L12型纳米颗粒增强以Al-Ca-Ni-La体系为基体的铝基复合材料
来源期刊:中国有色金属学报(英文版)2020年第4期
论文作者:Torgom K. AKOPYAN Nikolay A. BELOV Evgeniya A. NAUMOVA Nikolay V. LETYAGIN Tat’yana A. SVIRIDOVA
文章页码:850 - 862
关键词:Al-Ca合金;共晶;金属间化合物;相图;稀土元素;纳米复合材料;显微组织;力学性能
Key words:Al-Ca alloys; eutectic; intermetallics; phase diagram; rare earth element; nanocomposite; microstructure; mechanical properties
摘 要:结合Thermo-Calc热模拟程序计算和实验(电子显微镜、微探针分析和 X射线衍射)研究四元系Al-(2~4)Ca-Ni-La(质量分数,%)合金在铝角附近的结构。根据得到的相平衡数据,提出Al-Ca-Ni-La体系液相面和固相相场分布的实验投影。显微组织研究表明,含2%~4% Ca、2%~4% Ni和1%~3%La(质量分数)的合金具有超细的亚共晶组织,共晶金属间化合物的体积分数为30%,因此,可以将这些合金归类为天然Al基复合材料。超细共晶结构产生显著的强化作用,其机制可以很好地用修订的Orowan循环模型描述。在350~400 °C退火过程中,由于L12型相(Al3(Zr,Sc))纳米颗粒的形成,添加少量的Zr和Sc(分别为0.2%和0.1%,质量分数)具有显著的强化作用(提高约25%)。由于共晶金属间化合物的体积分数高,该新合金具有低的热膨胀系数、高的结构热稳定性和力学性能。
Abstract: The structure of the quaternary Al-(2-4)wt.%Ca-Ni-La system near the aluminum corner has been studied using computational analysis in the Thermo-Calc program and experimental studies (electron microscopy, microprobe analysis and X-ray diffraction). Based on the phase equilibria data obtained, the experimental projection of the liquidus surface and solid state phase-field distribution of the Al-Ca-Ni-La system have been proposed. Microstructure studies reveal that the alloys with the 2-4 wt.% Ca, 2-4 wt.% Ni and 1-3 wt.% La ranges have an ultra-fine hypoeutectic structure with 30% volume fraction of eutectic intermetallics, which allows one to classify these alloys as natural Al-matrix composites. The ultra-fine eutectic structure produces significant strengthening, the magnitude of which can be well described using the modified Orowan looping mechanism model. Small additives of Zr and Sc (0.2 and 0.1 wt.%, respectively) lead to significant strengthening (by ~25%) due to the formation of L12 type phase (Al3(Zr,Sc)) nanoparticles during annealing of the alloy at 350-400 °C. Due to the high volume fraction of eutectic intermetallics, the new alloys have low coefficients of thermal expansion and high thermal stability of the structure and mechanical properties.
Trans. Nonferrous Met. Soc. China 30(2020) 850-862
Torgom K. AKOPYAN, Nikolay A. BELOV, Evgeniya A. NAUMOVA, Nikolay V. LETYAGIN, Tat'yana A. SVIRIDOVA
National University of Science and Technology MISiS, 4 Leninsky pr., Moscow 119049, Russia
Received 16 August 2019; accepted 20 December 2019
Abstract: The structure of the quaternary Al-(2-4)wt.%Ca-Ni-La system near the aluminum corner has been studied using computational analysis in the Thermo-Calc program and experimental studies (electron microscopy, microprobe analysis and X-ray diffraction). Based on the phase equilibria data obtained, the experimental projection of the liquidus surface and solid state phase-field distribution of the Al-Ca-Ni-La system have been proposed. Microstructure studies reveal that the alloys with the 2-4 wt.% Ca, 2-4 wt.% Ni and 1-3 wt.% La ranges have an ultra-fine hypoeutectic structure with 30% volume fraction of eutectic intermetallics, which allows one to classify these alloys as natural Al-matrix composites. The ultra-fine eutectic structure produces significant strengthening, the magnitude of which can be well described using the modified Orowan looping mechanism model. Small additives of Zr and Sc (0.2 and 0.1 wt.%, respectively) lead to significant strengthening (by ~25%) due to the formation of L12 type phase (Al3(Zr,Sc)) nanoparticles during annealing of the alloy at 350-400 °C. Due to the high volume fraction of eutectic intermetallics, the new alloys have low coefficients of thermal expansion and high thermal stability of the structure and mechanical properties.
Key words: Al-Ca alloys; eutectic; intermetallics; phase diagram; rare earth element; nanocomposite; microstructure; mechanical properties
1 Introduction
Rare earth metals (RE) are widely used in various industrial fields for the production of structural materials, high corrosion resistant materials, superconductors, high performance magnets, hydrogen storage, etc [1,2]. Moreover, small additives of rare earth elements are used to modify the eutectic of conventional cast aluminum alloys [3-7]. However, technologies of new alloys on the basis of the Al-RE system are actively explored in the aluminum metallurgy [8-14]. Among them, alloys with RE and Ni exhibit the best combination of the strength and the heat resistance [15]. However, due to the high content of Ni and La, these alloys have many disadvantages. One of them is the high cost of alloys, which is associated with the high cost of Ni and La. Another disadvantage is the high density of alloys due to the high density of Al3Ni and Al11La3 intermetallic compounds which are in equilibrium with Al.
