Preparation and hygrothermal performance of composite phase change material wallboard with humidity control based on expanded perlite/diatomite/paraffin
来源期刊:中南大学学报(英文版)2018年第10期
论文作者:孔祥飞 杨华 LIU Yun(刘云) 陈万河 姚成强
文章页码:2387 - 2398
Key words:thermal storage; humidity control; phase change material; paraffin; expanded perlite; diatom mud
Abstract: Phase change material (PCM) can reduce the indoor temperature fluctuation and humidity control material can adjust relative humidity used in buildings. In this study, a kind of composite phase change material particles (CPCMPs) were prepared by vacuum impregnation method with expanded perlite (EP) as supporting material and paraffin as phase change material. Thus, a PCM plate was fabricated by mould pressing method with CPCMPs and then composite phase change humidity control wallboard (CPCHCW) was prepared by spraying the diatom mud on the surface of PCM plate. The composition, thermophysical properties and microstructure were characterized using X-ray diffraction instrument (XRD), differential scanning calorimeter (DSC) and scanning electron microscope (SEM). Additionally, the hygrothermal performance of CPCHCW was characterized by temperature and humidity collaborative test. The results can be summarized as follows: (1) CPCMPs have suitable phase change parameters with melting/freezing point of 18.23 °C/29.42 °C and higher latent heat of 54.66 J/g/55.63 J/g; (2) the diatom mud can control the humidity of confined space with a certain volume; (3) the combination of diatom mud and PCM plate in CPCHCW can effectively adjust the indoor temperature and humidity. The above conclusions indicate the potential of CPCHCW in the application of building energy efficiency.
Cite this article as: YANG Hua, LIU Yun, KONG Xiang-fei, CHEN Wan-he, YAO Cheng-qiang. Preparation and hygrothermal performance of composite phase change material wallboard with humidity control based on expanded perlite/diatomite/paraffin [J]. Journal of Central South University, 2018, 25(10): 2387–2398. DOI: https://doi.org/ 10.1007/s11771-018-3923-4.

J. Cent. South Univ. (2018) 25: 2387-2398
DOI: https://doi.org/10.1007/s11771-018-3923-4
YANG Hua(杨华), LIU Yun(刘云), KONG Xiang-fei(孔祥飞), CHEN Wan-he(陈万河), YAO Cheng-qiang(姚成强)
School of Energy and Environmental Engineering, Hebei University of Technology, Tianjin 300401, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: Phase change material (PCM) can reduce the indoor temperature fluctuation and humidity control material can adjust relative humidity used in buildings. In this study, a kind of composite phase change material particles (CPCMPs) were prepared by vacuum impregnation method with expanded perlite (EP) as supporting material and paraffin as phase change material. Thus, a PCM plate was fabricated by mould pressing method with CPCMPs and then composite phase change humidity control wallboard (CPCHCW) was prepared by spraying the diatom mud on the surface of PCM plate. The composition, thermophysical properties and microstructure were characterized using X-ray diffraction instrument (XRD), differential scanning calorimeter (DSC) and scanning electron microscope (SEM). Additionally, the hygrothermal performance of CPCHCW was characterized by temperature and humidity collaborative test. The results can be summarized as follows: (1) CPCMPs have suitable phase change parameters with melting/freezing point of 18.23 °C/29.42 °C and higher latent heat of 54.66 J/g/55.63 J/g; (2) the diatom mud can control the humidity of confined space with a certain volume; (3) the combination of diatom mud and PCM plate in CPCHCW can effectively adjust the indoor temperature and humidity. The above conclusions indicate the potential of CPCHCW in the application of building energy efficiency.
Key words: thermal storage; humidity control; phase change material; paraffin; expanded perlite; diatom mud
Cite this article as: YANG Hua, LIU Yun, KONG Xiang-fei, CHEN Wan-he, YAO Cheng-qiang. Preparation and hygrothermal performance of composite phase change material wallboard with humidity control based on expanded perlite/diatomite/paraffin [J]. Journal of Central South University, 2018, 25(10): 2387–2398. DOI: https://doi.org/ 10.1007/s11771-018-3923-4.
1 Introduction
With the increasing level for indoor thermal comfort, the building energy consumption has been increasing in recent years [1–3]. Along with the emergence of energy crisis, the energy conservation awareness in the world is growing. The building industry consumes nearly 40% of the total energy consumption of the world [4]. Thus, the technology for building energy efficiency has been focused on in the last several decades, especially the passive energy-saving technology [5, 6]. Phase change materials (PCM) [7–9] and humidity control materials [10, 11] are two important types of passive energy-saving materials that can be used in buildings. During the phase change process, phase change materials absorb or release large amounts of latent heat from or to the indoor air. In this way, phase change material can smooth the temperature fluctuation and increase the thermal inertia of buildings [12]. The humidity control material that relies on its own characteristics and the induction for the change of the temperature and humidity of surrounding can automatically control the air relative humidity.
In recent years, several researches have reported the desired effect of the phase change material in thermal storage [13, 14] and humidity control material in humidity buffering [15–17], and prove the energy-efficiency opportunity for the application of phase change material and humidity control material in the building [18, 19]. For the advantage of passively reducing the indoor temperature fluctuations, PCM has been widely studied to be incorporated within the building envelope [20]. The key point in practical application is to choose PCMs with low supercooling, appropriate phase change point and high thermal property [21, 22], such as paraffin, fatty acid, ester, alcohols, glycols, etc. In 1989, KEDL et al [23] had firstly developed thermal storage wallboards based on PCMs, which were used in the passive solar room, performing well in energy storage. BASF company [24] developed a new phase change mortar with paraffin mass fraction 10% to 25%, which can effectively adjust the room temperature and improve the thermal comfort of the indoor environment in the application on the building interior wall. The heat storage capacity of mortar with 2 cm thickness can be equivalent to that of brick structure with 20 cm thickness. However, PCM is very easy to leak out from building materials [25], resulting in operational difficulty of practical application. The method of vacuum impregnation for improving the phase change material leakage has been widely concerned. ZHANG et al [26] developed the vacuum impregnation method for loading porous materials with PCM. Furthermore, KARAIPEKLI et al [27] prepared novel form-stable PCM by the vacuum impregnation of capric acid-myristic acid eutectic mixture into vermiculite. In the above researches, it has proved the feasibility and reliability of vacuum impregnation method in improving the leakage of PCM.
