改性海泡石作为循环使用的吸附剂在酸性溶液中吸附Pd(II)的应用
来源期刊:中国有色金属学报(英文版)2020年第5期
论文作者:肖勇 冯宁宁 王润华 董海刚 郭子文 崔浩 武海艳 刘新星 谢建平
文章页码:1375 - 1386
关键词:改性海泡石;钯;吸附;脱附;动力学
Key words:modified sepiolite; palladium; adsorption; desorption; dynamics
摘 要:研究以改性海泡石作为吸附剂从酸性溶液中回收Pd(II)的吸附特性和机理;通过等温模型、动力学和热力学模型分析改性海泡石对Pd(II)的吸附特性;利用SEM-EDS、TEM和XPS技术研究改性海泡石对Pd(II)的吸附机理。Langmuir模型表明,当温度为30 °C时,改性海泡石对Pd(II)的最大吸附量为322.58 mg/g。动力学实验结果表明,准二级动力学模型能较好地模拟改性海泡石对Pd(II)的吸附过程,化学吸附为改性海泡石吸附Pd(II)的控速步骤。当Pd(II)的初始浓度为100 mg/L时,1 g/L改性海泡石可吸附99%的Pd(II)。吸附-脱附循环实验结果表明,改性海泡石具有良好的稳定性和重复使用性。本研究结果表明,改性海泡石是一种可高效且经济的Pd(II) 回收材料。
Abstract: The adsorption characteristics and mechanisms of modified sepiolite as an adsorbent to recover Pd(II) from acidic solutions were studied. The Pd(II) adsorption properties were analyzed through isotherm, kinetic and thermodynamic models. In addition, SEM-EDS, TEM and XPS were applied to investigating the Pd(II) adsorption mechanisms onto modified sepiolite. The equilibrium data were well fitted to Langmuir isotherm model with maximum Pd(II) adsorption capacity of 322.58 mg/g at 30 °C. The kinetic data could be satisfactorily simulated by the pseudo- second order model, indicating that the rate-controlling step was chemical adsorption. 99% of Pd(II) could be recovered using 1 g/L modified sepiolite when initial concentration of Pd(II) was 100 mg/L. The results of reusability studies indicated the modified sepiolite had an acceptable stability and reusability. This study indicated that the modified sepiolite might be an efficient and cost-effective material for Pd(II) recovery.
Trans. Nonferrous Met. Soc. China 30(2020) 1375-1386
Yong XIAO1,2, Ning-ning FENG1,2, Run-hua WANG1,2, Hai-gang DONG3, Zi-wen GUO1,2, Hao CUI3, Hai-yan WU 1,2, Xin-xing LIU1,2, Jian-ping XIE1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China;
3. State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals, Kunming Institute of Precious Metals, Kunming 650106, China
Received 7 July 2019; accepted 9 April 2020
Abstract: The adsorption characteristics and mechanisms of modified sepiolite as an adsorbent to recover Pd(II) from acidic solutions were studied. The Pd(II) adsorption properties were analyzed through isotherm, kinetic and thermodynamic models. In addition, SEM-EDS, TEM and XPS were applied to investigating the Pd(II) adsorption mechanisms onto modified sepiolite. The equilibrium data were well fitted to Langmuir isotherm model with maximum Pd(II) adsorption capacity of 322.58 mg/g at 30 °C. The kinetic data could be satisfactorily simulated by the pseudo- second order model, indicating that the rate-controlling step was chemical adsorption. 99% of Pd(II) could be recovered using 1 g/L modified sepiolite when initial concentration of Pd(II) was 100 mg/L. The results of reusability studies indicated the modified sepiolite had an acceptable stability and reusability. This study indicated that the modified sepiolite might be an efficient and cost-effective material for Pd(II) recovery.
Key words: modified sepiolite; palladium; adsorption; desorption; dynamics
1 Introduction
The platinum group metals are widely used in petrochemical industry catalysts, electronics industry and other industry fields due to their high strength, good corrosion resistance and catalytic performance [1-4]. Additionally, the demands for precious metals, especially platinum group metals, have been steadily increasing, but their deposits are mainly stored in only a few countries, such as South Africa (63000 t), Russia (3900 t), USA (900 t), and Canada (310 t) [5]. Therefore, from an economic point of view, the effective Pd(II) recovery from abandoned resources is of great economic interest.
There were several methods available in the literature for recovering precious metals from aqueous solutions such as ion exchange, membrane filtration, evaporation, solvent extraction, foam separation, precipitation, and adsorption [6-10]. The choice of these methods to treat precious metals wastewater depended on factors such as the initial concentration of noble metals, environmental protection issues and operating costs [9]. Compared with other methods, adsorption was a more promising method for the recovery of noble metals from aqueous solutions due to its higher effectiveness, lower cost and easier operation [11]. Additionally, this method could effectively recover noble metals at lower solution concentrations and reduce or even eliminate the secondary wastes produced in existing processes. However, treatment efficiency greatly depended on the capacity of the adsorbent. Therefore, it was very important to develop effective and abundant adsorbents for palladium recovery from wastewater.