In our opinion, the transition to the Al-Ca base alloys can help to solve the described disadvantages of the Al-La, Al-Ni and Al-Ni-La alloys. Indeed, calcium is an affordable metal and is widely used in metallurgy. Ca forms a eutectic-type diagram with Al; however, the density of Ca is one of the lowest metals (1.542 g/cm3). The Al4Ca phase in the binary eutectic exceeds 30 vol.%, which allows producing Al-matrix composites by simple melting and casting of alloys. Thus, it can be advisable to consider new metal matrix composites based on the Al-Ca eutectic [16-20], additionally containing Ni and rare-earth element, such as La or Ce. However, the design of Al-Ca-Ni-La base alloys is significantly constrained by the limited information on the quaternary phase diagram. Despite some data can be taken from works dealing with the binary and ternary systems [10,12,13,15,21-24], more complex systems have not been considered in the literature at all. Based on the above, the main purpose of this work is to analyze the structure of the Al-Ca-Ni-La quaternary system near the aluminum corner and evaluate the possibility of designing new aluminum matrix composite alloys based on it.
It should also be noted that, according to Ref. [25], in contrast to branded Al-Si alloys [26], the Al-Ca base alloys can be further strengthened with small additives of zirconium and scandium. In this case, the strengthening is associated with the decomposition of the aluminum solid solution (Al) and simultaneous formation of secondary coherent spherical nanoparticles of L12 type phases (Al3Zr, Al3Sc or Al3(Zr,Sc) [27-32]) during simple annealing of the as-cast alloy. In accordance with Ref. [33], to maintain a suitable balance of the alloy cost and maximum strengthening effect, it is advisable to combine alloying with Zr and Sc in amounts of 0.2 and 0.1 wt.%, respectively. Thus, the effect of the small additions on the mechanical properties and microstructure of new alloys is also considered in this work.
2 Experimental
To study the Al4CaNiLa system, we used seven model alloys presented in Table 1. The alloys were prepared on the basis of 99.99% aluminum in a resistance furnace (GRAFICARBO) with graphite crucible. Aluminum was placed in the crucible, after its melting, Al-15wt.%Ca and Al-20wt.%Ni binary master alloys were introduced, and after complete melting, lanthanum was introduced at a melt temperature of 730-740 °C. Zirconium and scandium were added using Al-15%wt.Zr and Al-2wt.%Sc master alloys at a melt temperature of 780 °С. After melting of the main components, the melt was held for 5-10 min for obtaining a homogeneous composition, the slag was raked off and the metal was cast into a graphite mold with working cavity dimensions of 10 mm × 20 mm × 180 mm for quaternary alloys and into a metal mold for tensile tests in the case of alloys with Zr and Sc addition. The cooling rate in the molds was about 10 K/s. The casting temperature was 750-820 °C.
Specimen No. 7 was heat treated in muffle electric furnaces with a maintenance temperature error of 3 K. To establish the temperature dependence of (Al) decomposition characteristics during the formation of the L12 phase nanoparticles, we performed step-by-step annealing in the range of 250-500 °C with 50 °C step for 3 h exposure (see Table 2) at each stage [23]. After each stage the sample was cooled in air. The stepwise mode allows us to carry out all studies of the influence of heating temperatures on one sample. This method was both informative and economical for the Al alloys.
The microstructure was studied by scanning electron microscopy (SEM, TESCAN VEGA 3) with electron microprobe analysis (EMPA, OXFORD AZtec) and transmission electron microscopy (TEM, JEM-2100). The specimens were prepared by mechanical and electrolytic polishing. The thin foils for TEM were prepared by ion thinning with a PIPS (Precision Ion Polishing System, Gatan) machine and studied at 160 kV.
Table 1 Chemical compositions of experimental alloys
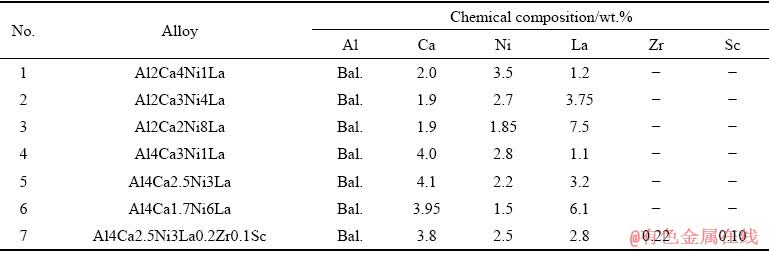
Table 2 Annealing modes

The Vickers hardness was determined on a DUROLINE MH-6 installation (load of 1 N, and dwell time of 30 s). Room-temperature tension tests were conducted for as-processed bar specimens with a universal testing machine, model Zwick Z250 (the loading rate was 10 mm/min).
X-ray diffraction (XRD) data were collected using Cu Kα radiation and treated by software package [34]. The objects of the X-ray diffraction (XRD) study were polished specimens cut from part of the ingots. Based on XRD data, the volume fraction and the periods of the crystal lattice of the phases have been determined. The relative error of a measurement is 10% for the volume fraction and 0.15% for the crystal lattice periods.
Differential scanning calorimetry (DSC) with heating and cooling rates of 10 K/min was performed using a high-temperature DSC Setaram Setsys Evolution (SETARAM Instrumentation, Caluire, France) for ~80 mg specimens. The experiment was carried out in a dynamic Ar atmosphere with a flow rate of 50 mL/min. A temperature accuracy of ±0.5 °C was obtained.
Dilatometric analysis with a heating rate of 5 K/min was carried out using a quenching dilatometer DIL 805A/D with the possibility of deformation of the sample by compression (TA Instruments, Germany). Dilatometric study was carried out using thermocouples (S-type) in vacuum (~0.013 Pa) on cylindrical samples with 6 mm in diameter and 12 mm in length.
To facilitate the preliminary analysis of the quaternary system, a thermodynamic calculation in the Thermo-Calc program and TTAL5 database was initially conducted [35].
