, lgJ, was also employed in this research (Fig. 5(b)). The overpotential data (η) used for the fitted Tafel lines of oxygen evolution were obtained from part of the anodic polarization curves (about 1.7 V to 2.1 V, vs SCE) using the following formula [8,13,25]:
η=E+0.231 V–1.240 V–JRs, (4)
where E (vs SCE) is the potential of oxygen evolution obtained in the anodic polarization curves, 0.231 V (vs SHE) is the potential of the SCE, 1.240 V (vs NHE) is the reversible potential of oxygen evolution calculated from the Nernst equation using a synthetic zinc electrowinning electrolyte of 50 g/L Zn2+ and 150 g/L H2SO4 at 40 °C, J is the Faradaic current, and Rs is the electrolyte resistance between the reference and working electrodes.
Figure 5(b) shows the JRs-corrected Tafel lines (η-lg J) of the anode samples obtained at different current densities. The lines mainly present a double-slope behavior except the line of composite electrode obtained at 2.5 A/dm2. These slope values and the potential intercepts of the five lines were separately analyzed using Origin 7.5 software and the results are displayed in Table 1. The η under a specific current density was calculated using the Tafel formula [32] and is listed in Table 1.
η=a+blg J (5)
where η and J represent the overpotential of oxygen evolution and the Faradaic current, respectively, and a and b are constants obtained by linear fitting of the relationship curve of η (Formula (4)) and lg J in Origin 7.5 software. Variables a1 and b1 correspond to a low potential area, whereas a2 and b2 correspond to a high potential area. In this work, η values under specific current densities and the electrode surface (exchange) current density were calculated in the low potential area (a1 and b1).
The electrode surface (exchange) current density, J0, was calculated using the Tafel equation (5) when η=0 [24].
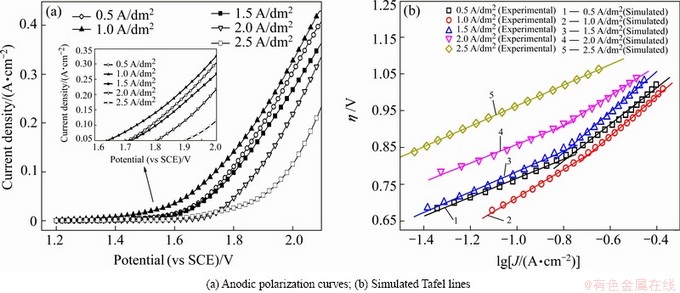
Fig. 5 Anodic polarization curves and Tafel lines for anode samples
Table 1 Overpotentials and kinetic parameters of oxygen evolution for composite electrode prepared at different current densities

As shown in Table 1, J0 mainly presented a declining trend with increasing current densities during electro- deposition. The J0 of composite electrode obtained at 2.5 A/dm2 was the lowest (3.510×10-5 A/cm2) and was the highest in the anode sample obtained at 1.0 A/dm2 (9.123×10-4 A/cm2). According to electrochemical theory, the electrode polarization and the reversibility of electrode reactions may be evaluated by J0; generally, a higher J0 implies that the electrode is not easily polarizable, electrode reversibility is improved, and the electrode reaction easily occurs.
The η values of the anode samples obtained at 1.0 A/dm2 during electro-deposition were 0.610, 0.638, 0.662, 0.682, 0.700, and 0.716 V at 500, 600, 700, 800, 900, and 1000 A/m2, respectively, corresponding to decreases of 270, 264, 259, 255, 251, and 248 mV, respectively, compared with anode samples obtained at 2.5 A/dm2 during electro-deposition. In summary, with increasing current densities during electro-deposition, the potential (vs SCE) and η mainly present rising trends whereas the J0 demonstrates a declining trend.
