Adsorption isotherm and inhibition effect of a synthesized di-(m-Formylphenol)-1,2-cyclohexandiimine on corrosion of steel X52 in HCl solution
来源期刊:中南大学学报(英文版)2016年第2期
论文作者:A. karimi I. Danaee H. Eskandari M. RashvanAvei
文章页码:249 - 257
Key words:corrosion; inhibitor; Schiff base; adsorption; Langmuir adsorption isotherm
Abstract: The potential of di-(m-Formylphenol)-1,2-cyclohexandiimine as an environmentally friendly corrosion inhibitor for steel was investigated in 1 mol/L HCl using potentiodynamic polarization, electrochemical impedance spectroscopy and chronoamperometry measurements. All electrochemical measurements suggest that this compound is an excellent corrosion inhibitor for mild steel and the inhibition efficiency increases with the increase in inhibitor concentration. The effect of temperature on the corrosion behavior of mild steel with the addition of the Schiff base was studied in the temperature range from 25 °C to 65 °C. It is found that the adsorption of this inhibitor follows the Langmuir adsorption isotherms. The value of activation energy and the thermodynamic parameters such as ΔHads, ΔSads, Kads and ΔGads were calculated by the corrosion currents at different temperatures using the adsorption isotherm. The morphology of mild steel surface in the absence and presence of inhibitor was examined by scanning electron microscopy (SEM) images.
J. Cent. South Univ. (2016) 23: 249-257
DOI: 10.1007/s11771-016-3068-2
A. karimi1, I. Danaee1, H. Eskandari1, M. RashvanAvei2
1. Abadan Faculty of Petroleum Engineering, Petroleum University of Technology, Abadan, Iran;
2. Department of Chemistry, K. N. Toosi University of Technology, Tehran, Iran
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: The potential of di-(m-Formylphenol)-1,2-cyclohexandiimine as an environmentally friendly corrosion inhibitor for steel was investigated in 1 mol/L HCl using potentiodynamic polarization, electrochemical impedance spectroscopy and chronoamperometry measurements. All electrochemical measurements suggest that this compound is an excellent corrosion inhibitor for mild steel and the inhibition efficiency increases with the increase in inhibitor concentration. The effect of temperature on the corrosion behavior of mild steel with the addition of the Schiff base was studied in the temperature range from 25 °C to 65 °C. It is found that the adsorption of this inhibitor follows the Langmuir adsorption isotherms. The value of activation energy and the thermodynamic parameters such as ΔHads, ΔSads, Kads and ΔGads were calculated by the corrosion currents at different temperatures using the adsorption isotherm. The morphology of mild steel surface in the absence and presence of inhibitor was examined by scanning electron microscopy (SEM) images.
Key words: corrosion; inhibitor; Schiff base; adsorption; Langmuir adsorption isotherm
1 Introduction
Corrosion is a fundamental process playing an important role in economics and safety, particularly for metals and alloys. Steel has found wide application in a broad spectrum of industries and machinery [1–2]. Steel X52 is used for pipeline transportation systems in the petroleum and natural gas industries. Internal corrosion in wells and pipelines is influenced by different parameters such as acidic content due to CO2 and H2S. Moreover, in most industrial processes, acidic solutions are commonly used for the pickling, industrial acid cleaning, acid descaling, oil well acidifying, etc. Unfortunately, iron and its alloys could corrode during these acidic applications particularly with the use of hydrochloric acid and sulphuric acid, which results in terrible waste of both resources and money [3–4].
Corrosion prevention systems favor the use of corrosion inhibitors with low or zero environmental impacts. Inhibitors are chemicals that react with a metallic surface or the environment. Inhibitors decrease corrosion processes by increasing the anodic or cathodic polarization behavior, decreasing the movement or diffusion of ions to the metallic surface and increasing the electrical resistance of the metallic surface [5–6].
The inhibiting actions of organic compounds are usually attributed to their interactions with the metal surface via their adsorption. These compounds in general are adsorbed on the metal surface, blocking the active corrosion sites. Three types of adsorption may take place by organic molecules at metal/solution interface: (1) electrostatic attraction between the charged molecules and the charged metal, (2) interaction of unshared electron pairs or π electron in the molecule with the metal, and (3) combination of (1) and (2) [7–8].
The choice of the inhibitor is based on two considerations, first is economic consideration, and second should contain the electron cloud on the aromatic ring or the electronegative atoms such as N, O in the relatively long chain compounds. Generally, the organic compounds containing hetero atoms like O, N, S, and P are found to work as very effective corrosion inhibitors. The efficiency of these compounds depends upon electron density present around the hetero atoms, the number of adsorption active centers in the molecule and their charge density, molecular size, mode of adsorption, and formation of metallic complexes [9–11]. These features obviously can be combined within the same molecule such as Schiff bases. In the past few years, the inhibition of various metals such as iron, copper, zinc, aluminum, and carbon steel corrosion in acid solutions by Schiff bases with environmental considerations has attracted more attention [12–13]. Schiff bases can conveniently be synthesized from relatively cheap starting materials. It is also reported that Schiff bases show more inhibition efficiency than corresponding amines [14].
