Recovery of magnetite from FeSO4·7H2O waste slag by co-precipitation method with calcium hydroxide as precipitant
来源期刊:中南大学学报(英文版)2017年第1期
论文作者:郑雅杰 余旺 彭映林
文章页码:62 - 70
Key words:FeSO4·7H2O; TiO2 industry; magnetite; co-precipitation; calcium hydroxide; magnetic seeding flocculation
Abstract: Proper utilization of the FeSO4·7H2O waste slag generated from TiO2 industry is an urgent need, and Fe3O4 particles are currently being widely used in the wastewater flocculation field. In this work, magnetite was recovered from ferrous sulphate by a novel co-precipitation method with calcium hydroxide as the precipitant. Under optimum conditions, the obtained spherical magnetite particles are well crystallized with a Fe3O4 purity of 88.78%, but apt to aggregate with a median particle size of 1.83 μm. Magnetic measurement reveals the obtained Fe3O4 particles are soft magnetic with a saturation magnetization of 81.73A·m2/kg. In addition, a highly crystallized gypsum co-product is obtained in blocky or irregular shape. Predictably, this study would provide additional opportunities for future application of low-cost Fe3O4 particles in water treatment field.
J. Cent. South Univ. (2017) 24: 62-70
DOI: 10.1007/s11771-017-3409-9
YU Wang(余旺)1, PENG Ying-lin(彭映林)2, ZHENG Ya-jie(郑雅杰)1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Chemistry and Environmental Engineering, Hunan City University, Yiyang 413000, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: Proper utilization of the FeSO4·7H2O waste slag generated from TiO2 industry is an urgent need, and Fe3O4 particles are currently being widely used in the wastewater flocculation field. In this work, magnetite was recovered from ferrous sulphate by a novel co-precipitation method with calcium hydroxide as the precipitant. Under optimum conditions, the obtained spherical magnetite particles are well crystallized with a Fe3O4 purity of 88.78%, but apt to aggregate with a median particle size of 1.83 μm. Magnetic measurement reveals the obtained Fe3O4 particles are soft magnetic with a saturation magnetization of 81.73 A·m2/kg. In addition, a highly crystallized gypsum co-product is obtained in blocky or irregular shape. Predictably, this study would provide additional opportunities for future application of low-cost Fe3O4 particles in water treatment field.
Key words: FeSO4·7H2O; TiO2 industry; magnetite; co-precipitation; calcium hydroxide; magnetic seeding flocculation
1 Introduction
Titanium dioxide is produced from raw materials as ilmenite, rutile, anatase and slags using “sulfate” or “chloride” routes. The sulphate route is based on FeTiO3 digestion by means of highly concentrated sulphuric acid and meanwhile a large amount of ferrous sulphate (FeSO4·7H2O) is generated [1]. The FeSO4·7H2O waste slag contains marked quantities of sulphuric acid and represents a hazardous waste. Wastes from the titanium dioxide industry are no longer allowed to dump into water bodies as noted in the directive 92/112/EEC [2]. Besides, this directive requires that the waste products should be reused or disposed of without endangering human health or harming the environment.
The common methods for utilization of ferrous sulphate are preparing iron oxide pigments and coagulants for water treatment [3-7]. Little work has been performed to utilize FeSO4·7H2O waste slag to prepare Fe3O4 particles for magnetic seeding flocculation. Magnetic particles are currently being widely studied in the wastewater flocculation field. LAKSHMANAN et al [8] used magnetic iron oxide nanoparticles for water content reduction in sewage wastewater sludge. While, JIANG et al [9] prepared a novel composite coagulant for microcystis aeruginosa removal in source water by adding magnetic Fe3O4 nanoparticles to polyferric chloride (PFC). Furthermore, magnetite was used for aqueous arsenic removal by superconducting magnetic separation [10]. In environmental engineering, however, the utilization of magnetite nanoparticles prepared from reagent-grade chemicals could not be cost-effective in the full-scale process, due to the characteristics of water or wastewater, such as high flow rate, and the presence of various pollutants and associated high consumption and loss of nanoparticles [11]. In this context, the present study aims at using FeSO4·7H2O waste slag to recover low-cost Fe3O4 particles for magnetic seeding flocculation in water and wastewater treatment.