In recent decades, clay minerals have been widely utilized as adsorbents for wastewater treatment, mainly due to their high efficiency and regeneration [12,13]. Sepiolite is a naturally fibrous clay mineral and a complex magnesium silicate with a typical unit cell formula of (Si12Mg8O30(OH)4(H2O)4·8H2O) [14,15]. The mineral reserves of sepiolite are also abundant throughout the world, such as in China, USA, Japan, Spain and India [16,17]. Sepiolite is widely used as an adsorbent to recycle uranium, nickel, copper, zinc and cadmium in wastewater because of its specific structure, large surface area, and various functional groups, which are responsible for metal adsorption [18,19]. However, to our knowledge, to date, no studies have been reported on the adsorption mechanism of palladium using sepiolite.
Therefore, the objectives of the current study are as follows: (1) to characterize the microscopic properties of sepiolite by XRF, XRD, and FTIR; (2) to describe the Pd(II) adsorption behavior under equilibrium experimental conditions using various kinetic, isotherm and thermodynamic models; (3) to investigate the effects of time, ionic strength, temperature on Pd(II) recovery and adsorption- desorption on sepiolite; and (4) to explore the mechanisms of Pd(II) adsorption to sepiolite by SEM-EDS, TEM and XPS.
2 Experimental
2.1 Materials
Raw sepiolite was obtained from the Jingui Mining Industry Co., Ltd. (Shijiazhuang, China). In order to obtain the modified sepiolite, firstly, raw sepiolite was purified by incubation with 0.1 mol/L acetic acid overnight; secondly, the obtained product was washed three times with distilled water, and the solid was dried at 220 °C. The chemical composition (in mass fraction) of raw sepiolite measured by XRF was 49.48% SiO2, 27.66% CaO, 15.29% MgO, 3.71% Al2O3, 0.93% Na2O, 0.64% K2O and 0.27% Fe2O3. The stock Pd(II) solutions were prepared by dissolving PdCl2 (99.99% purity) into a concentrated HCl solution. Other reagents were analytic grade.
2.2 Batch adsorption experiments
The pH edge experiments were carried out by contacting 0.1 g of modified sepiolite with 100 mL of palladium solutions at an initial Pd(II) concentration of 100 mg/L and different pH values. Then, the modified sepiolite-containing solutions were stirred for 3 h at 170 r/min and 30 °C.
The adsorption kinetic experiments were conducted using the same method as above, except that the pH was set to be 2 and the samples were collected at different time intervals for analysis.
Biosorption isotherm experiments were carried out by contacting 0.05 g of modified sepiolite with 50 mL of Pd(II) solution. The initial Pd(II) concentration was varied from 50 to 400 mg/L and pH was maintained to be 2. Then, the suspensions were stirred for 2 h at 170 r/min and 30 °C.
The adsorption thermodynamic experiment was performed as follows: 50 mL of 200 mg/L Pd(II) solution was transferred into 150 mL conical flasks containing 0.05 g of modified sepiolite, and the initial pH was maintained to be 2. The resulting suspensions were stirred for 2 h at 30, 40 and 50 °C, respectively.
After adsorption, the samples were centrifuged, and the supernatants were separated for analysis. Inductively coupled plasma optical emission spectrometry (ICP-OES, Shimadzu, ICP-7510, Japan) was used to measure the concentration of Pd(II) remaining in the aqueous phase after adsorption. The adsorption capacity of the sorbent for Pd(II) (qe, mg/g) was calculated as follows:
 (1)
(1)
where C0 (mg/L) and Ce (mg/L) are the initial and final concentrations of Pd(II), respectively, V (L) is the working volume, and m (g) is the mass of modified sepiolite.
2.3 Desorption and reusability
In the desorption operation, firstly 100 mL of 200 mg/L Pd(II) solution was mixed with 0.1 g of modified sepiolite and adsorption operation was completed under optimum conditions. After 2 h of adsorption, the sample was centrifuged for solid-liquid separation, and then an equal volume of acidified thiourea (0.1 mol/L HCl and 0.1 mol/L thiourea) was added to the system. Finally, the Pd(II) released from the adsorbent into aqueous phase was collected and analyzed at different time to obtain the desorption curve for Pd(II).
In reusability operation, 100 mL of acidified thiourea (0.1 mol/L HCl and 0.1 mol/L thiourea) was used as the eluent to desorb Pd(II) from sepiolite. After 2 h of adsorption, the sample was centrifuged, and the supernatant was separated for analysis. The sepiolite remaining after elution was reused in the sorption procedures, and the process was repeated for five cycles to investigate the reusability of sepiolite for Pd(II) adsorption. The amount of desorbed Pd(II) was analyzed, and the desorption efficiency was calculated according to the following equation:
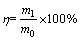 (2)
(2)
where η is the desorption efficiency, m1 is the amount of the desorbed Pd(II), and m0 is the amount of the initially adsorbed Pd(II).