3 Results
3.1 Analysis of quaternary Al-Ca-Ni-La system
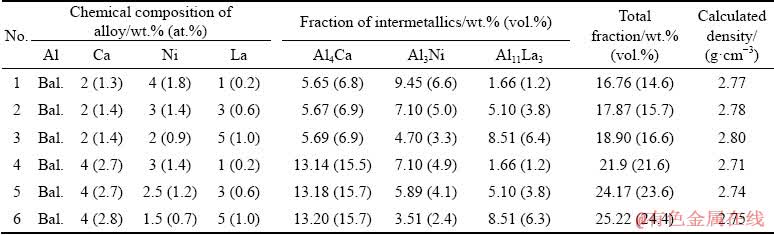
The TTAL5 database is designed to calculate the phase composition of the multi-component aluminum-based alloys, and contains the thermo- dynamic values of all the chemical elements of a given quaternary system and the expected phases. Liquidus data of the Al-Ca-Ni-La system at different Ca, Ni and La contents were calculated to define the areas of primary crystallization of phases in equilibrium. The calculated data were verified by analyzing the microstructure of the alloys. The specific type of primary crystals found in the alloys is marked on the calculated diagrams (Fig. 1(a)). In accordance with the obtained calculated data, the aluminum solid solution (Al) can be in equilibrium with three intermetallic compounds, Al4Ca, Al3Ni and Al11La3, the main characteristics of which are given in Table 3.

Fig. 1 Liquidus projection of Al-Ca-Ni-La system in aluminum corner (a) and isopleth at 4 wt.% Ca and 2.5 wt.% Ni (b) (The dashed line in Fig. 1(a) indicates the liquidus projection for the system at 4% Ca)
Depending on the actual chemical composition, the structure of hypoeutectic alloys can be formed through binary eutectic reaction either L → (Al) + Al3Ni or L → (Al) + Al11La3, followed by a ternary eutectic reaction either L → (Al) + Al3Ni + Al4Ca or L → (Al) + Al11La3 + Al3Ni, and the solidification ends via an invariant eutectic reaction L → (Al) + Al11La3 + Al3Ni + Al4Ca at ~601 °C (Fig. 1(b)). An increase in the content of Ca leads to a narrowing of the area of the (Al) (see dashed line in Fig. 1(a)). Moreover, analysis of the microstructure of experimental alloys revealed the wider area of primary crystallization of the Al11La3 phase compared to the calculated data (see Figs. 1 and 2). From the analysis of the microstructure, it can also be seen that the ultra-fine eutectic in hypoeutectic alloys with 4 wt.% Ca occupies more than half of the volume (Fig. 2). In accordance with the theoretical analysis (see Table 4), the total volume fraction of the eutectic intermetallics in these alloys can vary in the range of 15-25 vol.%. Due to the low density of the Al4Ca compound (Table 3), an increase in Ca content leads to a marked increase in the volume fraction of the intermetallic phase (Table 4). At the same time, we can observe a significant decrease in the alloys density (Table 4).
Table 3 Characteristics of phases in Al-rich corner of Al-Ca-Ni-La system [36,37]

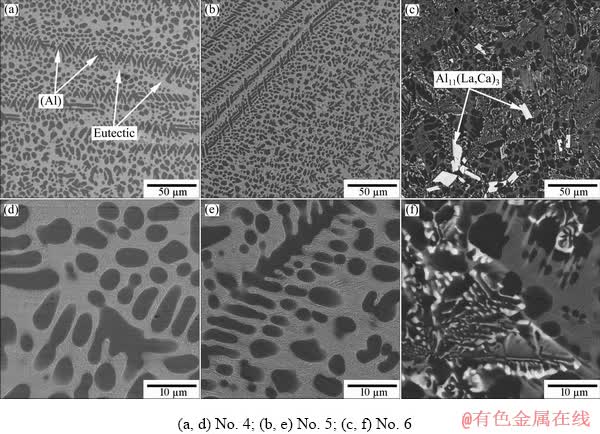
Fig. 2 SEM images showing as-cast microstructure of experimental alloys from Table 1 (obtained at regular melt cooling rates of about 10 K/min)
Table 4 Fractions of eutectic intermetallic phases in hypoeutectic alloys and their theoretical density
Indeed, the density of the Al4Ca3Ni1La alloy, totally containing 4 wt.% of heavy Ni and La, is close to that of pure aluminum. However, due to inconsistencies between the calculated and experimental data on the phase regions described above, a more detailed experimental analysis of the quaternary system is necessary for accurate determination of the phase equilibria in this system.