The aim of the present work is to investigate the effect of synthesized Schiff base corrosion inhibitor di-(m-Formylphenol)-1,2-cyclohexandiimine on the corrosion of mild steel in 1 mol/L HCl, evaluated by Tafel polarization and electrochemical impedance spectroscopy (EIS) data. The effects of concentration and temperature on the inhibition efficiencies of the selected Schiff base were studied. Thermodynamic parameters such as enthalpy, entropy and Gibbs free energy were calculated from experimental data of the inhibition process at different temperature.
2 Materials and methods
2.1 Preparation of di-(m-Formylphenol)-1,2- cyclohexandiimine (m-FP-DACH)
All chemicals used in the present work were of reagent-grade Merck product and used as received without further purification. The m-FP-DACH Schiff base (Fig. 1) was prepared in high yield (97%) by the condensation of m-Formylphenol (0.244 g, 2 mmol) with 1,2- Cyclohexanediamine (0.114 g, 1 mmol) in a stirred ethanolic solution and heated to reflux for 5 h according to the described procedure [6]. The resulting precipitate was filtered off, washed with warm ethanol and diethyl ether. Identification of structure of synthesized Schiff base was performed by IR and 1HNMR spectroscopy and elemental analysis. The resulting Schiff bases are shown in Fig. 1.

Fig. 1 General structure of investigated Schiff bases
2.2 Sample preparation
Working electrodes were prepared from steel X52 specimens of the chemical composition: C 0.3, Mn 1.350, P 0.03, S 0.03 (mass fraction, %), and the balance Fe. Samples were cut from a flat plate by the wire cut method. The dimension of each sample was 1 cm×1cm. Working electrodes were prepared by embedding the sample in epoxy resin, exposing a geometrical surface area of 1 cm2 to the aggressive electrolyte. The exposed areas of the electrodes were mechanically abraded with 400, 800, 1200 and 2000 grades of emery paper, rinsed by distilled water and degreased with acetone before each electrochemical experiment.
The measurements were performed in 1 mol/L HCl in the absence and presence of Schiff bases in the concentration range from 5×10–5 to 2×10–3 mol/L. Distilled water was used to prepare the test solutions.
2.3 Electrochemical measurements
Electrochemical measurements were carried out in a conventional three-electrode system. The reference electrode was a saturated calomel electrode (SCE) and the auxiliary electrode was a platinum sheet. All experiments were repeated three times. To reach the steady state condition, before each experiment, the working electrode was immersed in the test cell for 20 min. The electrochemical measurements were carried out using computer controlled ZAHNER potentiostat/ galvanostat. Electrochemical impedance spectroscopy (EIS) was recorded in frequency range of 100 kHz to 10 mHz with 0.01 V peak-to-peak amplitude using AC signals at open circuit potential. Polarization curves were scanned at a scan rate of 1 mV/s from –700 mV to –100 mV. All tests were done at constant temperature by controlling the cell temperature using a water bath. Polarization curve was used to calculate corrosion current density by the Tafel extrapolation method. The polarization resistance was calculated from the slope of the potential versus logarithm of current plots. Fitting of experimental impedance spectroscopy data to the proposed equivalent circuit was done by means of home written least square software based on the Marquardt method for the optimization of functions and Macdonald weighting for the real and imaginary parts of the impedance [15–16].
The morphology of steel surface after 12 h exposure to 1 mol/L HCl solution in the absence and presence of 2×10–3 mol/L m-FP-DACH was observed by scanning electron microscope (SEM) model VEGA\TESCAN.
3 Results and discussion
3.1 Tafel polarization measurements
Figure 2 shows the anodic and cathodic polarization plots of steel in 1 mol/L HCl in the absence and presence of different concentrations of inhibitor at 25 °C. Table 1 gives the electrochemical corrosion parameters such as corrosion potential (Ecorr vs SCE), corrosion current density (Jcorr), cathodic and anodic Tafel slopes (βa, βc), the degree of surface coverage (θ) and inhibition efficiency (IE, Ei) obtained by extrapolation of the Tafel lines. The degree of surface coverage and inhibition efficiency are calculated using the following equations [17]:
 (1)
(1)
Ei=θ (2)
where 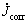 and Jcorr are the corrosion current densities determined by the intersection of the extrapolated Tafel lines in uninhibited and inhibited acid solution, respectively. Both the anodic and cathodic reactions of mild steel corrosion are inhibited in the presence of the inhibitor. This result suggests that the addition of the m-FP-DACH decreases the anodic dissolution and also retards the hydrogen evolution reaction. It can be seen that the IE increases by increasing inhibitor concentration which indicates that more inhibitor molecule are absorbed on the metal surface and provide wider surface coverage. Thus, these compounds are acting as an adsorption inhibitor.