As research continues, two difficult points to restrict the next research progress emerged. Firstly, some supporting materials [28] have the property of low thermal conductivity [29, 30], and the low heat transfer ability can be considered as an essential disadvantage for PCM completing the melting– freezing cycle. Secondly, although PCM can adjust the indoor temperature, it cannot regulate the humidity of indoor environment, limiting its building application [31]. The humidity control material [32] can passively improve the relative humidity in a closed environment, if the relative humidity is low in this space [33]. Diatom mud is receiving significant attention due to its unique physicochemical properties (such as high porosity, excellent absorption capacity, light weight, small particle size, high specific surface area, chemical inertness and unique microcellular pore structure). Diatom mud can be used in the field of building materials for humidity control. JANIS company prepared humidity control materials based on inorganic materials with inside micro-porous structure using (sepiolite, diatomite, zeolite) and light concrete as raw materials. VU et al [34] used a mixture of diatomite and volcanic ash as the substrate to prepare efficient humidity control materials, and in-depth analyzed the humidity control mechanism. It should be noted that most of the studies individually analyzed the performances of PCM plates or humidity control material, whereas the researches combing the characteristics of both were of lack.
Aiming to study the combination performance of PCM and humidity control material in a composite plate, a composite phase change material particles (CPCMPs) were prepared by liquid paraffin absorbed into expanded perlite (EP) with vacuum impregnation method. A kind of PCM plate was prepared by mould pressing method based on CPCMPs. And then, diatom mud was incorporated with PCM plate by spraying technique to fabricate the novel composite phase change humidity control wallboard (CPCHCW), which was assumed that it has the functions of heat storing and humidity buffering.
2 Preparation of CPCHCW
2.1 Material
Paraffin, an organic material with colorless and odorless, is a kind of suitable phase change material used in buildings due to its melting temperature within an appropriate range and large latent heat, and for different application fields, it can be selected according to the different type [35]. Therefore, 25# paraffin (purchased from Sinopec Nanyang Energy Chemical Co. Ltd.) is used as the phase change material in this study; expanded perlite (purchased from Licheng perlite factory in Xinyang city) with particle diameter of 4–6 mm is used as the supporting material; diatom mud (purchased from Liaoning Yingkou Panpan Environmental Diatom material Co. Ltd.) is used as the humidity control material. The performance parameters of materials are presented in Tables 1 and 2.
Table 1 Performance parameters of expanded perlite

Table 2 Performance parameters of diatom mud

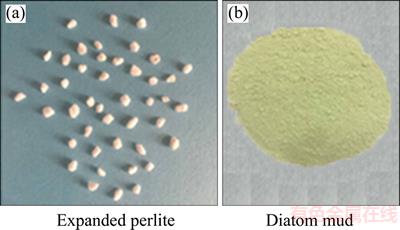
Figure 1 Expanded perlite (a) and diatom mud (b)
2.2 Preparation process
A certain quantity of EP was added into a suction bottle, which was connected with the vacuum pump through a vacuum rubber hose and a vacuum valve. Liquid paraffin was poured into a funnel with a closed valve that was in the middle part of the funnel. The mass ratio of paraffin vs expanded perlite was 60%:40%. Then, vacuum pump was started to maintain the pressure of 0.06 MPa for the inside suction bottle. After 30 min, the vacuum valve was closed. With the valve of funnel opened, liquid paraffin was released into the suction bottle, and absorbed by EP. In the mean time, the magnetic stirrer worked at a constant temperature of 70 °C, to uniformly blend paraffin and EP. 2 h later, the inside micropores of expanded perlite can be fully filled with paraffin, and composite particles were unloaded. The schematic diagram of vacuum adsorption is shown in Figure 3. Because of the air in the micropores of EP was removed, liquid paraffin can be sucked into the micropores under the capillary action, and firmly maintained in the internal three-dimensional structure of EP with the wall effect [36, 37].
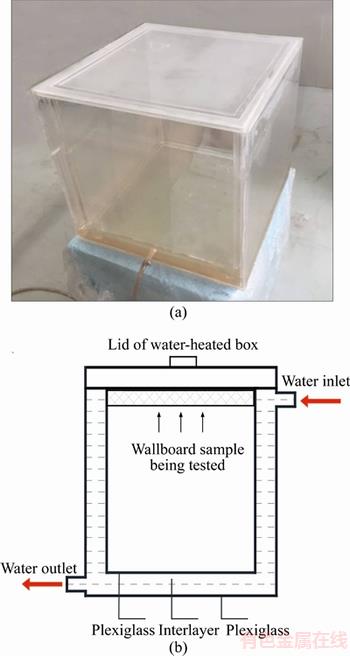
Figure 2 Water-heated box of external view (a) and schematic diagram (b)
Composite particles and styrene-acrylic emulsion were mixed according to the mass ratio of 8: 1 in the agitator tank. Besides, 5% of AP with the particle size of 15 μm was added into the mixture. After mixing uniformity, CPCMPs were finally finished. The CPCMPs were put into a self-made mould (500 mm×500 mm×60 mm).A pressure of 4 MPa was applied to the cover plate to maintain the plate shape for the mixture. After 72 h, the mould was removed. Finally, PCM plate was finished. Figure 3 shows the preparation process of PCM plate.