For a more detailed analysis of the quaternary system, additional studies of the microstructure of alloys (Nos. 4 and 5 in Table 1) obtained during slow solidification (melt cooling rate is ~0.01 K/min) and after prolonged high-temperature annealing of the as-cast specimens analyzed above (presented in Fig. 2) were carried out. In both cases, we can expect the formation of the phase composition close to the equilibrium one. According to EMPA, the microstructure of alloy No. 4 (Fig. 3(a)) contains two eutectic phases: predicted binary Al4Ca and ternary compound with the formula close to Al9CaNi. Lanthanum distributes among these compounds and does not form the Al11La3 phase. An increase in the content of La in alloy No. 5 (Fig. 3(b)) leads to the appearance of relatively coarse bright crystals of the Al11La3 phase. The chemical compositions of the phases as determined by EMPA are presented in Table 5. The data suggest a significant mutual solubility between the binary Al11La3 and Al4Ca compounds. The Ca and La atoms in the lattice interchange with each other in the equivalent positions. XRD analysis (Fig. 4) of the as-annealed samples (Figs. 3(c, d)) well confirms the results obtained by EMPA. Table 5 gives experimental data on the quantitative parameters of the phases, in particular, their fraction, crystal lattice parameters and densities calculated based on these data. It can be seen that both alloys consist of the Al4Ca and Al9CaNi phases, the total volume fraction of which is about 30%. Due to the high content of La, alloy No. 5 also contains a small amount of the Al11La3 phase. However, the main amount of La is dissolved in the Al4Ca phase. As can be seen, an increase in the content of La in Al4Ca leads to a marked increase in the a lattice parameter and density. Indeed, according to Table 3, the natural density of Al4Ca is about 2.35 g/cm3 while replacing 10% of calcium atoms by lanthanum (see Table 5) leads to an increase in the density of the compound to 2.49 g/cm3, i.e., by 8%. Replacing more than 20% calcium atoms increases the density by 13% (Table 6). Noticeable changes in the lattice parameter and density can also be observed for the Al11La3 compound. Replacing more than 32% of the lanthanum atoms by calcium leads to a decrease in the density of the compound to 3.51 g/cm3. It should be emphasized that the ternary Al-Ca-La system was not previously analyzed and therefore additional studies of the ternary system are required for a more detailed analysis of the observed mutual solubility. Analysis of the properties of the Al9CaNi compound revealed a certain increase of its density, which is associated with the dissolution of a relatively small amount of lanthanum. An additional increase in the content of lanthanum (see data for alloy No. 5 in Table 6) leads to a certain increase in the c parameter of the crystal lattice, while the density remains almost unchanged. This result is due to a slight increase of the lanthanum content, the presence of which leads to stretching (i.e., increase in the volume) of the crystal lattice.
Thus, based on the analysis above, three phases Al4(Ca,La), Al11(La,Ca)3 and Al9CaNi are in equilibrium with (Al) in the Al-Ca alloys. Taking into account the structure of the ternary Al-Ca-Ni system [23], the projection of the liquidus surface of the quaternary system (Fig. 5(a)) was proposed. In accordance with it, two invariant reactions can be expected near the aluminum corner: peretectic L + Al3Ni → Al9CaNi + Al11(La,Ca)3 (P) and eutectic L → Al + Al4(Ca,La) + Al9CaNi + Al11(La,Ca)3 (E) which, according to the results of DSC, takes place at ~603 °C. Accordingly, the expected solid state phase-field distribution is proposed in Fig. 5(b). As can be seen, the main part of the diagram consists of two four-phase areas: (Al) + Al4(Ca,La) + Al11(La,Ca)3 + Al9CaNi and (Al) + Al4(Ca,La) + Al11(La,Ca)3 + Al3Ni (marked as 1 and 2) and two three-phase areas: (Al) + Al11(La,Ca)3 + Al9CaNi and (Al) + Al4(Ca,La) + Al9CaNi (marked as 3 and 4). The Al4(Ca,La) phase is not in equilibrium with the Al3Ni phase and therefore the hypoeutectic quaternary alloys always contain the ternary Al9CaNi phase. Due to the relatively high solubility of La in Al4(Ca,La) phase, the four-phase area is sufficiently narrow and therefore the vast majority of the hypoeutectic alloy composites corresponds to the (Al) + Al4(Ca,La) + Al9CaNi three-phase area.

Fig. 3 SEM images showing microstructure of alloys No. 4 (a, c) and No. 5 (b, d) obtained during slow solidification at melt cooling rate of about 0.01 K/min (a, b) and after prolonged high-temperature annealing (at 500 °C for 10 h) of as-cast samples obtained at regular cooling rate of 10 K/s (c, d)
Table 5 Chemical compositions of phases in Al-Ca-Ni-La alloys determined by EMPA
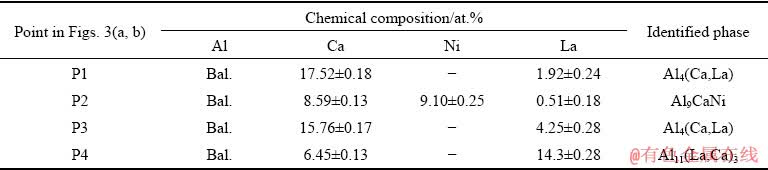
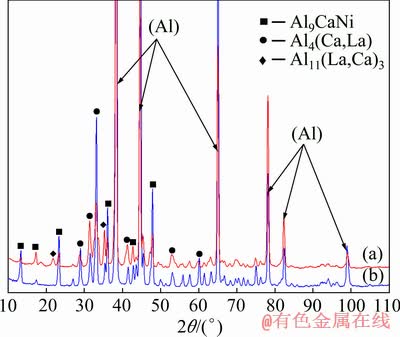
Fig. 4 XRD patterns of Al4Ca3La3Ni (a) and Al4Ca1La3Ni (b) alloys
3.2 Analysis of Al-Ca-Ni-La system base hypoeutectic alloy
As can be seen from Fig. 2, hypoeutectic alloys Nos. 4 and 5 at 4 wt.% Ca have an ultra-fine eutectic structure which is beneficial for obtaining high mechanical properties. On the other hand, an additional increase in strength can be achieved by alloying with small amounts of zirconium and scandium. The microstructure of the new alloy Al4Ca2.5Ni3La0.2Zr0.1Sc (No. 7) with zirconium and scandium additives in the as-cast state is shown in Fig. 6. As can be seen, no new structural components are detected compared with Fig. 2. The fine eutectic layers which were liquid during casting separate the (Al) solid grains from each other and also locate in the grain boundary triple junctions (Figs. 6(a, b)). TEM analysis reveals that the eutectic particles are slightly elongated (in the direction of heat removal in alloy solidification) and have a size of about 100-150 nm in the transverse direction and about 150-250 nm in the longitudinal direction (Figs. 6(c, d)). EMPA shows that zirconium and scandium distribute between the aluminum solid solution (Al) and the eutectic (Table 7). To confirm the possibility of alloy hardening, stepwise annealing of the alloy according to the mode in Table 2 was conducted. The Vickers hardness was measured after each step. The results are shown in Fig. 7. For the steps S300 and S400, we can observe a substantial hardening by ~25% compared to the as-cast state. In accordance with the TEM results (Fig. 7), this hardening is caused by the formation of nanoparticles of the L12 type phase (Al3(Zr,Sc)) with a diameter of about 10-15 nm.