2.4 Analytical methods
The mineralogical compositions of raw and modified sepiolite were identified by XRD (Scanting GLP-10195/07-S, Japan). The powder samples were scanned in the 2θ range of 5°-80° using a Ni-filtered Cu Kα radiation source at 40 kV and 150 mA. FTIR spectra were recorded in the wavenumber region of 400-4000 cm-1 on a KBr disc and measured on a Nicolet 6700 (USA) Fourier transform infrared spectrometer to identify the functional groups on the sepiolite surface. The surface morphologies of the samples were analyzed by SEM (Quanta-200, FEI, UK) at an accelerating voltage of 10 kV. Samples for SEM observations were prepared by coating with a thin film of gold before analysis. In addition, elemental analysis of the collected samples was conducted by EDS equipped on the Quanta-200. TEM analysis was performed using a Hitachi-7650 (Japan) at 100 kV. Ultrathin sections of biomass samples were obtained using an ultramicrotome and then placed onto a copper grid. XPS analysis was carried out by a Thermo Scientific ESCALAB 250 spectrometer (UK) using a monochromatic Al Kα X-ray source and a hemispherical analyzer.
3 Results and discussion
3.1 Characterization of adsorbent
XRD patterns of raw and modified sepiolite were presented in Fig. 1(a). The reflections indexed to the (331), (341), (530), (441) and (541) planes appearing at 2θ values of 28.19°, 29.31°, 30.35°, 33.51° and 40.12°, respectively, were consistent with the standard XRD data for the orthorhombic unitcell of sepiolite based on comparison with the standard pattern of sepiolite (JCPDS card No. 13-0595) [20,21]. The peak at 2θ value of 10.8° might be assigned to the (210) plane of talc (JCPDS card No. 19-0770) [22]. The peaks at 2θ values of 58.21° and 66.12° could be attributed to the (122) and (300) plane of calcite, respectively (JCPDS card No. 05-0586) [23]. After sepiolite was modified, the characteristic peaks of talc and calcite disappeared. The results showed that the raw sepiolite was purified by acetic acid.

Fig. 1 XRD patterns (a), FTIR spectra (b) and adsorption capacities (c) of raw and modified sepiolite (pH 2, C0=100 mg/L, V=100 mL)
Figure 1(b) showed the FTIR spectra of raw and modified sepiolite. The peaks of the raw sepiolite could be summarized as follows. The band at 3695 cm-1 was attributed to the octahedral Mg—OH groups [11]. The wide band around 3425 cm-1 was due to the O—H stretching vibration of zeolitic water [24]. The peak at 1645 cm-1 was represented the OH stretching vibration of the absorbed water [25]. The peak at 1428 cm-1 was corresponding to the calcite impurity [23]. The peak at 1018 cm-1 could be due to the Si—O—Si plane vibration [26]. The peak at 655 cm-1 was assigned to Mg—OH bending vibration [20]. The peak at 442 cm-1 could be assigned to the Si—O—Mg groups [24]. After acid treatment, the intensities of these peaks at 3695, 1645, and 655 cm-1 increased, respectively. At the same time, the peak at 1428 cm-1 representing calcite disappeared. These results also demonstrated that the raw sepiolite was purified by acetic acid.
As shown in Fig. 1(c), under the same adsorption conditions, the Pd(II) adsorption capacity by raw sepiolite was 79 mg/g, and that of the modified sepiolite was 103 mg/g. The results indicated that the modified sepiolite could increase the adsorption amount for Pd(II).
3.2 Effect of pH on adsorption of Pd(II) by modified sepiolite
The effect of pH on the adsorption performance of Pd(II) on modified sepiolite was shown in Fig. 2. The adsorption capacity of Pd(II) on modified sepiolite was significantly affected by the pH value of the system. The adsorption capacity of Pd(II) increased from 5.12 mg/g (pH 1) to 101.89 mg/g (pH 3), which was increased by approximately 20 times. The reason might be that at higher pH, the amount of negative charges on the surface of sepiolite also increased, which enhanced the adsorption capacity of the modified sepiolite through electrostatic attraction.
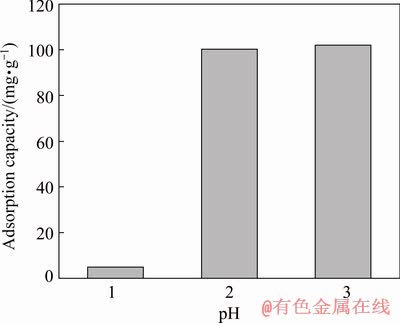
Fig. 2 Effect of pH on adsorption capacity of Pd(II) by modified sepiolite
3.3 Adsorption behavior of modified Pd(II) and sepiolite
3.3.1 Adsorption isotherms
Empirical models such as the Langmuir and Freundlich models could verify the biosorption mechanisms, and they were useful for estimating the maximum adsorption capacity. In these models, the isothermal adsorption curves were expressed as follows [27]:
Langmuir model: 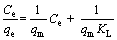 (3)
(3)
Freundlich model: 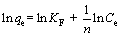 (4)
(4)
where qe is the amount of adsorbed metal (mg/g), Ce is the concentration of metal ion at equilibrium (mg/L), qm is the maximum adsorption capacity (mg/g), KL is the Langmuir constant (L/mg), KF is the Freundlich constant related to adsorption capacity (mg/g), and n is the Freundlich constant.