and Jcorr are the corrosion current densities determined by the intersection of the extrapolated Tafel lines in uninhibited and inhibited acid solution, respectively. Both the anodic and cathodic reactions of mild steel corrosion are inhibited in the presence of the inhibitor. This result suggests that the addition of the m-FP-DACH decreases the anodic dissolution and also retards the hydrogen evolution reaction. It can be seen that the IE increases by increasing inhibitor concentration which indicates that more inhibitor molecule are absorbed on the metal surface and provide wider surface coverage. Thus, these compounds are acting as an adsorption inhibitor.
No definite trend is observed in the shift of Ecorr values in the presence of different concentrations of the m-FP-DACH, suggesting that this compound behaves as mixed-type inhibitor [18]. Polarization resistance (Rp) values are determined using Stern–Geary equation which is given below [19]:
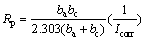 (3)
(3)
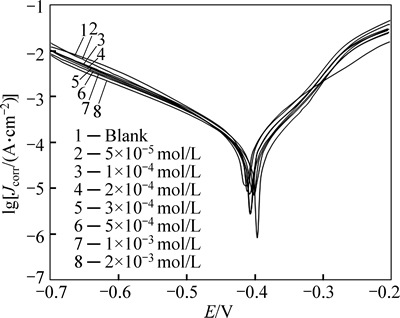
Fig. 2 Anodic and cathodic polarization curves of steel electrode in 1 mol/L HCl at 25 °C without and with different concentrations of inhibitor
By increasing the m-FP-DACH concentration, the polarization resistance increases in the presence of compound, indicating adsorption of the inhibitor on the metal surface to block the active sites efficiently and inhibit corrosion [20].
3.2 Electrochemical impedance spectroscopy
Figure 2 shows the Nyquist plots recorded for the corrosion of steel in 1 mol/L HCl solution without and with different concentrations of inhibitor obtained at Ecorr. The plots show a depressed capacitive loop which arises from the time constant of the electrical double layer and charge transfer resistance. The impedance of the inhibited steel increases with increasing the m-FP-DACH concentrations and consequently the inhibition efficiency increases. The equivalent circuit compatible with the Nyquist diagrams recorded in the presence of inhibitor is depicted in Fig. 4. The simplest approach requires the theoretical transfer function Z(ω) to be represented by a parallel combination of a resistance Rct and a capacitance Cdl, both in series with another resistance Rs [21]:
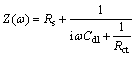 (4)
(4)
where ω is the frequency in rad/s, ω=2πf and f is frequency in Hz. To obtain a satisfactory impedance simulation of steel, it is necessary to replace the capacitor (C) with a constant phase element (CPE) Q in the equivalent circuit [22]. The most widely accepted explanation for the presence of CPE behavior and depressed semicircles on solid electrodes is microscopic roughness, causing an inhomogeneous distribution in the solution resistance as well as in the double layer capacitance [22]. Constant phase element Qdl, Rs and Rct can be corresponded to double layer capacitance, 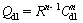 solution resistance and charge transfer resistance, respectively. To corroborate the equivalent circuit, the experimental data are fitted to equivalent circuit and the circuit elements are obtained. Table 2 illustrates the equivalent circuit parameters for the impedance spectra of corrosion of steel in 1 mol/L HClsolution. The results demonstrate that the presence of inhibitor enhances the value of Rct obtained in the pure medium while the values of Qdl decrease. The decrease in Qdl is caused by adsorption of inhibitor, indicating that the exposed area decreases. On the other hand, a decrease in Qdl, which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer, suggests that Schiff base inhibitor acts by adsorption at the metal/solution interface [23].
solution resistance and charge transfer resistance, respectively. To corroborate the equivalent circuit, the experimental data are fitted to equivalent circuit and the circuit elements are obtained. Table 2 illustrates the equivalent circuit parameters for the impedance spectra of corrosion of steel in 1 mol/L HClsolution. The results demonstrate that the presence of inhibitor enhances the value of Rct obtained in the pure medium while the values of Qdl decrease. The decrease in Qdl is caused by adsorption of inhibitor, indicating that the exposed area decreases. On the other hand, a decrease in Qdl, which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer, suggests that Schiff base inhibitor acts by adsorption at the metal/solution interface [23].