Table 6 Fractions of eutectic intermetallic phases and their lattice parameters and densities calculated based on XRD data


Fig. 5 Proposed phase diagrams of Al-Ca-Ni-La system

Fig. 6 SEM (a, b) and TEM (c, d) images showing microstructure of experimental Al4Ca2.5Ni3La0.2Zr0.1Sc alloy
Table 7 Chemical compositions of structural components in Al4Ca2.5Ni3La0.2Zr0.1Sc alloy


Fig. 7 Effect of stepwise annealing on Vickers hardness of Al4Ca2.5Ni3La0.2Zr0.1Sc alloy (a) and TEM image showing precipitates of Al3(Zr,Sc)-L12 phase forming during annealing (b)
According to uniaxial tensile tests (Table 8), the base alloy in the as-cast state has an average strength that is comparable to that of industrial cast aluminum alloy A356 [38] after quenching and artificial aging. The addition of zirconium and scandium significantly increases the strength of the alloy while maintaining an acceptable elongation.
4 Discussion
Due to the high volume fraction of eutectic intermetallic compounds, new Al-Ca-Ni-La hypoeutectic alloys have many advantages in comparison with branded Al-Si cast alloys. First of all, the strength of the as-cast new alloys (Table 8) is comparable to those of branded Al-Si alloys after artificial aging. In addition, new alloys, unlike Al-Si branded ones, can be strengthened by small additions of zirconium and scandium (Fig. 7(a)). A simple calculation shows that the dissolution of 0.2 wt.% Zr and 0.1 wt.% Sc in (Al) should lead to the formation of about 0.4 vol.% of the L12-type phase. Taking into account that the average diameter (DS) of the precipitates is about 15 nm, the interprecipitate spacing λ can be calculated using well known relationship [39]:
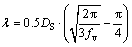 (1)
(1)
where fv is the particle volume fraction. According to Eq. (1), λ is about 165 nm. The modified Orowan model for spherical unshearable precipitates [40] can be used for analyzing the increase in yield strength associated with the nanoparticles. According to this model,
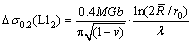 (2)
(2)
where M is the Taylor factor varying in 2.44-3.7 range, G and b are the shear modulus and the absolute value of Burgers vector of Al, respectively, 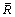 is the average radius of circular cross-section, ν is Poisson’s ratio of Al and r0=1.5b. Thus, if the average Taylor factor is 3.1, the increase in the yield strength of the aluminum matrix caused by the Al3(Zr,Sc) nanoparticles is about 73 MPa.
is the average radius of circular cross-section, ν is Poisson’s ratio of Al and r0=1.5b. Thus, if the average Taylor factor is 3.1, the increase in the yield strength of the aluminum matrix caused by the Al3(Zr,Sc) nanoparticles is about 73 MPa.
As can be seen from Table 8, the fine eutectic structure also contributes significantly to the strengthening. However, it will not be entirely correct to perform a similar calculation for the eutectic particles since they have an elongated shape. If we assume that the particles have a shape close to ellipsoid, Eq. (2) can be modified as follows:
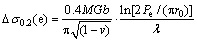 (3)
(3)
where Pe is the ellipse perimeter. According to quantitative metallographic analysis, λ for eutectic particles is 150 nm. If we also assume that the particles have an average length of 200 nm and a width of 150 nm, the increase in the yield strength (according to Eq. (3)) caused by the eutectic particles is 160 MPa. Additionally, we should also take into account that the aluminum phase in the eutectic is also reinforced by the L12 nanoparticles.
To calculate the resulting strength of the alloy, we can use the basic additivity principle for the composite properties. Based on it, the yield strength of the alloy can be calculated using simple equation:
σ0.2=V(Al)(σ0.2(Al)+Δσ0.2(L12))+Ve((σ0.2(Al)+Δσ0.2(L12))+Δσ0.2(e)) (4)
where σ0.2(Al) is the yield strength of pure aluminum (can be accepted at ~30 MPa) and V(Al) and Ve are the volume fractions of aluminum and eutectic, respectively. In accordance with quantitative metallographic analysis, the fraction of the eutectic is about 60 vol.% (Fig. 6(a)). Thus, the yield strength is about 130 MPa for the base alloy and 200 MPa for the alloy with small additives of Zr and Sc. The calculated result is quite close to the experimental data and can be used for estimating the yield strength of the eutectic composite alloys.
Table 8 Mechanical properties after uniaxial tensile tests of alloys

Another interesting feature of these alloys is their relatively low density due to calcium content. As can be seen from Table 3, the density of the Al4Ca phase is lower than that of pure aluminum. Dissolving La in Al4Ca increases its density up to 2.6 g/cm3, but it still remains lower than that of pure aluminum. The ternary Al9CaNi compound has a density of 2.85 g/cm3, which is slightly higher than that of aluminum but significantly lower than that of the binary Al3Ni compound (3.95 g/cm3). Taking into account the volume fraction and actual density of the phases (Table 6), the calculated density of the Al4Ca1La2.5Ni and Al4Ca3La2.5Ni alloys is 2.70 and 2.73 g/cm3, respectively, which is in a good agreement with the experimental data (2.69 and 2.72 g/cm3). As can be seen, even at 3 wt.% La, the density of the alloy barely exceeds the density of pure aluminum, despite the total volume fraction of intermetallic compounds in the structure is more than 30 vol. % (Table 6). It should be noted that despite the calculation in the Thermo-Calc program does not provide a correct analysis of the phase composition, the obtained calculated data can nevertheless be used for estimating the density of the alloys (see Table 4). The obtained result is explained, on the one hand, by an increase in the density (see Table 4) of the Al4Ca compound (as a result of the dissolution of La), and on the other hand, by the compensation of the lower density of the ternary Al9CaNi compound (compared with the Al3Ni compound) at the expense of its higher volume fraction, which leads to a decrease in the volume fraction of aluminum with a lower density.