The linear Langmuir and Freundlich isotherms of Pd(II) were shown in Fig. 3, and the parameters of isotherm were presented in Table 1. The correlation coefficient (R2) of Langmuir model was 0.995, which was higher than that of Freundlich model (0.972). The maximum uptake capacity (qm) according to the Langmuir model was 322.58 mg/g. In addition, the experimental data of Pd(II) adsorption on modified sepiolite fitted better to the Langmuir isotherm model. Therefore, these evidences could provide that the Langmuir model was more suitable for describing the adsorption of Pd(II) on modified sepiolite. The results indicated that the biosorption process was dominated by monolayer adsorption [28].

Fig. 3 Fitted curves of Langmuir (a) and Freundlich (b) models for Pd(II) adsorption by sepiolite
Table 1 Isotherm parameters of Langmuir and Freundlich models for Pd(II) adsorption by modified sepiolite
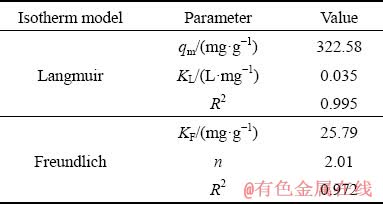
Generally, high maximum adsorption capacity (qm) and affinity (RL) were regarded as good qualities for an adsorbent. The value of RL indicated the affinity of the adsorption process, which could be unfavorable (RL>1), linear (RL=1), favorable (0
Table 2 RL values based on Langmuir equation

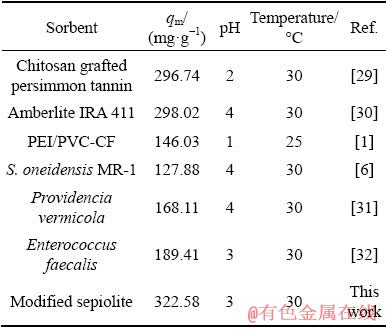
Table 3 showed that the qm values of Pd(II) for various adsorbents, such as Amberlite IRA 411, PEI/PVC-CF, S. oneidensis MR-1 and Enterococcus faecalis were 298.02, 146.03, 127.88 and 189.41 mg/g, respectively, which were lower than that of the modified sepiolite. These results indicated that the modified sepiolite could be considered as an effective adsorbent for Pd(II).
Table 3 Maximum adsorption capacities of Pd(II) for different biosorbents
3.3.2 Adsorption kinetics and thermodynamics
The kinetic experiments were performed to identify the mechanism of adsorption reactions and assess the adsorption performance in the adsorption process [33]. The experimental data were described by pseudo-first-order and pseudo-second-order kinetic models. These models were expressed as follows [34]:
Pseudo-first-order model:
 (5)
(5)
Pseudo-second-order model:
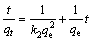 (6)
(6)
where qt and qe (mg/g) represent the adsorption capacity at time t and adsorption capacity in equilibrium, respectively. k1 (min-1) and k2 (g/(mg·min)) are the pseudo-first-order and pseudo second-order rate constants, respectively.
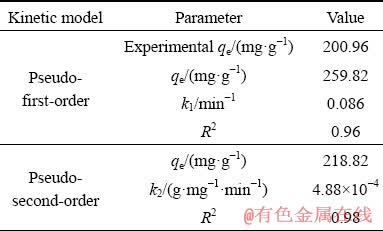
The values of kinetic parameters and correlation coefficients (R2) from both models were presented in Table 4. The correlation coefficient (R2) of the pseudo-second-order model (0.98) was higher than that of the pseudo-first-order model (0.96). In addition, the value of qe estimated through the pseudo-second-order model (218.82 mg/g) was closer to the experimental qe value (200.96 mg/g) than that of the pseudo-first- order model (259.82 mg/g). Furthermore, Fig. 4 also showed that the simulated curve fitted the experimental data well by pseudo-second-order model. Therefore, the adsorption kinetics of Pd(II) on modified sepiolite could be better predicted by pseudo-second-order model. This indicated that the controlling step of Pd(II) adsorption by modified sepiolite might be chemical adsorption, which could involve valence forces through the sharing or exchange of electrons between adsorbent and sorbate [35].
Table 4 Kinetic parameters of pseudo-first-order and pseudo-second-order models for Pd(II) adsorption by modified sepiolite
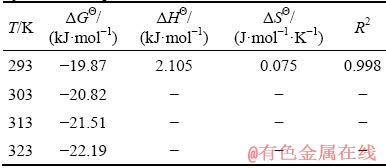
Thermodynamic parameters were used to investigate the nature of the adsorption. The Gibbs free energy change ΔGΘ (kJ/mol), enthalpy change ΔHΘ (kJ/mol), and entropy change ΔSΘ (J/(mol·K)) were calculated according to the following equations [36]:
ΔGΘ=-RTln KL (7)
ΔGΘ=ΔHΘ-TΔSΘ (8)
where R is the mole gas constant (8.314 J/(mol·K), T is the temperature (K), KL is the equilibrium constant of Langmuir, ΔHΘ is the enthalpy change, and ΔSΘ is the entropy change.