Table 1 Potentiodynamic polarization parameters for corrosion of steel in 1 mol/L HCl solution in absence and presence of different concentrations of inhibitor at 25 °C

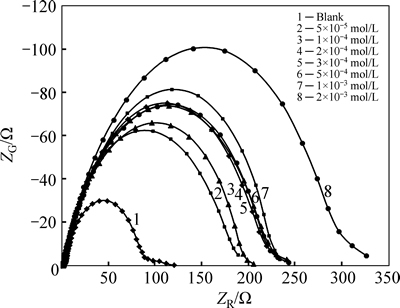
Fig. 3 Nyquist plots for steel in 1 mol/L HCl at 25 °C without and with various concentrations of inhibitor
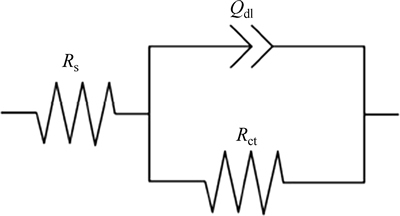
Fig. 4 Equivalent circuits compatible with experimental impedance data for corrosion of steel electrode in different inhibitor concentrations
Table 2 Impedance spectroscopy data for steel corrosion in 1 mol/L HCl solution with and without different concentrations of inhibitor at 25 °C
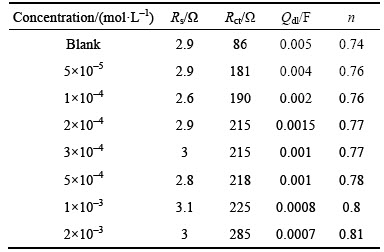
As the Qdl exponent (n) is a measure of the surface heterogeneity, values of n indicates that the steel surface become more and more homogeneous as the concentration of inhibitor increases as a result of its adsorption on the steel surface and corrosion inhibition [23]. The increase in values of Rct and the decrease in values of Qdl with increasing concentration also indicate that schiff base inhibitor acts as primary interface inhibitor and the charge transfer controls the corrosion of steel under the open circuit conditions.
3.3 Effect of temperature
The adsorption phenomenon has been successfully explained by thermodynamic parameters [24-25]. The change of the corrosion rate with the temperature was studied in 1 mol/L HCl. For this purpose, polarization curves were performed at different temperatures from 25 °C to 65 °C in the absence and presence of different concentrations of m-FP-DACH (Figs. 5 and 6). For 45 °C and 65 °C, the electrochemical parameters were extracted and summarized in Tables 3 and 4. It is obvious that the values of Jcorr increase and the efficiency value decreases by increasing the temperature. Figures 5 and 6 show that raising the temperature has no significant effect on the corrosion potentials but leads to increasing corrosion rate. According to the Arrhenius equation, the apparent activation energy (Ea) of metal corrosion in both media (blank and inhibited) can be calculated from the following equation [26]:
 (5)
(5)
where Ea represents the apparent activation energy, R is the gas constant, A is the pre-exponential factor, and T is the absolute temperature. Figure 7 shows the logarithm of Jcorr against the reciprocal of temperature T–1 in the absence and presence of m-FP-DACH. The activation energy Ea is calculated from the slope of the plots (-Ea/R). The calculated value of Ea in the absence of inhibitor is 57.47 kJ/mol, while in the presence of 2× 10–3 mol/L of inhibitor, it is 63.91 kJ/mol. It has been reported that higher Ea in the presence of the inhibitor for mild steel in comparison with blank solution typically shows physisorption [27]. Enthalpy and entropy ofactivation (ΔHa and ΔSa) are calculated from the transition state theory as [27]:
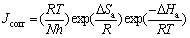 (6)
(6)
where h is the Plank constant and N is the Avogadro’s number. A plot of ln(Jcorr T–1) versus 1/T in straight lines is shown in Fig. 9 for mild steel dissolution in 1 mol/L HCl in the absence and presence of differentconcentrations of m-FP-DACH. Straight lines are obtained with a slope of –△Ha/R and an intercept of ln(R/Nh)+△Sa/R. The values of Ea, A, △Ha and △Sa are calculated and given in Table 5. The positive values of △Ha mean that the dissolution reaction is an endothermic process. Practically, Ea and △Ha are of the same order. Also, the entropy △Sa increases more positively in the presence of the inhibitor than the non-inhibited one, which reflects that the activated complex in the rate determining step represents dissociation rather than an association step, meaning that an increase in disordering takes place on going from reactants to the activated complex [6].