Another advantage of the new alloys is their relatively high thermal stability which is associated with a high volume fraction of intermetallic phases. Indeed, as can be seen from Fig. 8, during heating in the range of 50-600 °C, the coefficient of thermal expansion (CTE) of the new alloy varies slightly in the range of (22.5-24)×10-6 K-1. In contrast, the CTE of the branded A356 alloy increases significantly in the temperature range of 200-300 °C, reaching a maximum value of more than 27×10-6 K-1. The thermal stability of the mechanical properties can be evaluated by analyzing the microstructure and microhardness after prolonged high-temperature annealing. After annealing at 450 °C for 12 h, we can observe coarsening of the eutectic structure; however, the intermetallic particles are still less than 1 μm in diameter (see Fig. 9). The microhardness of the Al4Ca3La2.5Ni alloy is still high enough after annealing and decreases from HV 82 to 60, i.e, by 27%. It should be noted that the microhardness of the alloy with small additives of Zr and Sc, even after annealing at 500 °C (Fig. 7), remains higher than HV 85, which is directly related to the influence of the L12 nanoparticles.
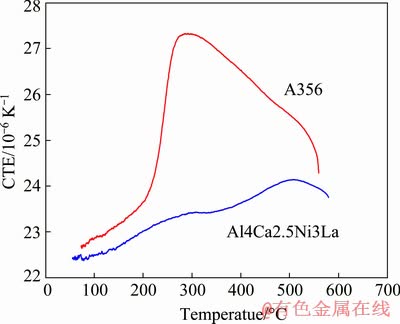
Fig. 8 Coefficient of thermal expansion (CTE) versus temperature for Al4Ca2.5Ni3La and A356 alloys

Fig. 9 Microstructures of alloys No. 4 (a) and No. 5 (b) after prolonged high-temperature annealing at 450 °C for 10 h
Thus, new hypoeutectic alloys based on the quaternary Al-Ca-Ni-La system have low density, high dispersion of the eutectic in the as-cast state, can be significantly strengthened by small additions of Zr and Sc, and have a low coefficient of thermal expansion and high thermal stability of the structure and properties. Due to these advantages, the new Al-Ca-Ni-La system base alloys can be considered as a promising replacement for the branded Al-Si alloys for the manufacture of critical products.
5 Conclusions
(1) Phase equilibria in the quaternary Al-Ca- Ni-La system near the aluminum corner have been analyzed using computational and experimental analysis. EMPA and XRD analyses have revealed significant mutual solubility between the binary Al4Ca (Al4(Ca,La)) and Al11La3 (Al11(La,Ca)3) compounds (Ca and La atoms interchange in the equivalent positions of the crystal lattice), which leads to changes in their lattice parameters and densities. The binary Al4(Ca,La) and Al11(La,Ca)3 and the ternary Al9CaNi compounds are in equilibrium with the aluminum solid solution (Al) in promising Al-Ca hypoeutectic alloys. The revealed phase equilibria imply the presence of two invariant transformations: peritectic L + Al3Ni → Al9CaNi + Al11(La,Ca)3 and eutectic L → Al4(Ca,La) + Al9CaNi + Al11(La,Ca)3 in the considered part of the system.
(2) The alloys of 2-4 wt.% Ca, 2-4 wt.% Ni and 1-3 wt.% La ranges have an ultra-fine hypoeutectic structure with an about 30 vol.% total volume fraction of eutectic intermetallics which allows one to classify these alloys as natural Al-matrix composites. The ultra-fine eutectic structure (the eutectic particles have a size of about 100-150 nm in the transverse direction and 150-250 nm in the longitudinal direction) produces significant strengthening, the magnitude of which can be well described using the modified Orowan looping mechanism model.
(3) The alloys can undergo additional strengthening (by ~25%) due to the introduction of small additives of Zr and Sc (0.2 and 0.1 wt.%, respectively). In accordance with TEM data, this result is associated with the decomposition of the aluminum solution (Al) and simultaneous formation of nanoparticles (the average diameter is about 10-15 nm) of the L12 (Al3(Zr,Sc)) phase during annealing of the alloy at 350-400 °C.
(4) Due to the high volume fraction of eutectic intermetallic phases, the new alloys have low coefficients of thermal expansion and high thermal stability of the structure and mechanical properties. Taking into account the advantages described above, the new Al-Ca-Ni-La base alloys can be considered as a promising replacement for the branded Al-Si alloys in the manufacture of critical parts.
Acknowledgments
The study was carried out by the financial support of the grant of the Russian Science Foundation (Project No. 18-79-00345) (preparation of alloys, electron microscopy (SEM, EMPA, TEM), tensile tests) and by the Ministry of Science and Higher Education of the Russian Federation in the framework of Increase Competitiveness Program of MISiS (No. P02-2017-2-10) (thermodynamic calculations, dilatometry, DSC and XRD).
References
[1] CAO Zu-jun, KONG Gang, CHE Chun-shan, WANG Yan-qi, PENG Hao-tang. Experimental investigation of eutectic point in Al-rich Al-La, Al-Ce, Al-Pr and Al-Nd systems [J]. Journal of Rare Earths, 2017, 35(10): 1022-1028.