Fig. 4 Fitted curves of pseudo-first-order (a) and pseudo- second-order models (b) for Pd(II) biosorption on modified sepiolite
Figure 5 showed a linear relationship in the plot of ΔGΘ versus T. As can be seen from Table 5, the ΔGΘ value was negative, meaning the feasible and spontaneous nature of Pd(II) adsorption on modified sepiolite. The positive value of ΔHΘ (2.105 kJ/mol) demonstrated the present adsorption was an endothermic process [37]. The ΔSΘ value was 0.075 J/(mol·K), which indicated the increased randomness at the solid-solution interface during the adsorption process.

Fig. 5 Plot of ΔGΘ versus T to estimate of thermo- dynamic parameters for Pd(II) adsorption by modified sepiolite
Table 5 Parameters of thermodynamic model for Pd(II) by modified sepiolite
3.4 Desorption and reusability
From an economic point of view, the reusability of an adsorbent was a very important parameter. According to recent literature, acidified thiourea has been used to elute Pd(II) from sorbents with high efficiency [38]. AWUAL et al [38,39] used a mixed solution of 0.1 mol/L HCl with thiourea to recover Pd(II) from loaded adsorbent with high efficiency (>95%) and without damaging the material. Therefore, the desorption efficiency of Pd-loaded sepiolite was investigated using acidified thiourea (0.1 mol/L HCl and 0.1 mol/L thiourea). As can be seen from Fig. 6(a), the released Pd(II) concentration dramatically increased in the first 30 min. The desorption efficiency increased up to 99% at about 60 min. The results revealed that mixed solution (0.1 mol/L HCl and 0.1 mol/L thiourea) was suitable to desorb the Pd(II) from the adsorbent.
In the reuse study, the mixed solution (0.1 mol/L HCl and 0.1 mol/L thiourea) was selected to evaluate the reusability of modified sepiolite. The adsorption-desorption experiment for Pd(II) on the modified sepiolite was performed consecutively 5 times. The data clarified that the adsorption efficiency decreased slightly after five cycles as shown in Fig. 6(b). The result indicated that modified sepiolite as an absorbent had high mechanical stability and functionality in adsorption-desorption process.

Fig. 6 Relationships of released Pd(II) concentration versus time (a) and modified sepiolite adsorption/ desorption efficiency versus cycle number (b)
3.5 Adsorption mechanism for Pd(II) by modified sepiolite
3.5.1 SEM-EDS images and EDS spectra
The morphologies and microstructures of the modified sepiolite before and after Pd(II) adsorption were characterized by SEM-EDS. Figure 7(a) showed the smooth surfaces of modified sepiolite. As shown in Fig. 7(b), the surfaces of the sepiolite after Pd(II) adsorption became irregular and rough. Figures 7(c) and (d) showed the EDS spectra of the modified sepiolite before and after Pd(II) adsorption, respectively. The sample treated with PdCl2 solution showed peaks of palladium and chloride, further confirming that Pd(II) could be adsorbed by the modified sepiolite. Additionally, Table 6 indicated the mass fractions of palladium and chloride reached 27.38% and 2.03%, respectively. These results suggested the sediment on the sepiolite surface might be a form of palladium complexes.

Fig. 7 SEM images (a, b) and EDS spectra (c, d) of modified sepiolite before (a, c) and after (b, d) Pd(II) adsorption
Table 6 Surface elemental compositions of Pd(II)-loaded modified sepiolite by SEM-EDS analysis

3.5.2 TEM images
The morphology changes of modified sepiolite before and after Pd(II) adsorption were analyzed by TEM (Fig. 8). As shown in Figs. 8(b) and (c), the metal precipitates could be clearly observed on the modified sepiolite surface after Pd(II) adsorption. In addition, small particles were detected on the modified sepiolite surface (Fig. 8(d)). This phenomenon could be explained that hydroxyl group on modified sepiolite surface was capable of reducing Pd(II) to Pd(0) through the strong reduction power. In other research studies a similar adsorption-reduction mechanism was also reported [40-42].
Therefore, the Pd(II) adsorption on modified sepiolite could occur in two steps: (1) the initial adsorption of Pd(II) trapped by apertures on the sepiolite fiber; (2) Pd precipitates (Pd nanoparticles) formed by reduction and surface complexation by functional group on the modified sepiolite fiber.
3.5.3 XPS spertra
The elemental compositions of samples before and after Pd(II) adsorption and the chemical status of recovered palladium on the modified sepiolite surface were determined by XPS analysis. As shown in Figs. 9(a) and (b), C, N, O, Mg, Ca, K and Si could be found in the survey spectrum of modified sepiolite, and Pd element was detected on the modified sepiolite fiber surface after adsorption. Figure 9(c) illustrated the Pd 3d photoelectron peak. The curve fitting of the Pd 3d core-level spectrum was performed by two spin-orbit split Pd 3d5/2 and Pd 3d3/2 components [43]. The characteristic peaks corresponding to Pd 3d5/2 and 3d3/2 appeared at 337.8 and 343.2 eV, respectively. Each peak was divided into two different palladium states of Pd(II) and Pd(0). From Fig. 9(c), two Pd 3d5/2 components appeared at binding energies of 335.8 and 337.2 eV, which were assigned to Pd(0) and Pd(II) species, respectively [44]. The binding energies of two Pd 3d3/2 components were at 341.3 and 342.8 eV, which were assigned to Pd(0) and Pd(II) species, respectively [45]. There was only one way to produce Pd(0) during the adsorption process: Pd(II) anchored on the surface of the modified sepiolite was reduced to Pd(0) by a functional group. This explanation was consistent with the result of TEM-EDS.