Diatom mud and water were mixed according to mass fraction ratio of 1:0.7, and then the mixture was sprayed on the PCM plate through a d5 mm caliber spray gun at a distance of distance of 45 cm. Firstly, the diatom mud mixture was coated on the surface of PCM plate by the spray gun; secondly, when the coated mixture was dried, diatom mud mixture was spray again. It was to ensure that the diatom mud can uniformly cover the surface of PCM plate. The thickness of diatom mud on the PCM plate was 3 mm. Figure 3 shows the flowchart of the preparation process.

Figure 3 Flowchart of preparation process
3 Performance characterization
3.1 XRD diffraction test
The material composition of the expanded perlite and diatom mud was characterized with X-ray diffraction instrument (XRD, D8Advance, Germany). The ray source was Cu target. The scanning mode was step-scan and scanning speed was 5 (°)/min.
3.2 Pore size test
Pore structures of expanded perlite and diatom mud were tested by physical adsorption analyzer (ASAP2020, America).
3.3 DSC test
Thermal physical properties of paraffin and CPCMPs, inclusive of phase change points and latent heat, were studied by differential scanning calorimeter (DSC, 2910, America) with a heating rate of 5 °C/min under nitrogen protection.
3.4 SEM test
The microstructure and surface morphology of expanded perlite and diatom mud were observed by scanning electron microscope (SEM, Nova Nano SEM450, Netherlands).
3.5 Diatom mud control humidity test
Diatom mud control humidity test was conducted in a water-heated box that was fabricated by transparent plexiglass (5 mm thickness) with an internal space size of 600 mm×600 mm×600 mm, as shown in Figure 2(a). The inside wall was hollow and fully filled with water. The inside temperature can be conveniently controlled through the circulated water that was heated by a thermostat water bath.
3.5.1 Equilibrium moisture content test of diatom mud
The diatom mud was placed in the oven until the mass change was less than 0.001 g to prepare dried samples. The mass of each sample was 10 g. The equilibrium moisture content tests were carried out in the 25 °C constant temperature water-heated box with the RH of 35%, 55%, 75%, 85% and 98%, respectively. Besides, electronic balance (BS110S, Germany) with accuracy (±0.1 mg) measured data until the mass of sample was no longer changed.
3.5.2 Cyclic moisture absorption and moisture desorption performance test
In this test, two relative humidity values of 35% and 98% were selected for cyclic moisture absorption and moisture desorption performance tests. The mass of sample was recorded after reaching the moisture balance. The tests were repeated three times.
3.5.3 Control humidity test of diatom mud on different environment
Flattened bottles containing 10 g diatom mud were placed in an environment with the RH of 30%. When the quality of diatom mud did not change or change little (±0.001 g), diatom mud reached equilibrium humidity, and then the treated diatom mud was reserved as sample.
The tests of humidity control, including two stages of temperature change and humidity change, were also conducted by the water-heated box. In the stage of temperature change, humidity control performance of diatom mud sample was studied under three temperature conditions, i.e., 10, 25 and 40 °C, and the initial relative humidity (RH) of inside box was 90%. On the contrary, in the stage of humidity change, the temperature of inside box remained unchanged with 30 °C, but RH of inside box changed between three values, i.e., 75%, 85% and 90%. Besides, the temperature and humidity logger (DSR-TH, Tianjin Opine Instruments Co. Ltd.), with accuracy (±0.3 °C, ±3% RH) and measure range (–30 °C–70 °C, 0%–100% RH) was installed in the box to record the temperature and humidity data.
3.6 Combination test of heat storage and humidity control for CPCHCW
Two wallboard samples, CPCHCW and reference wallboard (diatom mud wallboard without paraffin), were tested. The samples were also installed on the internal surface of cover of water- heated box. Under temperature and humidity changing, the comprehensive regulatory effects of CPCHCW on temperature and humidity have been contrastively studied with reference wallboard. The detailed process was presented as follows:
a) CPCHCW was placed in an environment with the relative humidity of 30%. CPCHCW achieved humidity balance, when the quality did not change or change little (±0.001 g), and then reserved the treated CPCHCW;
b) The treated CPCHCW was sealed in the water-heated box of the initial temperature of 15 °C and the relative humidity of 80%; then the temperature of water-heated box was raised to 40 °C;
c) After 3 h, the temperature of water-heated box was reduced to 15 °C;
d) The test was finished 3 h later.
4 Results and discussion
4.1 Material composition analysis
As shown in Figure 4, the shape of the diffraction peak is wide and diffuse, but not sharp, indicating that the structure of expanded perlite is vitreous. In the figure, the amorphous main peak with the larger diffraction intensity at 2θ=21.7° is the diffraction peak of SiO2, indicating SiO2 is the main component of expanded perlite. In addition, expanded perlite contains a small amount of feldspar, quartz porphyry, microcrystalline, flash angle stone and so on. Therefore, there are some overlapping small crystal peaks, besides the amorphous main peak.
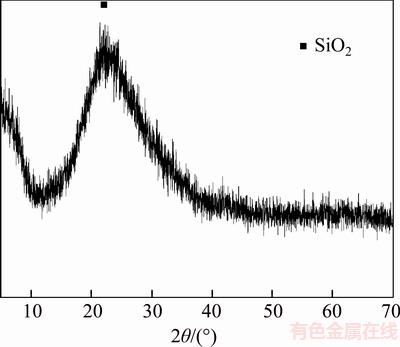
Figure 4 XRD pattern of expanded perlite
Diatom mud is mainly composed of diatomite, whose main component is water-containing amorphous SiO2. The XRD pattern of diatom has a wide asymmetric diffraction peak with low diffraction intensity, whose shape is flattened like steamed bread peak. Diatom mud in the preparation process went through high temperature firing, which accelerated the crystallization of amorphous SiO2, so the flattened steamed bread peak gradually became sharp towering diffraction peak. As shown in Figure 5(b), the compositions of diatom mud are more complex, mainly contain SiO2 (the characteristic diffraction peaks near 2θ=9.8°, 19.7°, 27°), CaCO3 (the characteristic diffraction peaks near 2θ=30°, 39°, 43°, 47°), Na2Al2Si3O10·2H2O (the characteristic diffraction peaks near 2θ=9.8°, 19.7°, 27°). The result shows that the main compositions of diatom mud are diatomite, calcium bicarbonate and sodium zeolite. Among them, as a kind of zeolite, sodium zeolite has high specific surface area, which can also effectively enhance the adsorption property of diatom mud.