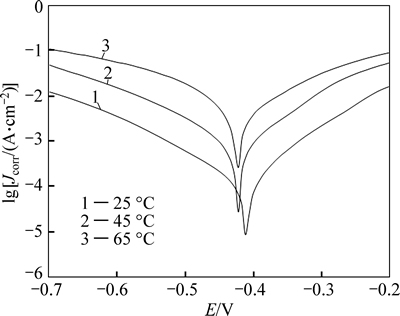
Fig. 5 Anodic and cathodic polarization curves of steel electrode in 1 mol/L HCl without inhibitor at different temperatures
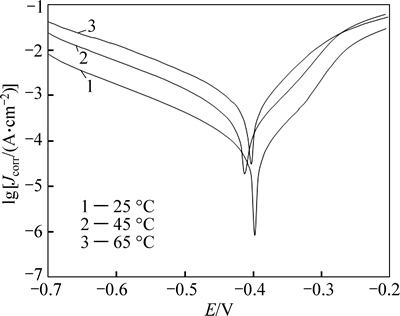
Fig. 6 Anodic and cathodic polarization curves of steel electrode in presence of 2×10–3 mol/L of inhibitor at different temperatures
Table 3 Potentiodynamic polarization parameters for corrosion of steel in 1 mol/L HCl solution in absence and presence of different concentrations of inhibitor at 45 °C
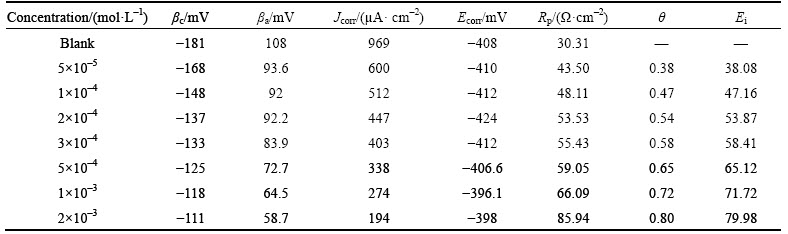
Table 4 Potentiodynamic polarization parameters for corrosion of steel in 1 mol/L HCl solution in absence and presence of different concentrations of inhibitor at 65 °C

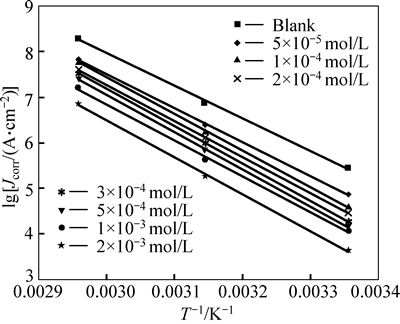
Fig. 7 Typical Arrhenius plots of lnJcorr vs 1/T for steel in 1 mol/L HCl at different concentrations of inhibitor

Fig. 8 Variation of lnJcorr/T vs 1/T for steel in 1 mol/L HCl at different concentrations of inhibitor
3.4 Adsorption isotherm
Adsorption isotherms provide information about the interaction of the adsorbed molecules with the metal surface. The adsorption of organic compounds can be expressed by two main types of interactions: physical adsorption and chemical adsorption. There are some factors that influence the adsorption processes including the nature and charge of metal, the chemical of inhibitor, and the type of electrolyte [28].
The adsorption of an organic adsorbate at metal/solution interface can be presented as a substitution adsorption process between the organic molecules in aqueous solution, Org(sol), and the water molecules on metallic surface, H2O(ads):
Org(sol)+xH2O(ads) Org(ads)+xH2O(sol) (7)
Org(ads)+xH2O(sol) (7)
where Org(sol) and Org(ads) are the organic species dissolved in the aqueous solution and adsorbed onto the metallic surface, respectively, H2O(ads) is the water molecule adsorbed on the metallic surface, H2O(sol) is the water molecule in solution, and x is the size ratio and represents the number of molecules of water replaced by the inhibitor molecule.
Different adsorption isotherms, Langmuir, Temkin, Freundlich, Frumkin, Modified, Langmuir, Henry, Viral, Damaskin, Volmer, and Flory-Huggins, [29–30] were tested for their fit to the experimental data. The linear regression coefficient values (R2) were determined from the plotted curves. According to these results, it is found that the experimental data obtained from polarization readings could be fitted by Langmuir’s adsorption isotherm. According to this isotherm, the surface coverage is related to inhibitor concentration by [30–31]:
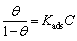 (8)
(8)
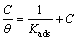 (9)
(9)
where Kads is the equilibrium constant of the inhibitor adsorption process, C is the inhibitor concentration and θ is the surface coverage calculated by Eq. (1).