Fig. 8 TEM images of modified sepiolite (a), Pd-loaded modified sepiolite (b, c) and Pd nanoparticles (d)
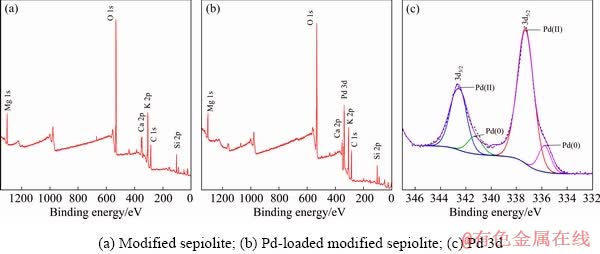
Fig. 9 XPS spectra of modified sepiolite fiber
4 Conclusions
(1) Through the Langmuir isotherm analysis, modified sepiolite showed the maximum Pd(II) adsorption capacity of 322.58 mg/g.
(2) Kinetic studies indicated the experimental data were fitted well by pseudo-second-order model.
(3) The results of reusability studies demonstrated that the mixed solution (0.1 mol/L HCl and 0.1 mol/L thiourea) was an excellent eluent for desorbing Pd(II) from modified sepiolite.
(4) The results of SEM-EDS, TEM and XPS confirmed that the mechanism of Pd(II) adsorption by modified sepiolite might complicated, and ion exchange, electrostatic adsorption and reduction were involved in the adsorption process.
References
[1] CHOI H A, PARK H N, WON S W. A reusable adsorbent polyethylenimine/polyvinyl chloride crosslinked fiber for Pd(II) recovery from acidic solutions [J]. Journal of Environmental Management, 2017, 204: 200-206.
[2] GALLUCCI F, FERNANDEZ E, CORENGIA P, ANNALAND M V S. Recent advances on membranes and membrane reactors for hydrogen production [J]. Chemical Engineering Science, 2013, 92(14): 40-66.
[3] CATALDO S, GIANGUZZA A, PETTIGNANO A. Sorption of Pd(II) ion by calcium alginate gel beads at different chloride concentrations and pH: A kinetic and equilibrium study [J]. Arabian Journal of Chemistry, 2016, 9: 656-667.
[4] FIRMANSYAH M L, KUBOTA F, YOSHIDA W, GOTO M. Application of a novel phosphonium-based ionic liquid to the separation of platinum group metals from automobile catalyst leach liquor [J]. Industrial & Engineering Chemistry Research, 2017, 9: 3845-3852.
[5] MORCALI M H, ZEYTUNCU B. Investigation of adsorption parameters for platinum and palladium onto a modified polyacrylonitrile-based sorbent [J]. International Journal of Mineral Processing, 2015, 137: 52-58.
[6] XU H, TAN L, CUI H, XU M Y, XIAO Y, WU H Y, DONG H G, LIU X X, QIU G Z, XIE J P. Characterization of Pd(II) biosorption in aqueous solution by Shewanella oneidensis MR-1 [J]. Journal of Molecular Liquids, 2018, 255: 333-340.
[7] CHEN H, GUAN X, LIN Z, PAN X H. Investigation of lead(II) uptake by Bacillus thuringiensis 016 [J]. World Journal of Microbiology and Biotechnology, 2015, 31: 1729-1736.
[8] WON S W, LIM A, YUN Y S. Recovery of high-purity metallic Pd from Pd(II)-sorbed biosorbents by incineration [J]. Bioresource Technology, 2013, 137: 400-403.
[9] NAGIREDDI S, KATIYAR V, UPPALURI R. Pd(II) adsorption characteristics of glutaraldehyde cross-linked chitosan copolymer resin [J]. International Journal of Biological Macromolecules, 2017, 94: 72-84.
[10] LIU P, LIU G F, CHEN D L, CHENG S Y, TANG N. Adsorption properties of Ag(I), Au(III), Pd(II) and Pt(IV) ions on commercial 717 anion-exchange resin [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(6): 1509-1513.
[11] JING Q X, CHAI L Y, HUANG X D, TANG C J, GUO H, WANG W. Behavior of ammonium adsorption by clay mineral halloysite [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(7): 1627-1635.
[12] SUN Y B, LI J X, WANG X K. The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques [J]. Geochimica et Cosmochimica Acta, 2014, 140: 621-643.
[13] ZHANG H X, WANG X Y, LIANG H H, TAN T S, WU W S. Adsorption behavior of Th(IV) onto illite: Effect of contact time, pH value, ionic strength, humic acid and temperature [J]. Applied Clay Science, 2016, 127-128: 35-43.