4.2 Pore size analysis
As shown in Figure 6, according to the IUPAC classification nitrogen adsorption-desorption isotherms of expanded perlite presented type IV isotherms with a hysteresis loop [38, 39], whose adsorption curve does not coincide with the desorption curve. In the low pressure section with relative pressure below 0.8, the adsorption curve is relatively gentle, because the nitrogen molecules are filled on the surface of the channels by the way of monolayer adsorption, and then the amount of adsorption increased rapidly with the relative pressure increasing, which was caused by multilayer adsorption and capillary condensation in the mesoporous channels. Between the relative pressure of 0.6–0.9, the desorption curve has an obvious hysteresis loop close to H3 type [40], indicating that the expanded perlite particles have slit pores formed by irregular flaky structures. Figure 7 shows that the pore sizes of expanded perlite are concentrated in the range of 3–20 nm, and have peaks at 3.7 nm, 5.0 nm, 6.2 nm, 10.0 nm, indicating that the pore size distribution of expanded perlite is not uniform, and the pores are mainly mesopores.
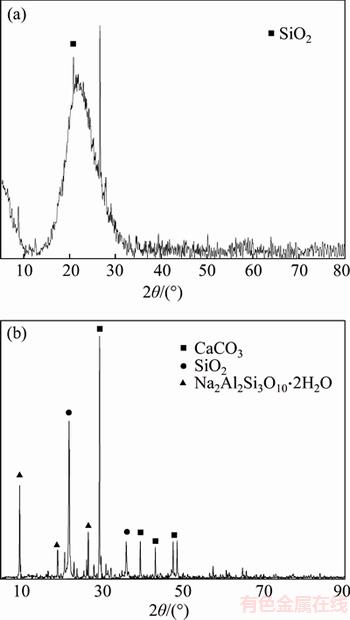
Figure 5 XRD patterns of diatom (a) and diatom mud (b)
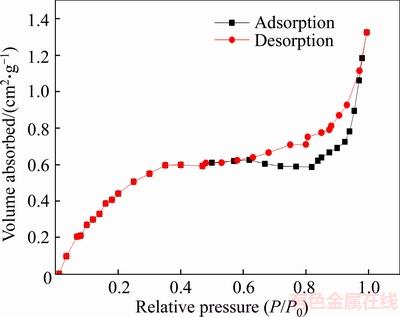
Figure 6 Nitrogen adsorption–desorption isotherms of expanded perlite
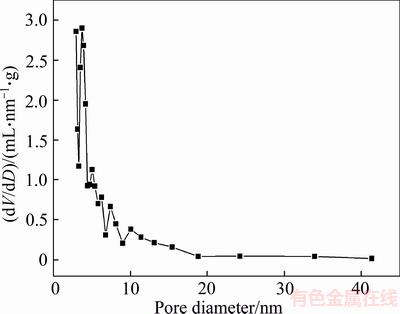
Figure 7 Pore size distribution of expanded perlite
As shown in Figure 8, nitrogen adsorption- desorption isotherms of diatom mud belong to the typical type IV isotherms. In the low pressure section (0–0.1) and high pressure section (0.9–1.0), the amount of adsorption increased drastically, indicating that diatom mud has a certain amount of micropores (pore size <2 nm) and large pores (pore size>50 nm). The hysteresis loop of diatom mud is narrow and long, and the type of hysteresis loop is the mixed type of H3 and H4, showing that there are irregular stacking pores and regular slit pores in the diatom mud. Figure 9 shows that the pores of diatom mud are mainly mesopores [41], which can form capillary condensation at lower pressure, so that the water molecules adsorbed on the surface of the diatom mud are easier to enter the pores.
The specific surface area of the sample was calculated by multipoint BET method, and the pore volume was calculated by BJH method. The results are shown in Table 3.
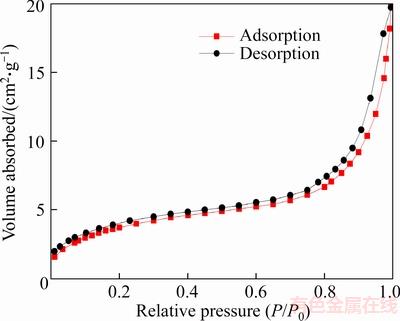
Figure 8 Nitrogen adsorption–desorption isotherms of diatom mud
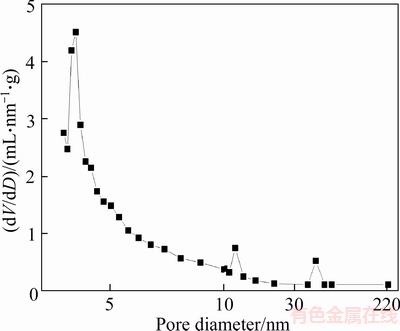
Figure 9 Pore size distribution of diatom mud
Table 3 Performance parameters of pore structure

4.3 DSC
As shown in Figure 10, DSC curves have a significant melting peak and freezing peak. During the process of temperature rise, paraffin absorbs heat. The melting point is 18.74 °C and the melting latent heat is 104.69 J/g. Paraffin releases heat in the process of temperature reduction. The freezing point is 29.38 °C and the freezing latent heat is 103.42 J/g. The trend of the DSC curve of the CPCMPs is consistent with that of the DSC curve. The melting and freezing latent heat of CPCMPs are 54.66 J/g and 55.63 J/g, respectively. The latent heat ratio of CPCMPs to pure paraffin are 52.21% and 53.79%, respectively, which are closed to paraffin mass percent of 60%. Meanwhile, the melting and freezing point of CPCMPs are 18.23 °C and 29.42 °C, respectively. Compared with the result of paraffin, the phase change points of CPCMPs have little shift, which is due to the weak attractive interaction of wall effect between paraffin molecule and EP internal faces. However, the little shift of phase change point does not affect the application of CPCMPs. Therefore, CPCMPs still maintain the original thermal properties of paraffin and potential in the application of building materials.