A fitted straight line is obtained for the plot of C/θ versus C with slopes close to 1 as seen in Fig. 9. The strong correlation (R2>0.99) suggests that the adsorption
of m-FP-DACH on the mild steel surface obeys Langmuir’s adsorption isotherm. This isotherm assumes that the adsorbed molecules occupy only one site and there are no interactions with other adsorbed species [30]. The Kads values can be calculated from the intercept lines on the C/θ-axis. This is related to the standard free energy of adsorption (ΔGads) with the following equation [32]:
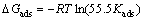 (10)
(10)
where R is the gas constant and T is the absolute temperature. The constant value of 55.5 is the concentration of water in solution in molar. The enthalpy and entropy of adsorption (ΔHads and ΔSads) can be calculated using the following equations [23]:
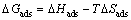 (11)
(11)
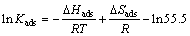 (12)
(12)
Table 5 Activation parameters of dissolution of steel in 1 mol/L HCl solution in absence and presence of inhibitor
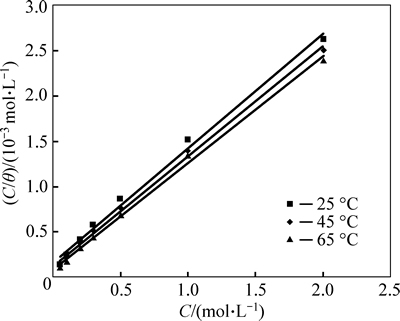
Fig. 9 Langmuir adsorption isotherm C/θ vs C of inhibitor in 1 mol/L solution
The negative values of △Gads suggest that the adsorption of m-FP-DACH on the steel surface is spontaneous. Generally, the values of -△Gads around or less than 20 kJ/mol are associated with the electrostatic interaction between charged molecules and the charged metal surface (physisorption); while those around or higher than 40 kJ/mol mean charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate type of metal bond (chemisorption). The values of Kads and △Gads are listed in Table 6. The ΔGads values are around –28 kJ/mol, which means that the absorption of inhibitor on the steel surface belongs to both physisorption and chemisorption, and the adsorptive film has an electrostatic character [20]. Figure 10 represents the plots of lnKads versus 1/T for adsorption m-FP-DACH. The obtained lines can provide valuable information about the mechanism of corrosion inhibition. An endothermic adsorption process △Hads>0 is attributed unequivocally to chemisorption, and an exothermicadsorption process △Hads<0 may involve either physisorption or chemisorption or a mixture of both processes [33]. The calculated values of △Hads and △Sads are –1.46 kJ/mol and 84.59 J/(mol·K), respectively. The calculated △Gads and ΔHads values for inhibitor show that the adsorption mechanism is not completely physical or chemical, and a combination of physisorption and chemisorption exists between the inhibitor and metal surface. The positive sign of △Sads arises from the substitution process, which can be attributed to the increase in the solvent entropy and more positive water desorption entropy. It is also interpreted with an increase of disorders due to the more water molecules which can be desorbed from the metal surface by one inhibitor molecule [21].
Table 6 Thermodynamic and equilibrium adsorption parameters for adsorption of inhibitor on steel surface in 1 mol/L HCl solution


Fig. 10 ln Kads vs 1/T for inhibitor adsorption on steel surface
3.5 Chronoamperometry
In order to gain more insight about the effect of m-FP-DACH on the electrochemical behavior of steel in 1 mol/L HCl solution, potentiostatic current–time transients were recorded. Figure 11 shows the current transients of steel electrode at –0.3 V (vs SCE) applied anodic potential. Initially the current decreases monotonically with time, and the decrease in the current density is due to the formation of corrosion products layer on the electrode surface. However, in later time, the current reaches to a steady state value due to the steel dissolution depending on applied potential (Fig. 11). In the presence of inhibitor, the dissolution current decreases and electrode inhibits from corrosion due to inhibitor adsorption.

Fig. 11 Current transients of steel electrode at -0.3 V vs SCE
3.6 Scanning electron microscopy
In order to evaluate the conditions of the steel surfaces in contact with hydrochloric acid solution, surface analysis was carried out. Surface was observed before and after 12 h of immersion in 1 mol/L HCl in the absence and presence of m-FP-DACH at 25 °C. Scanning electron microscopy (SEM) studies of the surface in the absentce and presence of inhibitor are presented in Fig. 12. It is revealed that the presence ofcorrosion attack and some pits on the surface in the absence of inhibitor while such damages are diminished in the presence of inhibitor.
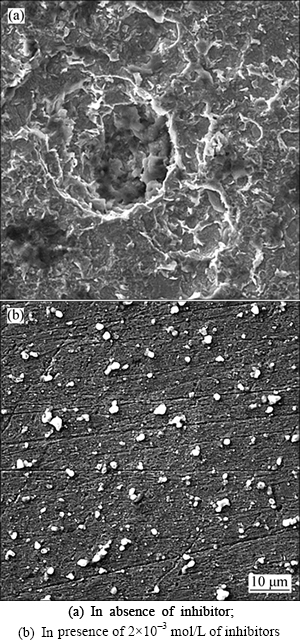
Fig. 12 Scanning electron microscopy images of steel exposed to 1 mol/L HCl solution:
4 Conclusions
1) The synthetic Schiff base m-FP-DACH acts as an inhibitor for mild steel corrosion in 1 mol/L HCl solution, especially in high concentration.