[14] SABAH E, TURAN M, CELIK M S. Adsorption mechanism of cationic surfactants onto acid- and heat-activated sepiolites [J]. Water Research, 2002, 36(16): 3957-3964.
[15] LEMIC J, TOMASEVIC C M, DJURICIC M, STANIC T. Surface modification of sepiolite with quaternary amines [J]. Journal of Colloid and Interface Science, 2005, 292: 11-19.
[16] BENLI B, YALM C. The influence of silver and copper ions on the antibacterial activity and local electrical properties of single sepiolite fiber: A conductive atomic force microscopy (C-AFM) study [J]. Applied Clay Science, 2017, 146: 449-456.
[17] TEKIN N, KAYA A U, ESMER K, KARA A. Adsorption and dielectric properties of poly(1-vinylimidazole) on sepiolite [J]. Applied Clay Science, 2012, 57: 32-38.
[18] GREGORIO M C D, NEEFF D V D, JAGER A V, CORASSIN C H, CARAO A C D P, ALBUQUERQUE R D, AZEVEDO A C D, OLIVEIRA C A F. Mineral adsorbents for prevention of mycotoxins in animal feeds [J]. Toxin Reviews, 2014, 33(3): 1-11.
[19] WANG Z H, LINA L, ANDREW H, NA S, REN B. Sepiolite-based adsorbents for the removal of potentially toxic elements from water: A strategic review for the case of environmental contamination in Hunan, China [J]. International Journal of Environmental Research and Public Health, 2018, 15: 1660-4601.
[20] FAYAZI M, AFZALI D, TAHER M A, MOSTAFAVI A, GUPTA V K. Removal of safranin dye from aqueous solution using magnetic mesoporous clay: Optimization study [J]. Journal of Molecular Liquids, 2015, 212: 675-685.
[21] HU B W, HU Q Y, CHEN C G, SUN Y B, XU D, SHENG G D. New insights into Th(IV) speciation on sepiolite: Evidence for EXAFS and modeling investigation [J]. Chemical Engineering Journal, 2017, 322: 66-72.
[22] WU X P, ZHANG Q X, LIU C, ZHANG X L, CHUNG D D. Carbon-coated sepiolite clay fibers with acid pre-treatment as low-cost organic adsorbents [J]. Carbon, 123: 259-272.
[23] QIU Y, YU S M, SONG Y F, WANG Q, ZHONG S S, TIAN W X. Investigation of solution chemistry effects on sorption behavior of Sr(II) on sepiolite fibers [J]. Journal of Molecular Liquids, 2013, 180: 244-251.
[24] FAYAZI M. Facile hydrothermal synthesis of magnetic sepiolite clay for removal of Pb(II) from aqueous solutions [J]. Analytical and Bioanalytical Chemistry Research, 2019, 6: 125-136.
[25] SOHEILMOGHADDAM M, WAHIT M U, YUSSUF A A, AL-SALEH M A, WHYE W T. Characterization of bio regenerated cellulose/sepiolite nanocomposite films prepared via ionic liquid [J]. Polymer Testing, 2014, 33: 121-130.
[26] FAYAZI M, AFZALI D, REZA G M, IRAJI A. Synthesis of novel sepiolite–iron oxide–manganese dioxide nano- composite and application for lead(II) removal from aqueous solutions [J]. Environmental Science and Pollution Research, 2019, 26: 18893-18903.
[27] XU H, TAN L, DONG H G, HE J, LIU X X, QIU, G Z, HE Q F,XIE J P. Competitive biosorption behavior of Pt(IV) and Pd(II) by Providencia vermicola [J]. RSC Advances, 2018, 7: 32229-32235.
[28] SARI A, MENDIL D, TUZEN M, SOYLAK M. Biosorption of palladium(II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies [J]. Journal of Hazardous Materials, 2009, 162(2-3): 874-879.
[29] ZHOU Z, LIU F, HUANG Y, WANG Z, LI G. Biosorption of palladium(II) from aqueous solution by grafting chitosan on persimmon tannin extract [J]. International Journal of Biological Macromolecules, 2015, 77: 336-343.
[30] GANDHI M R, YAMADA M, KONDO Y, SHIBAYAMA A, HAMADA F. p-Sulfonatothiacalix[6]arene-impregnated resins for the sorption of platinum group metals and effective separation of palladium from automotive catalyst residue [J]. Journal of Industrial and Engineering Chemistry, 2015, 30: 20-28.
[31] TAN L, DONG H G, LIU X X, HE J, XU H, XIE J P. Mechanism of palladium(II) biosorption by Providencia vermicola [J]. RSC Advances, 2017, 7: 7060-7072.
[32] CUI J Y, ZHU N W, KANG N X, HA C, SHI C H, WU P X. Biorecovery mechanism of palladium as nanoparticles by Enterococcus faecalis: From biosorption to bioreduction [J]. Chemical Engineering Journal, 2017, 328: 1051-1057.
[33] FUJIWARA K, RAMESH A, MAKI T, HASEGAWA H, UEDA K. Adsorption of platinum(IV), palladium(II) and gold(III) from aqueous solutions onto glycine modified crosslinked chitosan resin [J]. Journal of Hazardous Materials, 2007, 146(1-2): 39-50.