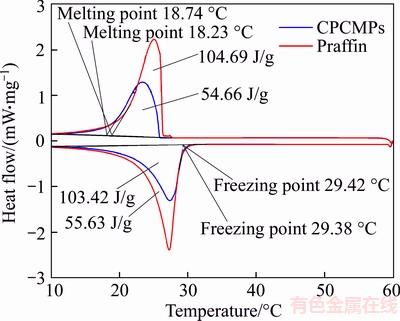
Figure 10 DSC curves of paraffin and CPCMPs
4.4 SEM
Figure 11(a) shows the microstructure of diatom mud. There are two kinds of pores in the diatom mud, of which one is the irregular pore stacked by diatom shells, and another is the regular pore on the surface of the diatom particles. In the SEM picture of Figure 11(a), the material morphology and pore structure of diatom mud can be observed very clearly. The content of diatom shells in diatom mud is higher and the integrity of diatom shells is also better. The morphology of diatom mud is mainly round sieve, and the diameter of diatomite particles range from 1 μm to 20 μm. The central portion of the round sieve shell surface has regular cylindrical pores with the pore size of about 0.5 μm, and the edge is the ink bottle-shaped pores with the pore size of about 0.1 μm.
Figure 11(b) shows that the microstructure of expanded perlite was irregular sheet, which provides adequate adsorption space and good mechanical strength. The light-colored region is the pore skeleton, and dark area represents the pores. These pores used to absorb paraffin are numerous with unfixed shapes, thin wall, and size range of a few micrometers to several hundred micrometers. What is more, due to the function of capillary action and surface tension, the leakage of liquid paraffin in the phase change process was prevented. As shown in Figure 11(c), the pores of the expanded perlite are basically filled with paraffin, making themselves into dense particles.
4.5 Analysis of diatom mud humidity control test
4.5.1 Equilibrium moisture content test of diatom mud
The equilibrium moisture absorption content of diatomite increased with the increase of RH. The equilibrium moisture desorption content is lower than the equilibrium moisture absorption content, which is due to the fact that the internal channels of diatom mud have a certain amount of micropores, the adsorbed moisture because of the capillary phenomenon are not easily desorbed; secondly, the moisture is attracted by the hydrophilic groups in the structure, which is adsorbed on the outer surface or the internal channels, and this part of the chemical adsorption is difficult to desorption.
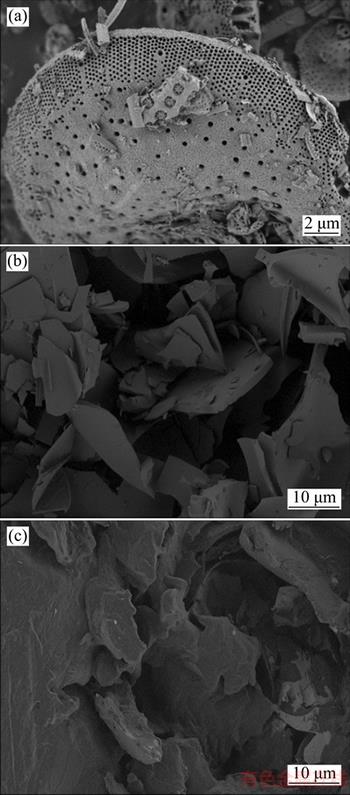
Figure 11 SEM pictures of diatom mud (a), expanded perlite (b) and CPCMPs (c)
4.5.2 Cyclic moisture absorption and moisture desorption performance test
From the comparison of the chart, it can be found that the moisture desorption of sample is significantly weaker than their own moisture absorption capacity, and the moisture absorption content and moisture desorption content of the sample has a certain degree of attenuation. However, there is not much of downward trend. Overall, the humidity control performance of diatom mud is relatively stable, and diatom mud has a continuous humidity control capacity.
4.5.3 Control humidity test of diatom mud on different environment
As shown in Figure 14, in the 0–2 h, there is no obvious difference in the effect of diatom mud on environmental humidity control under different temperatures (10 °C, 25 °C, 40 °C). The decline scope of environmental humidity is smaller with the increase of temperature, because the increase of temperature is not conducive to adsorption of water molecules by diatom mud. The fundamental reason is that the adsorption of water molecules is based on physical adsorption, which contains single layer adsorption, multi-layer adsorption and capillary condensation. The increase of temperature causes the collision of the gaseous water molecules to strengthen, and the residence time of the water molecules on the surface of the diatom mud is reduced, which directly affects the capillary condensation in the mesopores, resulting in the decrease of moisture content, thus the ability to control humidity of the environment declines.