As shown in Fig. 5 and Table 1, at a fixed current density, the A1/conductive coating/α-PbO2-CeO2-TiO2/ β-PbO2-MnO2-WC-ZrO2 electrodes deposited at 1 A/dm2 show the lowest potential and overpotential and possess the best electrocatalytic activity. The electrocatalytic activity of the anode depends on electronic and geometric factors [33]. At a given current density, the corresponding electrode potential is a result of multiple factors affecting the OER kinetics (e.g. anode kind, electrode morphology, and electrolyte). Furthermore, the limiting catalytic activity is reached when the content of WC and nano-ZrO2 particles is at maximum (Fig. 4(a)). The overpotential mainly increases as the MnO2 amount decreases in the composite coating (Fig. 4(b)). Therefore, the incorporated MnO2 can improve the catalytic activity of the composite coating.
EIS measurements were performed by applying an AC voltage of 5 mV over the frequency range of 105 Hz to 10-1 Hz. The results analyzed by Zsimpwin software are shown in Figs. 6(a) and (b)). Apparently, an electrochemical responsive semicircle and a straight line are observed simultaneously. Thus, the electrochemical process was controlled by both the electrochemical reaction and the diffusion step. An equivalent circuit model [34] is proposed in Fig. 6(c). In the equivalent circuit, Rs stands for the electrolyte resistance, Cdl stands for the double layer capacitance, Rct represents the charge transfer resistance in the electrochemical process, CPE stands for the constant phase element of the interface between electrode and electrolyte, Ra is equivalent resistance associated with the adsorption of intermediate, and W0 is the Warburg resistance of the diffusion step. Table 2 shows the simulation result by Zsimpwin software. The charge transfer resistances Rct are 1.983 and 5.659 Ω·cm2 when the composite electrode is obtained at 0.5 and 2.5 A/dm2, respectively. The charge transfer resistance of composite electrode obtained at 0.5 A/dm2 is the minimum. However, the composite coating obtained at 0.5 A/dm2 presents large cracks which also fall off easily (Fig. 7(a′)). The charge transfer resistance of composite electrode obtained at 1 A/dm2 is the minimum (3.135 Ω·cm2) except that of composite electrode obtained at 0.5 A/dm2. Furthermore, the composite coating obtained at 1.0 A/dm2 presents the compact microstructures.
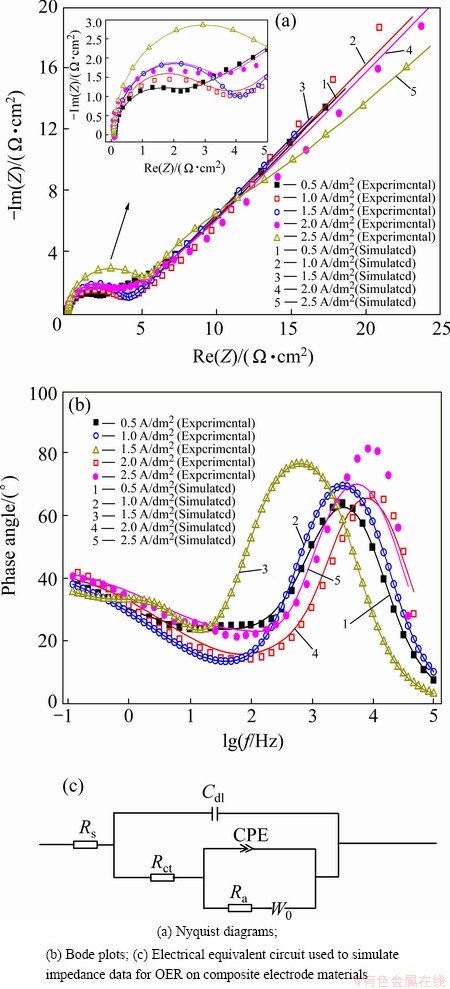
Fig. 6 Experimental EIS patterns and electrical equivalent circuit for composite electrode materials
The experimental (scatters) and simulated (lines) Bode patterns for Al/conductive coating/α-PbO2-CeO2- TiO2/β-PbO2-MnO2-WC-ZrO2 composite electrode materials obtained at different current densities (corresponding to Fig. 6(a)) are shown in Fig. 6(b). The experimental and simulated data reach a very good agreement.
3.3 Microscopic structure and phase composition
Figure 7 shows the morphologies of the β-PbO2- MnO2-WC-ZrO2 composite coatings obtained by anodic deposition at different current densities with 200- and 10000-time magnifications.