2) Inhibition efficiency of this compound increases with increasing their concentrations due to the formation of a film on the steel. The inhibitor decreases both anodic and cathodic currents, which shows the mixed mode of action of the inhibitor molecules.
3) The inhibition of mild steel in 1 mol/L HCl solution at different temperatures is found to obey the Langmuir adsorption isotherm.
4) The negative values of △G indicate the spontaneous adsorption of the inhibitor on the surface of mild steel.
5) Surface studies show that the surface of sample in solution with inhibitor molecules looks more flat and more uniform with lower roughness than that in the uninhibited solution.
References
[1] LI J Y, LIU N H,HUANG P W. Effects of pre-precipitation of Cr2N on microstructures and properties of high nitrogen stainless steel [J]. Journal of Central South University, 2012, 19: 1189–1195.
[2] LIU J H, ZHAN Z W, LI S M, YU M. Corrosion resistance of waterborne epoxy coating pigmented by nano-sized aluminium powder on steel [J]. Journal of Central South University, 2012, 19: 46–54.
[3] OKENIYI J O,OLADELE I O,AMBROSE I J,OKPALAK S O, OMONIYI O M,LOTO C A,POPOOLA A P. Analysis of inhibition of concrete steel-rebar corrosion by Na2Cr2O7 concentrations: Implications for conflicting reports on inhibitor effectiveness [J]. Journal of Central South University, 2013, 20: 3697–3714.
[4] GHASEMI O, DANAEE I, RASHED G R, RASHVANDAVEI M, MADDAHY M H. Inhibition effect of a synthesized N, N′-bis(2-hydroxybenzaldehyde)-1, 3-propandiimine on corrosion of mild steel in HCl [J]. Journal of Central South University, 2013, 20: 301–311.
[5] MUSA A Y,KADHUM A A H, MOHAMAD A B, TAKRIFF M S, DAUD A R, KAMARUDIN S K. Adsorption isotherm mechanism of amino organic compounds as mild steel corrosion inhibitors by electrochemical measurement method [J]. Journal of Central South University, 2010, 17: 34–39.
[6] JAFARI H, DANAEE I, ESKANDARI H, RASHVANDAVEI M. Electrochemical and Theoretical Studies of Adsorption and Corrosion Inhibition of N,N′-Bis(2 hydroxyethoxyacetophenone)-2,2-dimethyl- 1,2-propanediimine on Low Carbon Steel (API 5L Grade B) in Acidic Solution [J]. Ind Eng Chem Res, 2013, 52: 6617–6632.
[7] SHI S C,WANG X Y,YI P G,CAO C Z,DENG T T, SU J S. Influence of alkyl group of imidazolinyl-quaternary-ammonium-salt on corrosion inhibition efficiency [J]. Journal of Central South University of Technology, 2006, 13: 393–398.
[8] SOLMAZ R, ALTUNBAS E, KARDAS G. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1, 3, 4-thiadiazol-2-ylimino) methyl) phenol Schiff base on mild steel [J]. Mater Chem Phys, 2011, 125: 796–801.
[9] HASANOV R. Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution [J]. Appl Surf Sci, 2007, 253: 3913–3921.
[10] GHOLAMI M, DANAEE I, MADDAHY M H, RASHVANDAVEI M. Correlated ab initio and electroanalytical study on inhibition behavior of 2-mercaptobenzothiazole and its thiole-thione tautomerism effect for the corrosion of steel (API 5L X52) in sulphuric acid solution [J]. Ind Eng Chem Res 2013, 52: 14875-14889.
[11] BADIEA A M, MOHANA K N. Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2-hydrazino-4, 7-dimethylbenzothiazole in industrial water medium [J]. Corros Sci, 2009, 51: 2231–2241.
[12] STANLY J K, PARAMESWARAN G. Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furointhiosemicarbazone [J]. Corros Sci, 2010, 52: 224–228.
[13] DANAEE I, GHASEMI O, RASHED G R, RASHVANAVEI M, MADDAHY M H. Effect of hydroxyl group position on adsorption behavior and corrosion inhibition of hydroxybenzaldehyde Schiff bases: electrochemical and quantum calculations [J]. J Mol Struct, 2013, 1035: 247–259.
[14] PRABHU R A, VENKATESHA T V, SHANBHAG A V, KULKARNI G M, KALKHAMBKAR R G. Inhibition effects of some Schiff bases on the corrosion of mild steel in hydrochloric acid solution [J]. Corros Sci, 2008, 50: 3356–3362.
[15] MACDONALD J R. Note on the parameterization of the constant-phase admittance element [J]. Solid State Ion, 1984, 13: 147–149.