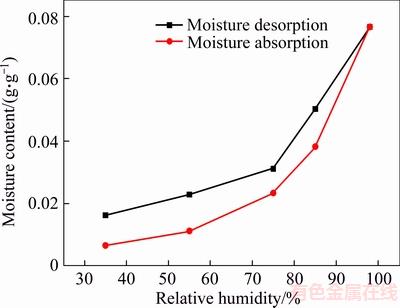
Figure 12 Equilibrium moisture absorption and desorption content of diatom mud
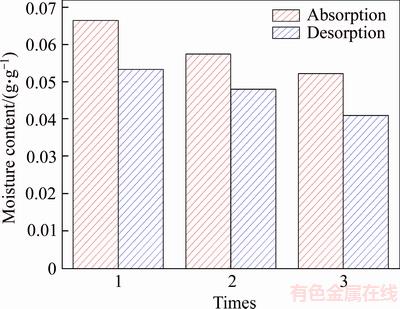
Figure 13 Cyclic attenuation chart of moisture absorption and desorption
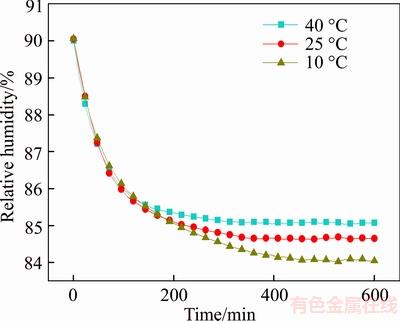
Figure 14 Humidity change curves at different temperatures
It can be seen from Figure 15 that the relative humidity in the experimental box decreases rapidly within 2 h. When the test time exceeds 2 h, the relative humidity curve tends to be gentle, indicating that the amount of moisture absorbed by the diatom mud is reduced, and in the test time of about 10 h moisture absorption is basically saturated. The maximum moisture absorption is about 3.975%, 5.255% and 5.958%, which shows that the moisture absorption capacity of diatom mud is improved with the increase of environment humidity.
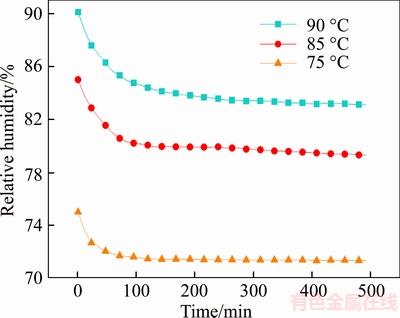
Figure 15 Humidity change curves on different relative humidities
4.6 Combination test of heat storage and humidity control for CPCHCW
In Figure 16, comparing the temperature change of water-heated box with the reference wallboard, that of CPCHCW has obvious lag. PCM absorbed the heat from box during the melting process, resulting in the relatively slow increase of temperature; meanwhile, PCM released heat to the box during the solidification process, resulting in a slower temperature reduction, indicating that the CPCHCW can do well in heat storage transfer, and plays a role in regulating the temperature.
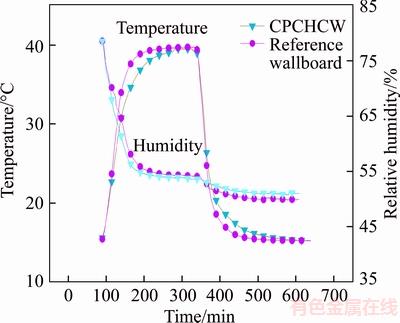
Figure 16 Curves of combination test
In the warming stage of 15 °C to 40 °C, diatom mud absorbed moisture of water-heated box, and the relative humidity decreased. It should be pointed out that the melting of the paraffin during the warming stage absorbed heat, which slowed down the increase of the surface temperature of the diatom mud and the desorption process of the adsorbed molecules to a certain extent, so the relative humidity of water-heated box where reference wallboard was located was slightly higher than that of water-heated box where the CPCHCW was located. In the cooling stage of 40 °C to 15 °C, because the reduction of the temperature was conducive to the adsorption of water molecules, diatom mud again adsorbed water molecules, and then the relative humidity of the experimental room decreased. At the same time, because the adsorption of the diatom mud on water molecules was the low exothermic process, the exothermic effect of CPCHCW was enhanced and the decline of room temperature was delayed to a certain extent. The coupling of PCM and diatom mud can effectively adjust the indoor temperature and humidity, which indicates the potential of CPCHCW in the application of building energy efficiency.
5 Conclusions
In this study, the CPCMPs were prepared by vacuum impregnation method with expanded perlite as carrier material, paraffin as phase change material, and its thermal conductivity was promoted by AP additive. PCM plate was produced by mould pressing method with CPCMPs and then CPCHCW was prepared by spraying the diatom mud into the PCM plate. In addition, the related properties of expanded perlite, paraffin and diatom mud and the hygrothermal performance of composite wall was studied and analyzed, and the following conclusions are obtained:
1) The CPCMPs had suitable phase change point 18.23 °C/29.42 °C and higher latent heat 54.66 J/g/55.63 J/g.
2) After the tests of cyclic moisture absorption and moisture desorption, the moisture absorption content and moisture desorption content of the sample has a certain degree attenuation, but the humidity control performance of diatom mud was relatively stable. In addition, the adsorption content was always more than the desorption content no matter in high or low humidity environment.
3) The humidity control performance of diatom mud at different temperatures (T=10 °C,25 °C, 40 °C; RH=85%) decreased due to the increase of temperature, and at different humidities (RH=75%, 85%, 90%; T=25 °C) enhanced due to the increase of humidity.
4) The coupling of PCM plate and diatom mud could effectively adjust the indoor temperature and humidity, which indicated the potential of CPCHCW in the application of building energy efficiency.
References
[1] ISAAC M, van VUUREN D P. Modeling global residential sector energy demand for heating and air conditioning in the context of climate change [J]. Energy Policy, 2009, 37(2): 507–521.
[2] ZHANG Yan, HE Chen-qi, TANG Bao-jun, WEI Yi-ming. China’s energy consumption in the building sector: A life cycle approach [J]. Energy and Buildings, 2015, 94: 240–251.
[3] ZHANG Tao, LIU Xiao-hua, JIANG Yi. Development of temperature and humidity independent control (THIC) air-conditioning systems in China—A review [J]. Renewable and Sustainable Energy Reviews, 2014, 29: 793–803.