[2] AMARAL J C B S, SA M L C G, MORAIS C A. Recovery of uranium, thorium and rare earth from industrial residues [J]. Hydrometallurgy, 2018, 181: 148-155.
[3] SONG Xian-chen, YAN Hong, ZHANG Xiao-jun. Microstructure and mechanical properties of Al-7Si-0.7Mg alloy formed with an addition of (Pr+Ce) [J]. Journal of Rare Earths, 2017, 35(4): 412-418.
[4] LIN Gao-yong, LI Kun, FENG Di, FENG Yong-ping, SONG Wei-yuan, XIAO Meng-qiong. Effects of La-Ce addition on microstructure and mechanical properties of Al-18Si- 4Cu-0.5Mg alloy [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(8): 1592-1600.
[5] LI Diao-feng, Cui Chun-xiang, Wang Xin, Wang Qing-zhou, Chen Cheng, Liu Shui-qing. Microstructure evolution and enhanced mechanical properties of eutectic Al–Si die cast alloy by combined alloying Mg and La [J]. Materials and Design, 2016, 90: 820-828.
[6] LIAO Heng-cheng, HU Yi-yun. Effect of RE addition on solidification process and high-temperature strength of Al-12%Si-4%Cu-1.6%Mn heat-resistant alloy [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(6): 1117-1126.
[7] Jiang Wen-ming, Fan Zi-tian, Dai Yu-cheng, Li Chi. Effects of rare earth elements addition on microstructures, tensile properties and fractography of A357 alloy [J]. Materials Science and Engineering A, 2014, 597: 237-244.
[8] SIMS Z C, WEISS D, MсCALL S K, MсGUIRE M A, OTT R T, GEER T, RIOS O, TURCHI P A E. Cerium-based, intermetallic-strengthened aluminum casting alloy: High-volume co-product development [J]. JOM, 2016, 68: 1940-1947.
[9] SIMS Z C, RIOS O, MсCALL S K, van BUUREN T, OTT R T. Characterization of near net-shape castable rare earth modified aluminum alloys for high temperature application [C]//WILLIAMS E. Light Metals 2016. Springer, Cham, 2016.
[10] SIMS Z C, RIOS O, TURCHI P E A, PERRON A, LEE J R I, LI T T, HAMMONS J A, BAGGE-HANSEN M, WILLEY T M, AN K, CHEN Y, KING A H, McCALL S C. High performance aluminum–cerium alloys for high-temperature applications [J]. Materials Horizons, 2017, 4: 1070-1078.
[11] WEISS D, RIOS O, SIMS Z C, MсCALL S K, OTT R T. Casting characteristics of high cerium content aluminum alloys [C]//RATVIK A. Light Metals 2017. The Minerals, Metals & Materials Series. Springer, Cham, 2017.
[12] He Yang, Liu Jian-hua, Qiu Sheng-tao, Deng Zhen-qiang, Zhang Jie, Shen Yao-zu. Microstructure evolution and mechanical properties of Al-La alloys with varying La contents [J]. Materials Science and Engineering A, 2017, 701: 134-142.
[13] MANCA D R, CHURYUMOV A Y, POZDNIAKOV A, PROSVIRYAKOV A, RYABOV D, KROKHIN A, KOROLEV V, DAUBARAYTE D. Microstructure and properties of novel heat resistant Al-Ce-Cu alloy for additive manufacturing [J]. Metals and Materials International, 2019, 25(3): 633-640.
[14] COURY F G, KIMINAMI C S, BOTTA W J, BOLFARINI C, KAUFMANA M J. Design and production of Al-Mn-Ce alloys with tailored properties [J]. Material and Design, 2016, 110: 436-448.
[15] AKOPYAN T K, BELOV N A, NAUMOVA E A, LETYAGIN N V. New in-situ Al matrix composites based on Al-Ni-La eutectic [J]. Materials Letters, 2019, 245: 110-113.
[16] BELOV N A, NAUMOVA E A, ALABIN A N, MATVEEVA I A. Effect of scandium on structure and hardening of Al-Ca eutectic alloys [J]. Journal of Alloys and Compounds, 2015, 646: 741-747.
[17] TIAN L, KIM H, ANDERSON I, RUSSELL A. The microstructure-strength relationship in a deformation processed Al-Ca composite [J]. Materials Science and Engineering A, 2013, 570: 106-113.
[18] BELOV N A, NAUMOVA E A, DOROSHENKO V V, AVXENTIEVA N N. Combined effect of calcium and silicon on the phase composition and structure of Al-10%Mg Alloy [J]. Russian Journal of Non-Ferrous Metals, 2018, 59(1): 67-75.
[19] BELOV N A, NAUMOVA E A, AKOPYAN T K, DOROSHENKO V V. Design of multicomponent aluminium alloy containing 2 wt% Ca and 0.1 wt% Sc for cast products [J]. Journal of Alloys and Compounds, 2018, 762: 528-536.
[20] CHAUBEY A K, SCUDINO S, MUKHOPADHYAY N K, KHOSHKHOO M S, MISHRAC B K, ECKERTA J. Effect of particle dispersion on the mechanical behavior of Al-based metal matrix composites reinforced with nanocrystalline Al-Ca intermetallics [J]. Journal of Alloys and Compounds, 2012, 536: 134-137.
[21] Jiang Yu-rong, Shi Xi, Bao Xiao-heng, He Ye, Huang Shuai-xiong, Wu Di, Bai Wei-min, Liu Li-bin, Zhang Li-gang. Experimental investigation and thermodynamic assessment of Al-Ca-Ni ternary system [J]. Journal of Materials Science, 2017, 52(20): 12409-12426.
[22] SUWANPREECHA C, PANDEE P, PATAKHAM U, LIMMANEEVICHITR C. New generation of eutectic Al-Ni casting alloys for elevated temperature services [J]. Materials Science and Engineering A, 2018, 709: 46-54.