[16] DANAEE I. Kinetics and mechanism of palladium electrodeposition on graphite electrode by impedance and noise measurements [J]. J Electroanal Chem, 2011, 662: 415–420.
[17] NEGM N A, ELKHOLY Y M, ZAHRAN M K, TAWFIK S M. Corrosion inhibition efficiency and surface activity of benzothiazol- 3-ium cationic Schiff base derivatives in hydrochloric acid [J]. Corros Sci, 2010, 52: 3523–3536.
[18] HEGAZY M A. A novel Schiff base-based cationic Gemini surfactants: Synthesis and effect on corrosion inhibition of carbon steel in hydrochloric acid solution [J]. Corros Sci, 2009, 51: 2610–2618.
[19] KELES H. Electrochemical and thermodynamic studies to evaluate inhibition effect of 2-[(4-phenoxy- phenylimino) methyl]-phenol in 1 M HCl on mild steel [J]. Mater Chem Phys, 2011, 130: 1317–1324.
[20] EMREG L K C, ATAKOL O. Corrosion inhibition of mild steel with Schiff base compounds in 1 M HCl [J]. Mater Chem Phys, 2003, 82: 188–193.
L K C, ATAKOL O. Corrosion inhibition of mild steel with Schiff base compounds in 1 M HCl [J]. Mater Chem Phys, 2003, 82: 188–193.
[21] HOSEINZADEH A R, DANAEE I, MADDAHY M H. Thermodynamic and adsorption behaviour of vitamin B1 as a corrosion inhibitor for AISI 4130 steel alloy in HCl solution [J]. Z Phys Chem, 2013, 227: 403-417.
[22] DANAEE I, NIKNEJAD K M, ATTAR A A. Corrosion behavior of AISI 4130 steel alloy in ethylene glycol-water mixture in presence of molybdate [J]. Mater Chem Phys, 2012: 135, 658–667.
[23] HOSEINZADEH A R, DANAEE I, MADDAHY M H. Thermodynamic and adsorption behaviour of medicinal nitramine as a corrosion inhibitor for AISI steel alloy in HCl solution [J]. J Mater Sci Technol, 2013, 29: 884–892.
[24] BADR G E. The role of some thiosemicarbazide derivatives as corrosion inhibitors for C-steel in acidic media [J]. Corros Sci, 2009, 51: 2529–2536.
[25] HEGAZY M A, AHMED H M, EL-TABEI A S. Investigation of the inhibitive effect of p-substituted4-(N, N, N-dimethyldodecy- lammonium bromide) benzylidene- benzene-2-yl-amineon corrosion of carbon steel pipelines in acidic medium [J]. Corros Sci, 2011, 53: 671–678.
[26] ALJOURANI J, RAEISSI K, GOLOZAR M A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution [J]. Corros Sci, 2009, 51: 1836–1843.
[27] HERRAG L, CHETOUANI A, ELKADIRI S, HAMMOUTI B, AOUNITI A. Pyrazole derivatives as corrosion inhibitors for steel in hydrochloric acid [J]. Portugal Ectrochim Acta, 2008, 26: 211–220.
[28] OGUZIE E E, UNAEGBU C, OGUKWE C N, OKOLUE B N, ONUCHUKWU A I. Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives [J]. Mater Chem Phys 2004, 84: 363–368.
[29] MU G, LI X, QU Q, ZHOU J. Molybdate and tungstate as corrosion inhibitors for cold rolling steel in hydrochloric acid solution [J]. Corros Sci, 2006, 48: 445–459.
[30] BAYOL E, GURTENB T, GURTENA A A, ERBIL M. Interactions of some Schiff base compounds with mild steelsurface in hydrochloric acid solution [J]. Mater Chem Phys, 2008, 112: 624–630.
[31] KELES H, KELES M, DEHRI I, SERINDAG O. The inhibitive effect of 6-amino-m-cresol and its Schiff base on the corrosion of mild steel in 0.5 M HCl medium [J]. Mater Chem Phys, 2008, 112: 173–179.
[32] DOGRU M B, ERMAN M M, KARDAS G, YAZICI B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole- 5-thiol as a corrosion inhibitor for carbon steel in HCl medium [J]. Corros Sci, 2011, 53: 4265-4272.
[33] LI X H, DENG S D, FU H, MU G N. Synergistic inhibition effect of rare earth cerium(IV) ion and sodium oleate on the corrosion of cold steel in H2SO4 solution [J]. Corros Sci, 2009, 51: 2639–2651.
(Edited by FANG Jing-hua)
Received date: 2015-03-10; Accepted date: 2015-06-09
Corresponding author: I. Danaee; Tel: +98–6153329937; E-mail: danaee@put.ac.ir