[4] P REZ-LOMBARD L, ORTIZ J, POUT C. A review on buildings energy consumption information [J]. Energy and Buildings, 2008, 40(3): 394–398.
REZ-LOMBARD L, ORTIZ J, POUT C. A review on buildings energy consumption information [J]. Energy and Buildings, 2008, 40(3): 394–398.
[5] ZHOU Dan, ZHAO Chang-ying, TIAN Yuan. Review on thermal energy storage with phase change materials (PCMs) in building applications [J]. Applied Energy, 2012, 92: 593–605.
[6] BAETENS R, JELLE B P, GUSTAVSEN A. Phase change materials for building applications: A state-of-the-art review [J]. Energy and Buildings, 2010, 42(9): 1361–1368.
[7] KUZNIK F, DAVID D, JOHANNES K, ROU X J. A review on phase change materials integrated in building walls [J]. Renewable and Sustainable Energy Reviews, 2011, 15(1): 379–391.
[8] SHARMA A, TYAGI V V, CHEN C R, BUDDHI D. Review on thermal energy storage with phase change materials and applications [J]. Renewable and Sustainable Energy Reviews, 2009, 13(2): 318–345.
[9] RATHOD M K, BANERJEE J. Thermal stability of phase change materials used in latent heat energy storage systems: A review [J]. Renewable and Sustainable Energy Reviews, 2013, 18: 246–258.
[10] HU Zhi-bo, YAN Yang, ZHENG Shui-lin, SUN Qin, YIN Sheng-nan. Preparation and characterization of humidity control material based on diatomite/ground calcium carbonate composite [J]. Journal of Inorganic Materials, 2016, 1: 14.
[11] MADDISON M, MAURING T, KIRSIM E K, MANDER U. The humidity buffer capacity of clay–sand plaster filled with phytomass from treatment wetlands [J]. Building and Environment, 2009, 44(9): 1864–1868.
E K, MANDER U. The humidity buffer capacity of clay–sand plaster filled with phytomass from treatment wetlands [J]. Building and Environment, 2009, 44(9): 1864–1868.
[12] LIU Fan-han, XU Jian-xin, WANG Hui-tao, WANG Hua. Numerical method and model for calculating thermal storage time for an annular tube with phase change material [J]. Journal of Central South University, 2017, 24(1): 217–226.
[13] RAO Zhong-hao, WANG Shuang-feng, ZHANG Zheng-guo. Energy saving latent heat storage and environmental friendly humidity-controlled materials for indoor climate [J]. Renewable and Sustainable Energy Reviews, 2012, 16(5): 3136–3145.
[14] FELDMAN D, BANU D, HAWES D, GHANBARI E. Obtaining an energy storing building material by direct incorporation of an organic phase change material in gypsum wallboard [J]. Solar Energy Materials, 1991, 22(2, 3): 231–242.
[15] VU D H, WANG K S, BAC B H. Humidity control porous ceramics prepared from waste and porous materials [J]. Materials Letters, 2011, 65(6): 940–943.
[16] AGYENIM F, HEWITT N. The development of a finned phase change material (PCM) storage system to take advantage of off-peak electricity tariff for improvement in cost of heat pump operation [J]. Energy and Buildings, 2010, 42(9): 1552–1560.
[17] ARUNDEL A V, STERLING E M, BIGGIN J H, STERLING T D. Indirect health effects of relative humidity in indoor environments [J]. Environmental Health Perspectives, 1986, 65: 351.
[18] AL-SAADI S N, ZHAI Z J. Modeling phase change materials embedded in building enclosure: A review [J]. Renewable and Sustainable Energy Reviews, 2013, 21: 659–673.
[19] CABEZA L F, CASTELLON C, NOGUES M, MEDRANO M, LEPPERS R, ZUBILLAGA O. Use of microencapsulated PCM in concrete walls for energy savings [J]. Energy and Buildings, 2007, 39(2): 113–119.
[20] SHILEI L V, ZHU Neng, FENG Guo-hui. Impact of phase change wall room on indoor thermal environment in winter [J]. Energy and Buildings, 2006, 38(1): 18–24.
[21] SOL A, MIR
A, MIR L, BARRENECHE C, MARTORELL I, CABEZA L F. Review of the T-history method to determine thermophysical properties of phase change materials (PCM) [J]. Renewable and Sustainable Energy Reviews, 2013, 26: 425–436.
L, BARRENECHE C, MARTORELL I, CABEZA L F. Review of the T-history method to determine thermophysical properties of phase change materials (PCM) [J]. Renewable and Sustainable Energy Reviews, 2013, 26: 425–436.
[22] SUN J Z, WU Z Z. Study on evaluation method of exudation of phase transition working substance for building materials [J]. New Build Mater, 2004, 7: 43–46.
[23] KEDL R J, STOVALL T K. Activities in support of the wax-impregnated wallboard concept [R]. Oak Ridge National Lab, TN (USA), 1989.
[24] SHAPIRO A B. Solar thermal energy storage using a paraffin wax phase change material [J]. Energy Production and Conversion, 1980, 2(6): 65–72.
[25] LI Hui-qiang, CHEN Hui-su, LI Xiang-yu, SANJAYAN J. Development of thermal energy storage composites and prevention of PCM leakage [J]. Applied Energy, 2014, 135: 225–233.
[26] ZHANG Dong, ZHOU Jian-ming, WU Ke-yu, LI Zong-jin. Granular phase changing composites for thermal energy storage [J]. Solar Energy, 2005, 78(3): 471–480.
[27] KARAIPEKLI A, SARI A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage [J]. Solar Energy, 2009, 83(3): 323–332.
[28] WEN Zhi-hong, CHEN Bin. Performance analysis and application of adiabatic insulation product made from expanded perlite [J]. Journal of Zhengzhou Institute Technology, 2005, 4: 12. (in Chinese)
[29] SARI A, KARAIPEKLI A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material [J]. Applied Thermal Engineering, 2007, 27(8): 1271–1277.