Table 2 Equivalent circuit simulation results for composite coatings obtained at different current densities in Zn2+ 50 g/L, H2SO4 150 g/L solution


Fig. 7 Influence of current density on surface microstructures of composite coatings
The surface morphologies of the coatings are considerably different from one another. Coarse grains and numerous agglomerations are observed on the surface. The grains mainly consist of square-, triangular-, and rectangular-shaped subgrains with different orientations [35]. The subgrain size increases with increasing current density. The exact change in subgrain size depends on the composition on the surface, which determines the stacking fault energy [36]. More solid particle contents are obtained at higher current density. A coating morphology with no obvious cracks is obtained at the current density of 1 A/dm2. Appropriately, increasing the current density is beneficial to the absorption between the coating and particles. The highest content of solid particles on the coating is achieved at a current density of 1 A/dm2, resulting in the maximal dispersion effect of the composite coating. Consequently, the inner stress of the coatings is eliminated and the cracks between coatings are reduced or even completely covered. Therefore, the current density should be controlled appropriately at 1 A/dm2.
Figure 8 shows the XRD patterns for the β-PbO2- MnO2-WC-ZrO2 composite coatings prepared at different current densities. Attribution of the peaks is performed using the JCPDS cards. The surface layer of β-PbO2-MnO2-WC-ZrO2 is mainly composed of β-PbO2 (JCPCDS card 41-1492), α-PbO2 (JCPCDS card 45-1416), and MnO2 (JCPCDS card 24-0735) with a small amount of WC (JCPCDS card 51-0939) and ZrO2 (JCPCDS card 37-1484). The current density does not change the phase in the composite coating but changes their relative abundances. The highest contents of WC and nano-ZrO2 are obtained at 1 A/dm2, resulting in minimum peak intensity. The reason may be that the high content of WC and nano-ZrO2 can modify the crystal structure of the composite coating by decreasing its grain size.
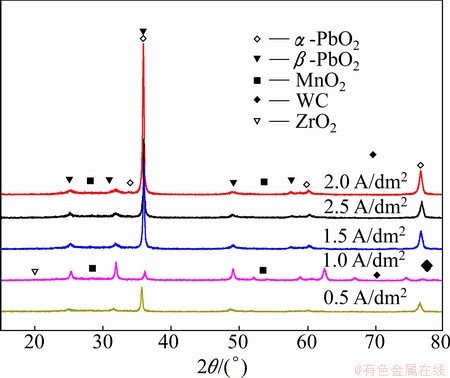
Fig. 8 XRD patterns of composite coating at different current densities
3.4 Service life
Figure 9 shows the accelerated life tests of the composite electrodes obtained at different current densities in 150 g/L H2SO4 solution under 2 A/cm2 at 40 °C. The composite electrode material was subjected to accelerated life tests to compare their electrochemical stability. For composite electrode materials obtained at 2.5 A/dm2 after 20 h, the cell voltage rapidly increased. A sharp potential increase was observed during the last few hours. The conductive composite oxide film and aluminum substrate were thus eroded. Therefore, the composite electrode materials obtained at 2.5 A/dm2 had a service life of 20 h under accelerated life test conditions.

Fig. 9 Accelerated life tests of composite coating obtained at different current densities in 150 g/L H2SO4 solution under 2 A/cm2 at 40 °C
Electrode deactivation includes metal base passivation, film consumption, film detachment, and mechanical damage [17,37]. The β-PbO2-MnO2-WC- ZrO2 composite coatings disappeared at the end of the accelerated life test. Examination of the surface of the failed electrode shows that some areas of the aluminum substrate were exposed to the solution and showed pitting corrosion. Furthermore, a black deposit was observed at the cell bottom. The new composite electrode deactivation was the result of film detachment. The composite electrode materials obtained at 1 A/dm2 displayed excellent stability under accelerated life test conditions up to 360 h. A sharp potential increase was also observed for composite electrode materials obtained at 1 A/dm2. This result indicates that the service life of the composite electrode materials obtained at 1 A/dm2 is approximately 360 h, which is longer than that of composite electrode materials obtained at 0.5, 1.5, 2, and 2.5 A/dm2 under the same conditions. Moreover, no black deposit was observed at the cell bottom, and no area of the aluminum substrate was exposed to the solution. Thus, no film detachment occurred, and the film and substrate exhibited good bonding. The most likely reason for electrode deterioration is film consumption during the test [17].