[23] BELOV N A, ALABIN A N, MATVEEVA I A, ESKIN D G. Effect of Zr additions and annealing temperature on electrical conductivity and hardness of hot rolled Al sheets [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(9): 2817-2826.
[24] BELOV N A, NAUMOVA E A, ESKIN D G. Casting alloys of the Al-Ce-Ni system: Microstructural approach to alloy design [J]. Materials Science and Engineering A, 1999, 271: 134-142.
[25] BELOV N A, NAUMOVA E A, ALABIN A N, MATVEEVA I A. Effect of scandium on structure and hardening of Al-Ca eutectic alloys [J]. Journal of Alloys and Compounds, 2015, 646: 741-747.
[26] TOROPOVA L S, ESKIN D G, KHARAKTEROVA M L, DOBATKINA T V. Advanced aluminum alloys containing scandium: structure and properties [M]. Amsterdam: Gordon and Breach Science Publishers, 1998.
[27] JIA Zhi-hong, ROYSET J, SOLBERG J K, LIU Qing. Formation of precipitates and recrystallization resistance in AlScZr alloys [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(8): 1866-1871.
[28] ZHOU W W, CAI B, LI W J, LIU Z X, YANG S. Heat resistant Al-0.2Sc-0.04Zr electrical conductor [J]. Materials Science and Engineering A, 2012, 552: 353-358.
[29] BOOTH-MORRISON C, MAO Z, DIAZ M, DUNAND D C, WOLVERTON C, SEIDMAN D N. Role of silicon in accelerating the nucleation of Al3(Sc,Zr) precipitates in dilute Al-Sc-Zr alloys [J]. Acta Materialia, 2012, 60(12): 4740-4752.
[30] MARQUIS E A, SEIDMAN D N. Nanoscale structural evolution of Al3Sc precipitates in Al (Sc) alloys [J]. Acta Materialia, 2001, 49: 1909-1919.
[31] GUAN Ren-guo, JIN Hong-mei, JIANG Wen-sen, WANG Xiang, WANG Yu-xiang, LI Zheng, ZHANG Jian, LIU Hui-nan. Quantitative contributions of solution atoms, precipitates and deformation to microstructures and properties of Al-Sc-Zr alloys [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(5): 907-918.
[32] FULLER C B, SEIDMAN D N. Temporal evolution of the nanostructure of Al(Sc, Zr) alloys: Part II-Coarsening of Al3(Sc1-xZrx) precipitates [J]. Acta Materialia, 2005, 53: 5415-5428.
[33] KNIPLING K E, KARNESKY R A, LEE C P, DUNAND D C, SEIDMAN D N. Precipitation evolution in Al-0.1Sc, Al-0.1Zr and Al-0.1Sc-0.1Zr (at.%) alloys during isochronal ageing [J]. Acta Materialia, 2010; 58: 5184-5195.
[34] SHELEKHOV E V, SVIRIDOVA T A. Programs for X-ray analysis of polycrystals [J]. Metal Science and Heat Treatment, 2000, 42: 309-313.
[35] Information on www.thermocalc.com. [EB/OL]. 2019-03- 30.
[36] MONDOLFO L F. Aluminium alloys: Structure and properties [M]. London: Butterworths, 1976.
[37] MANYAKO M B, ZARECHNYUK O S, YANSON T I. Crystal structure of CaNiAl9 [J]. Soviet Physics Crystallography (translated from Kristallografiya), 1987, 32: 816-817.
[38] POLMEAR I J. Light metals: From traditional alloys to nanocrystals [M]. 4th ed. Oxford, UK: Elsevier, 2006.
[39] KNIPLING K E, DUNAND D C, SEIDMAN D N. Precipitation evolution in Al-Zr and Al-Zr-Ti alloys during aging at 450–600 °C [J]. Acta Materialia, 2008, 56: 1182-1195.
[40] ZHU A W, STARKE E A. Strengthening effect of unshearable particles of finite size: A computer experimental study [J]. Acta Materialia, 1999, 47: 3263-3269.
Torgom K. AKOPYAN, Nikolay A. BELOV, Evgeniya A. NAUMOVA, Nikolay V. LETYAGIN, Tat'yana A. SVIRIDOVA
National University of Science and Technology MISiS, 4 Leninsky pr., Moscow 119049, Russia
摘 要:结合Thermo-Calc热模拟程序计算和实验(电子显微镜、微探针分析和 X射线衍射)研究四元系Al-(2~4)Ca-Ni-La(质量分数,%)合金在铝角附近的结构。根据得到的相平衡数据,提出Al-Ca-Ni-La体系液相面和固相相场分布的实验投影。显微组织研究表明,含2%~4% Ca、2%~4% Ni和1%~3%La(质量分数)的合金具有超细的亚共晶组织,共晶金属间化合物的体积分数为30%,因此,可以将这些合金归类为天然Al基复合材料。超细共晶结构产生显著的强化作用,其机制可以很好地用修订的Orowan循环模型描述。在350~400 °C退火过程中,由于L12型相(Al3(Zr,Sc))纳米颗粒的形成,添加少量的Zr和Sc(分别为0.2%和0.1%,质量分数)具有显著的强化作用(提高约25%)。由于共晶金属间化合物的体积分数高,该新合金具有低的热膨胀系数、高的结构热稳定性和力学性能。
关键词:Al-Ca合金;共晶;金属间化合物;相图;稀土元素;纳米复合材料;显微组织;力学性能
(Edited by Bing YANG)
Corresponding author: Torgom K. AKOPYAN; E-mail: nemiroffandtor@yandex.ru
DOI: 10.1016/S1003-6326(20)65259-1