[34] YANG S B, DING C C, CHENG W C, JIN Z X, SUN Y B. Effect of microbes on Ni(II) diffusion onto sepiolite [J]. Journal of Molecular Liquids, 2015, 204: 170-175.
[35] GONG R M, ZHU S X, ZHANG D, CHEN J, NI S J, GUAN R. Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: Kinetic and thermodynamic profile [J]. Desalination, 2008, 230: 220-228.
[36] WU L S, YE Y, LIU F Q, TAN C M, LIU H, WANG S F, WANG J, YI W J, WU W S. Organo-bentonite-Fe3O4 poly (sodium acrylate) magnetic superabsorbent nanocomposite: Synthesis, characterization, and thorium(IV) adsorption [J]. Applied Clay Science, 2013, 84: 405-414.
[37] HESHMATI H, GILANI H G, TORAB-MOSTAEDI M, HAIDARY A. Adsorptive removal of thorium(IV) from aqueous solutions using synthesized polyamidoxime chelating resin: Equilibrium, kinetic, and thermodynamic studies [J]. Journal of Dispersion Science and Technology, 2014, 35(4): 501-509.
[38] AWUAL M R, KHALEQUE M A, RATNA Y, ZNAD H. Simultaneous ultra-trace palladium(II) detection and recovery from wastewater using new class meso-adsorbent [J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 405-413.
[39] AWUAL M R. Solid phase sensitive palladium(II) ions detection and recovery using ligand based efficient conjugate nanomaterials [J]. Chemical Engineering Journal, 2016, 300: 264-272.
[40] MELE E, ANYFANTIS G C, FRAGOULI D, RUFFILLI R, ATHANASSIOU A. Localized synthesis of gold nanoparticles in anisotropic alginate structures [J]. RSC Advances, 2014, 4: 20449-20453.
[41] GAO X P, ZHANG Y, ZHAO Y M. Biosorption and reduction of Au(III) to gold nanoparticles by thiourea modified alginate [J]. Carbohydrate Polymers, 2018, 159: 108-115.
[42] TAO R T, SUN Z Y, XIE Y, ZHANG H Y, HUANG C L, ZHAO Y F, LIU Z M. In situ loading of palladium nanoparticles on mica and their catalytic applications [J]. Journal of Colloid and Interface Science, 2011, 353(1): 269-274.
[43] TUO Y, LIU G, DONG B, YU H, ZHOU J, WANG J, JIN R. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol [J]. Environmental Science and Pollution Research, 2016, 24(6): 1-10.
[44] RAMAKRISHNAN A, DUMBUYA K, OFILI J, STEINRCK H P, GOTTFRIED J M, SCHWIEGER W. Highly dispersed Pd nanoparticles within silica: Synthesis and characterization [J]. Applied Clay Science, 2011, 51: 8-14.
[45] WAN J, RAN R, WU X D, CAO Y D, LI M, WENG D, HUANG K S. Re-dispersion of Pd on Ce0.5Zr0.5O2 upon cooling in the presence of oxygen [J]. Catalysis Today, 2015, 253: 51-56.
肖 勇1,2,冯宁宁1,2,王润华1,2,董海刚3,郭子文1,2,崔 浩3,武海艳1,2,刘新星1,2,谢建平1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083;
3. 昆明贵金属研究所 铂金属综合利用先进技术国家重点实验室,昆明 650106
摘 要:研究以改性海泡石作为吸附剂从酸性溶液中回收Pd(II)的吸附特性和机理;通过等温模型、动力学和热力学模型分析改性海泡石对Pd(II)的吸附特性;利用SEM-EDS、TEM和XPS技术研究改性海泡石对Pd(II)的吸附机理。Langmuir模型表明,当温度为30 °C时,改性海泡石对Pd(II)的最大吸附量为322.58 mg/g。动力学实验结果表明,准二级动力学模型能较好地模拟改性海泡石对Pd(II)的吸附过程,化学吸附为改性海泡石吸附Pd(II)的控速步骤。当Pd(II)的初始浓度为100 mg/L时,1 g/L改性海泡石可吸附99%的Pd(II)。吸附-脱附循环实验结果表明,改性海泡石具有良好的稳定性和重复使用性。本研究结果表明,改性海泡石是一种可高效且经济的Pd(II) 回收材料。
关键词:改性海泡石;钯;吸附;脱附;动力学
(Edited by Wei-ping CHEN)
Foundation item: Projects (51871250, 51504106) supported by the National Natural Science Foundation of China; Project (SKL-SPM- 201809) supported by the State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals, China; Projects (502211852, 502211906) supported by the Fundamental Research Funds for the Central Universities of Central South University, China; Project (SKYAM005-2016) supported by State Key Laboratory of Applied Microbiology Southern China; Projects (2015FB204, 2016BA006, 2017FA030) supported by the Yunnan Science and Technology Plan Project of China
Corresponding author: Jian-ping XIE; E-mail: xiejianping@csu.edu.cn
DOI: 10.1016/S1003-6326(20)65303-1