The composite electrode obtained at 1 A/dm2 presents the best electrochemical stability. To assess the actual life, an evaluation is conducted using the method proposed by CHEN et al [37]. In this method, a simple relationship between the electrode service life (Ls) and the current density (J) is proposed as follows:
Ls=1/Jm (6)
where m ranges from 1.4 to 2.0. Assuming a lower m of 1.4 for the composite electrode, the predicted service life is 7 a at a current density of 50 mA/cm2.
3.5 Cell voltage
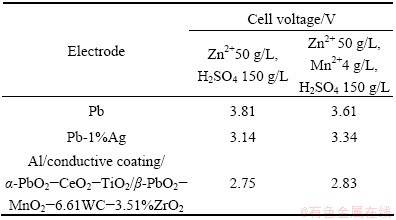
The electrolysis conditions used in this experiment are as follows: 50 g/L Zn2+, 4 g/L Mn2+ (or without Mn2+), 150 g/L H2SO4, 40 °C temperature, 3 cm cathode–anode distance, 500 A/m2 current density, and 24 h electrolytic time. The measured cell voltage is shown in Table 3. The A1/conductive coating/α-PbO2- CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 obtained at 1 A/dm2 possesses the lowest cell voltage with and without Mn2+. This result shows that the composite electrode has superior advantages over the conventional Pb-1%Ag alloy with regard to reducing cell voltage [8,9,17,38]. The possible reasons are as follows. Firstly, the surface layer of composite electrode was composed of β-PbO2, MnO2, WC and ZrO2. These solid particles and β-PbO2 possess higher catalytic activity for oxygen evolution [17]. Secondly, intermediate layer of composite electrode was composed of α-PbO2, CeO2 and TiO2. The intermediate layer improves the adhesion of surface layer and undercoating. And finally, the lower cell voltage may be due to the good electric conduction of the aluminum substrates.
Table 3 Cell voltages of different electrodes in ZnSO4-H2SO4 solution
4 Conclusions
1) Good catalytic activity of A1/conductive coating/ α-PbO2-CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 inert node was successfully prepared by composite electro- deposition of MnO2, PbO2, WC, and ZrO2 particles on A1/conductive coating/α-PbO2-CeO2-TiO2. The composite coating possesses the best combination property at current density of 1 A/dm2.
2) With increasing current density, the coating exhibits grain growth and the content of MnO2 is reduced. However, the crystalline structure is only slightly affected. The highest content of solid particles is observed at the current density of 1 A/dm2, indicating the maximal dispersion effect of the composite coating. Thus, the inner stress of the coatings is eliminated and the cracks between coatings are reduced or even completely covered.
3) At a fixed current density, the A1/conductive coating/α-PbO2-CeO2-TiO2/β-PbO2-MnO2- WC-ZrO2 electrodes deposited at 1 A/dm2 exhibit the lowest potential and overpotential and possess the best electrocatalytic activity.
4) The composite electrode obtained at the current density of 1 A/dm2 has the longest service life. At high current density (2 A/dm2), its lifetime reaches 360 h. The cell voltage is 0.4 V lower than that of the Pb-1% Ag alloy anode in 50 g/L Zn2+, 4 g/L Mn2+, and 150 g/L H2SO4.
References
[1] LIU Yuan, LIU Hui-ling, LI Yan. Comparative study of the electrocatalytic oxidation and mechanism of nitrophenols at Bi-doped lead dioxide anodes [J]. Applied Catalysis B: Environmental, 2008, 84(1): 297-302.
[2] da SILVA L M, BOODTS J F C, de FARIA L A. Oxygen evolution at RuO2(x)+Co3O4(1-x) electrodes from acid solution [J]. Electrochimica Acta, 2001, 46(9): 1369-1375.