Many methods have been developed to synthesize magnetic particles of magnetite, such as co-precipitation [12-14], microemulsions [15], sol–gel syntheses [16], and hydrothermal or solvothermal reactions [17]. However, the most common method for producing synthetic magnetite particles is the co-precipitation of Fe2+/Fe3+ ions (molar ratio 1:2) by sodium hydroxide or ammonia solution [18].
In the present study, we proposed a novel, simple, low cost and effective co-precipitation method to synthesize Fe3O4 particles. In this method, calcium hydroxide was selected as a precipitator and added to the solution of ferrous sulphate. By controlling the pH value, Fe2+ ions in the reaction solution were precipitated in the form of Fe(OH)2, then Fe(OH)2 was converted into Fe3O4 under the condition of aeration and heating. Finally, Fe3O4 particles were separated from the reaction solution by a self-designed magnetic separator.
2 Experimental
2.1 Materials
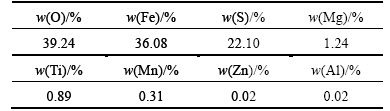
The dried ferrous sulphate sample used for this study was obtained from Guangdong Huiyun Titanium Industry Corporation Limited, China. Its crystalline phase was examined by XRD as shown in Fig. 1. Rozenite (FeSO4·4H2O) was identified to be the most abundant phase. The dried ferrous sulphate sample was dried in a vacuum atmosphere at 80 °C for 12 h, and then its chemical composition was analyzed by XRF as shown in Table 1. It is observed that iron content of dried ferrous sulphate is 36.08% and the main impurities are Mg, Ti, Mn, Zn and Al.
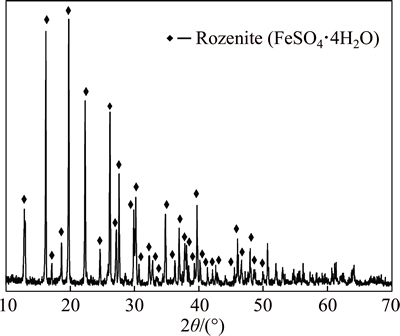
Fig. 1 XRD pattern of dried ferrous sulphate
Table 1 Chemical composition of dried ferrous sulphate
2.2 Experimental device
As shown in Fig. 2, a set of self-designed experimental devices was used to recover magnetite from ferrous sulphate. A 500mL high-type beaker was used as the reactor that would be heated in a water bath. A vent pipe connected to an adjustable flow meter that could control flow rate coming from a small air pump. A magnetic separator with permanent magnets was composed of stainless-steel stirring axis, cylinder and stirring paddle. The cylinder had sizes of outer diameter 27mm, inner diameter 21mm and height 115mm. The upper end of the stirring axis could be fixed to an electric mixer which could adjust stirring rate, while the lower end was welded to the cylinder cover. The cylinder cover could be screwed to the cylinder surface, and a stirring paddle was welded to the cylinder bottom. The permanent magnets were constituted by 4 identical magnet rings that superimposed together, and stuck into the cylinder with the help of cylinder cover. The magnet ring was made of N45H Ru-Fe-B magnet with sizes of outer diameter 20mm, inner diameter 12mm and thickness 25mm, and radially magnetized with a maximum surface magnetic induction of 0.509T.
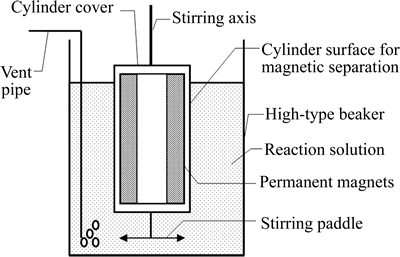
Fig. 2 Schematic diagram of experimental device
2.3 Magnetite recovery
The typical procedure used to recover magnetite from ferrous sulphate is summarized in Fig. 3. Magnetite was synthesized by using a solution of ferrous sulphate with Ca(OH)2 as the precipitant. To obtain lime milk, an amount of purified water was added to calcium oxide (CaO) with a 3:1 mass ratio of liquid to solid under vigorous stirring. The lime milk was dropwise added to the ferrous sulphate solution under constant stirring until pH reached to 8.5. Consequently, Fe2+ ions contained in the solution were precipitated in the form of Fe(OH)2. Then, the reaction solution was heated up to 80 °C by using water bath, and Fe(OH)2 was converted into Fe3O4 at a air flow rate of 1L/min. Finally, Fe3O4 particles were separated from the reaction solution by the self-designed magnetic separator (Fig. 2). The obtainedFe3O4 particles were washed several times with distilled water and then dried in a vacuum atmosphere at 80 °C for 12h. To recover gypsum, the residual reaction solution was filtrated, and then air dried at 105 °C for 4h.