[30] EVOLA G, MARLETTA L, SICURELLA F. A methodology for investigating the effectiveness of PCM wallboards for summer thermal comfort in buildings [J]. Building and Environment, 2013, 59: 517–527.
[31] FANG L, CLAUSEN G, FANGER P O. Temperature and humidity: Important factors for perception of air quality and for ventilation requirements/Discussion [J]. ASHRAE Transactions, 2000, 106: 503.
[32] OHASHI F, MAEDA M, INUKAI K, SUZUKI M, TOMURA S. Study on intelligent humidity control materials: Water vapor adsorption properties of mesostructured silica derived from amorphous fumed silica [J]. Journal of Materials Science, 1999, 34(6): 1341–1346.
[33] JIA Yu-xin, HAN Wei, XIONG Guo-xing, YANG Wei-shen. Diatomite as high performance and environmental friendly catalysts for phenol hydroxylation with H2O2 [J]. Science and Technology of Advanced Materials, 2007, 8(1): 106–109.
[34] VU D H, WANG K S, BAC B H, NAM B X. Humidity control materials prepared from diatomite and volcanic ash [J]. Construction and Building Materials, 2013, 38: 1066–1072.
[35] HUMPHREYS M A, NICOL J F, RAJA I A. Field studies of indoor thermal comfort and the progress of the adaptive approach [J]. Advances in Building Energy Research, 2007, 1(1): 55–88.
[36] KONG Xiang-fei, ZHONG Yu-liang, RONG Xian, MIN Chun-hua, QI Cheng-ying. Building energy storage panel based on paraffin/expanded perlite: Preparation and thermal performance study [J]. Materials, 2016, 9(2): 70.
[37] KONG Xiang-fei, YAO Cheng-qiang, JIE Peng-fei, LIU Yun, QI Cheng-ying, RONG Xian. Development and thermal performance of an expanded perlite-based phase change material wallboard for passive cooling in building [J]. Energy and Buildings, 2017, 152: 547–557.
[38] THOMMES M, KANEKO K, NEIMARK A V, OLIVIER J P, PODRIGUEZ F, ROUQUEROL J, SING K S W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) [J]. Pure and Applied Chemistry, 2015, 87(9, 10): 1051–1069.
[39] WANG Wen-wen, ZHOU Jia-bin, ACHARI G, YU Jia-guo, CAI Wei-quan. Cr (VI) removal from aqueous solutions by hydrothermal synthetic layered double hydroxides: Adsorption performance, coexisting anions and regeneration studies [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 457: 33–40.
[40] ZHOU Jia-bin, TANG Chuan, CHENG Bei, YU Jia-guo, JARONIEC M. Rattle-type carbon–alumina core–shell spheres: Synthesis and application for adsorption of organic dyes [J]. ACS applied Materials & Interfaces, 2012, 4(4): 2174–2179.
[41] GREGG S J, SING K S W, SALZBERG H W. Adsorption surface area and porosity [J]. Journal of the Electrochemical Society, 1967, 114(11): 279C.
(Edited by HE Yun-bin)
中文导读
基于膨胀珍珠岩/硅藻土/石蜡的复合相变蓄热调湿强板的制备及热湿性能研究
摘要:随着人们对室内热舒适度要求的不断提高,近年来建筑能耗逐渐上升。因此,被动节能材料受到广泛关注。相变材料和调湿材料是可用于建筑物的两种重要的被动节能材料。相变材料通过在相变过程中吸收或释放热量来调节室内温度,调湿材料可以依据自身特性自动调节室内的相对湿度。值得注意的是,现有的研究大多数都是单独分析相变材料或调湿材料,结合两者性能的研究却很少,限制了它们在建筑中的应用。为了研究相变材料和调湿材料的复合性能,本文以石蜡为相变材料,膨胀珍珠岩为载体材料,通过真空吸附法制备复合相变颗粒。将复合相变颗粒在自制的模具里压制成相变板材,最后在相变板材上喷涂调湿材料硅藻泥,制得具有调湿性能的复合相变墙板。利用XRD、物理吸附分析仪、SEM、DSC对物质组成、孔径、微观结构、热物理性能进行了表征,复合相变颗粒对调湿材料的平衡含湿量和循环吸放湿性能进行了测试,同时,对具有调湿性能的复合相变墙板进行性能测试。结果表明:(1)复合相变墙板具有适宜的相变参数,熔点/凝固点分别为18.23 °C/29.42 °C,高潜热值分别为54.66 J/g和55.63 J/g。(2)经过循环吸放湿测试后,硅藻泥的吸湿量总是高于放湿量,且吸湿量和放湿量有微弱的衰减,但硅藻泥的调湿性能相对稳定。(3)硅藻泥在不同温度下(T=10 °C,25 °C,40 °C; RH=85%)的调湿性能随着温度的升高而降低,在不同湿度下(RH=75%,85%,90%;T=25 °C)随着湿度的增加而增强。(4)相变材料和硅藻泥耦合,两者相互作用对各自的效果有正向加强作用,可以有效调节室内温湿度。
关键词:蓄热;调湿;相变材料;石蜡;膨胀珍珠岩;硅藻土
Foundation item: Project(51408184) supported by the National Natural Science Foundation of China; Project(E2017202136) supported by the Natural Science Foundation of Hebei Province, China; Project(BSBE2017-05) supported by the Opening Funds of State Key Laboratory of Building Safety and Built Environment and National Engineering Research Center of Building Technology, China; Project(QG2018-3) supported by Hebei Provincial Department of Transportation, China
Received date: 2017-07-11; Accepted date: 2017-12-07
Corresponding author: KONG Xiang-fei, PhD, Associate Professor; Tel: +86–22–60435787; E-Mail: xfkong@hebut.edu.cn; ORCID: 0000- 0002-1550-4071