[3] CASELLATO U, CATTARIN S, MUSIANI M. Preparation of porous PbO2 electrodes by electrochemical deposition of composites [J]. Electrochimica Acta, 2000, 48(27): 3991-3998.
[4] BORRAS C, LAREDO T, SCHARIFKER B R. Competitive electrochemical oxidation of p-chlorophenol and p-nitrophenol on Bi-doped PbO2 [J]. Electrochim Acta, 2003, 48(19): 2775-2780.
[5] AMADELLI R, SAMIOLO L, VELICHKO A B, KNYSD V A, LUK’YANENKO T V, DANILOV F I. Composite PbO2-TiO2 materials deposited from colloidal electrolyte: Electrosynthesis, and physicochemical properties [J]. Electrochim Acta, 2009, 54(22): 5239-5245.
[6] DAN Yuan-yuan, LU Hai-yan, LIU Xiao-lei, LIN Hai-bo, ZHAO Jing-zhe. Ti/PbO2+nano-Co3O4 composite electrode material for electrocatalysis of O2 evolution in alkaline solution [J]. International Journal of Hydrogen Energy, 2011, 36(3): 1949-1954.
[7] ZHANG Zhao-xian. Engineering technology of titanium electrode [M]. Beijing: Metallurgical Industry Press, 2003. (in Chinese)
[8] LAI Yan-qing, LI Yuan, JIANG Liang-xing, XU Wang, LV Xiao-jun, LI Jie, LIU Ye-xiang. Electrochemical behaviors of co-deposited Pb/Pb-MnO2 composite anode in sulfuric acid solution-Tafel and EIS investigations [J]. Journal of Electroanalytical Chemistry, 2012, 671: 16-23.
[9] LI Yuan, JIANG Liang-xing,  Xiao-jun, LAI Yan-qing, ZHANG H L, LI Jie, LIU Ye-xiang. Oxygen evolution and corrosion behaviors of co-deposited Pb/Pb-MnO2 composite anode for electrowinning of nonferrous metals [J]. Hydrometallurgy, 2011, 109(3/4): 252-257.
Xiao-jun, LAI Yan-qing, ZHANG H L, LI Jie, LIU Ye-xiang. Oxygen evolution and corrosion behaviors of co-deposited Pb/Pb-MnO2 composite anode for electrowinning of nonferrous metals [J]. Hydrometallurgy, 2011, 109(3/4): 252-257.
[10] SU Ling-hao, FAN Shao-huan. Capacitance performance of MnO2/PbO2 as composite electrode materials [J]. Chinese Journal of Power Sources, 2005, 29(4): 462-465. (in Chinese)
[11] XIA Xi, GONG Liang-yu. Synthesis of nanophase PbO2 by solid state reaction and its influence on MnO2 electrode [J]. Acta Chimica Sinica, 2002, 60(1): 87-92. (in Chinese)
[12] YU Neng-feng, GAO Li-jun. Electrodeposited PbO2 thin film on Ti electrode for application in hybrid supercapacitor [J]. Electrochemistry Communications, 2009, 11(1): 220-222.
[13] LIANG Wen-yan, QU Jiu-hui, CHEN Li-bin, LIU Hui-juan, LEI Peng-ju. Inactivation of Microcystis aeruginosa by continuous electrochemical cycling process in tube using Ti/RuO2 electrodes [J]. Environmental Science and Technology, 2005, 39(12): 4633-4639.
[14] HU Ji-ming, ZHANG Jian-qing, CAO Chun-nan. Oxygen evolution reaction on IrO2-based DSA type electrodes: Kinetics analysis of Tafel lines and EIS [J]. International Journal of Hydrogen Energy, 2004, 29(8): 791-797.
[15] LI Jia-qing, ZHENG Lei, LI Luo-ping, SHI Guo-yue, XIAN Yue-zhong, JIN Li-tong. Photoelectro-synergistic catalysis at Ti/TiO2/PbO2 electrode and its application on determination of chemical oxygen demand [J]. Electroanalysis, 2006, 18(22): 2251-2256.
[16] LIANG Zhen-hai, SUN Yan-ping. Properties of Ti/SnO2+ Sb2O3+MnO2/PbO2 anode [J]. Journal of Inorganic Materials, 2001, 16(1): 183-187.
[17] SONG Yue-hai, WEI Gang, XIONG Rong-chun. Structure and properties of PbO2–CeO2 anodes on stainless steel [J]. Electrochimica Acta, 2007, 52(24): 7022-7027.
[18] CHEN Bu-ming, GUO Zhong-cheng, YANG Xian-wan, CAO Yuan-dong. Morphology of alpha-lead dioxide electrodeposited on aluminum substrate electrode [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 97-103.
[19] CHEN Bu-ming, GUO Zhong-cheng, HUANG Hui, YANG Xian-wan, CAO Yuan-dong. Effect of the current density on electrodepositing alpha-lead dioxide coating on aluminum substrate [J]. Acta Chimica Sinica, 2009, 22(5): 373-382.
[20] CHEN Bu-ming, GUO Zhong-cheng, XU Rui-dong. Electrosynthesis and physicochemical properties of α-PbO2-CeO2-TiO2 composite electrodes [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(4): 1191-1198.
[21] PAJONK G M, MANZALJI T. Synthesis of acrylonitrile from propylene and nitric oxide mixtures on PbO2-ZrO2 aerogel catalysts [J]. Catalysis letters, 1993, 21(3/4): 361-369.
[22] MIAO Zhi-guang, GUO Zhong-cheng. Preparation of a novel PbO2-WC-ZrO2 composite electrode material with stainless steel as substrate [J]. Electroplating and Finishing, 2007, 26(4): 15-17. (in Chinese)
[23] YAO Ying-wu, ZHAO Chun-mei, ZHU Jin. Preparation and characterization of PbO2–ZrO2 nanocomposite electrodes [J]. Electrochimica Acta, 2012, 69: 146-151.
[24] XU Rui-dong, HUANG Li-ping, ZHOU Jian-feng, ZHAN Peng, GUAN Yong-yong, KONG Ying. Effects of tungsten carbide on electrochemical properties and microstructural features of Al/Pb- PANI-WC composite inert anodes used in zinc electrowinning [J]. Hydrometallurgy, 2012, 125-126: 8-15.
[25] ZHAN Peng, XU Rui-dong, HUANG Li-ping, CHEN Bu-ming, ZHOU Jian-feng. Effects of polyaniline on electrochemical properties of composite inert anodes used in zinc electrowinning [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1693-1700.
[26] LIU Shu-lan, YU De-long, QIN Qi-xian. Investigation on the PbO2-WC composite anode [J]. Chinese Journal of Applied Chemistry, 1995, 12(5): 46-49. (in Chinese)
[27] GUO Zhong-cheng. Preparation method of energy saving and inert anodic material for non-ferrous metals electrowinning: China, 200810058194. 5 [P]. 2008-03-15. (in Chinese)
[28] DALCHIELE E A, CATTARIN S, MUSIANI M, CASELLATO U, GUERRIERO P. Electrodeposition studies in the MnO2+PbO2 system: Formation of Pb3Mn7O15 [J]. J Appl Electrochem, 2000, 30(1): 117-120.
[29] VELICHENKO A B, KNYSH V A, LUK’YANENKO T V, DEVILLY D, DANILOV F I. PbO2-TiO2 composites: Electrosynthesis and physicochemical properties [J]. Russian Journal of Applied Chemistry, 2008, 81(6): 994-999.
[30] STROUMBOULI M, GYFTOU P, PAVLATOU EA, SPYRELLIS N. Codeposition of ultrafine WC particles in Ni matrix composite electrocoatings [J]. Surface and Coatings Technology, 2005, 195(2): 325-332.
[31] VELICHENKO A B, KNYSH V A, LUK’YANENKO T V, DEVILLIERS D, DANILOV F I. Electrodeposition of PbO2-ZrO2 composite materials [J]. Russian Journal of Electrochemistry, 2008, 44(11): 1251-1256.
[32] SCHLESINGER M, PAUNOVIC M. Modern Electroplating [M]. 4th ed. New York: The Electro-chemical Society Inc, 2000.
[33] DA SILVA L M, FRANCO D V, FARIA L D, BOODTS J F C. Surface, kinetics and electrocatalytic properties of Ti/(IrO2+Ta2O5) electrodes, prepared using controlled cooling rate, for ozone production [J]. Electrochimica Acta, 2004, 49(22/23): 3977-3988.
[34] ZHU Xiao-dong, LI Ning, LI De-yu, YUE Qiang, HE Li-ge, LIU Wer-hua. Efects of 88 series additives on the quality of high-speed galvanization coatings [J]. Chinese Electroplating and Pollution Control, 2005, 25(5): 6-8.(in Chinese)
[35] RAINFORTH W M. Microstructural evolution at the worn surface: A comparison of metals and ceramics [J]. Wear, 2000, 245(1): 162-177.
[36] MARTELLI G N, ORNELAS R, FAITA G. Deactivation mechanisms of oxygen evolving anodes at high current densities [J]. Electrochimica Acta, 1994, 39(11): 1551-1558.
[37] CHEN Xue-ming, CHEN Guo-hua, YUE P L. Stable Ti/IrOx- Sb2O5-SnO2 anode for O2 evolution with low Ir content [J]. The Journal of Physical Chemistry B, 2001, 105(20): 4623-4628.
[38] IVANOV I, STEFANOV Y, NONCHEVA Z, PETROVA M, DOBREV Ts, MIRKOVA L, VERMEERCH R, DEMAEREL J P. Insoluble anodes used in hydrometallurgy: Part I. Corrosion resistance of lead and lead alloy anodes [J]. Hydrometallurgy, 2000, 57(2): 109-124.
杨海涛1,陈步明1,郭忠诚1, 2,刘焕荣3 ,张永春1,黄 惠1, 4,徐瑞东1, 4,付仁春5
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 昆明理工恒达科技有限公司,昆明 650022;
3. 昆明理工大学 国土资源工程学院,昆明 650093;
4. 昆明理工大学 复杂有色金属资源清洁利用省部共建国家重点实验室培育基地,昆明 650093;
5. 昆明理工大学 理学院,昆明 650093
摘 要:通过电化学氧化共沉积技术在A1/导电涂层/α-PbO2-CeO2-TiO2 基体上,制备了A1/导电涂层/α-PbO2- CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2复合阳极材料。通过能量色散X射线光谱(EDXS)、阳极极化曲线、暂稳态极化曲线(Tafel)、交流阻抗谱(EIS)、扫描电子显微镜(SEM)以及X射线衍射(XRD)等方法研究电流密度对复合阳极材料的化学组分、电化学活性和稳定性的影响。研究结果表明:在电流密度为1 A/dm2条件下制备的A1/导电涂层/α-PbO2-CeO2-TiO2/β-PbO2-MnO2-WC-ZrO2 复合材料具有最低的析氧过电位(0.610 V,条件:500 A/m2),最好的电化学活性,最长的使用寿命(360 h,条件:150 g/L H2SO4, 2 A/cm2, 40 °C)以及最低的槽电压(2.75 V,条件:500 A/m2)。而且,随着电流密度的增加,涂层晶粒逐渐增大,MnO2含量也逐渐降低,晶体结构几乎没有变化。
关键词:复合电极材料;铝基体;β-PbO2-MnO2-WC-ZrO2;电化学共沉积;电流密度
(Edited by Yun-bin HE)
Foundation item: Projects (51004056, 51004057) supported by the National Natural Science Foundation of China; Project (KKZ6201152009) supported by the Opening Foundation of Key Laboratory of Inorganic Coating Materials, Chinese Academy of Sciences; Project (2010ZC052) supported by the Applied Basic Research Foundation of Yunnan Province, China; Project (20125314110011) supported by the Specialized Research Fund for the Doctoral Program of Higher Education, China; Project (2010247) supported by Analysis & Testing Foundation of Kunming University of Science and Technology, China
Corresponding author: Bu-ming CHEN; Tel: +86-871-68352598; E-mail: 272601291@qq.com
DOI: 10.1016/S1003-6326(14)63482-8