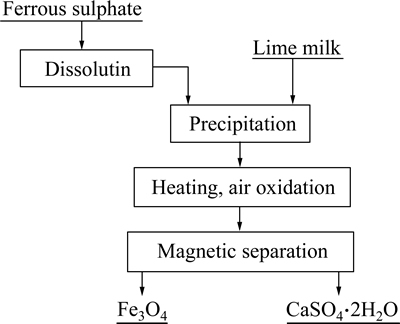
Fig. 3 Flow sheet of magnetite recovery from ferrous sulphate
2.4 Characterization
The experimental materials and products were analyzed to examine the ferrous and ferric ion content according to GB/T 1863—2008 (Chinese National Standard). Phase analysis of samples was conducted by Rigaku D/max-TTR III X-ray diffractometer (XRD) with Cu Kα radiation (λ=1.54056 ), voltage 40kV, current 250mA and at the scanning rate of 10°/min from 10° to 70°. For identification of functional groups present in the sample, Fourier transform infrared (FT-IR) spectrometer (WQF-510A, Beijing Beifen-Ruili Analytical Instrument (Group) Co., Ltd.) was used to record the spectrum from 400 to 4000cm-1. The major elements contained in samples were measured by X-ray fluorescence (XRF) with a Bruker S4 pioneer system equipped with two X-ray detectors. Surface morphology of the products was observed by using a FEI Quanta 200 scanning electron microscope (SEM). Laser particle size analyzer (LS-pop(6), Zhuhai OMEC instrument Co., Ltd.) was used to analyze the size distribution of obtained product. Magnetic properties of the sample were measured with a vibrating sample magnetometer (VSM, quantum design) at room temperature.
), voltage 40kV, current 250mA and at the scanning rate of 10°/min from 10° to 70°. For identification of functional groups present in the sample, Fourier transform infrared (FT-IR) spectrometer (WQF-510A, Beijing Beifen-Ruili Analytical Instrument (Group) Co., Ltd.) was used to record the spectrum from 400 to 4000cm-1. The major elements contained in samples were measured by X-ray fluorescence (XRF) with a Bruker S4 pioneer system equipped with two X-ray detectors. Surface morphology of the products was observed by using a FEI Quanta 200 scanning electron microscope (SEM). Laser particle size analyzer (LS-pop(6), Zhuhai OMEC instrument Co., Ltd.) was used to analyze the size distribution of obtained product. Magnetic properties of the sample were measured with a vibrating sample magnetometer (VSM, quantum design) at room temperature.
After the experiment, the residual rate, η1, of iron in reaction solution and the recovery rate, η2, of iron in magnetite product were calculated based on the mass of iron in raw material and defined as follows:
η1=m1/m×100% (1)
η2=m2/m×100% (2)
where m1 is the mass of iron in reaction solution after the experiment; m2 the mass of iron in magnetite product; and m the mass of iron in raw material. The grade, η, of iron in magnetite product was calculated based on the mass of magnetite product and defined as follows:
η=m2/m3×100% (3)
where m3 is the mass of magnetite product.
3 Results and discussion
3.1 Magnetite recovery
As mentioned previously, different experimental conditions, such as initial pH, stirring rate, reaction temperature, ferrous ion concentration and reaction time, can affect the recovery of magnetite. Therefore, it is necessary to optimize these conditions in order to obtain high-quality magnetite. In this study all these five experimental conditions were investigated one by one by keeping the four other conditions constant. The effects of these five conditions on the grade and recovery rate of iron in magnetite product are shown in Fig. 4.
3.1.1 Effect of initial pH on recovery of magnetite
To investigate the influence of initial pH, a series of experiments were performed with the initial pH ranging from 7 to 10, while the other conditions were kept at constant: temperature 80 oC, stirring rate 200r/min, ferrous ion concentration 0.2mol/L, time 3h.
XRD was used to determine crystal structures of the magnetite products obtained at different initial pH as shown in Fig. 5. The spectrum of the spinel structure of magnetite with a lattice parameter of a= 8.396 (JCPDS, No. 19-0629) can be seen in all the XRD spectra, and its seven different characteristic reflections correspond to (111), (220), (311), (400), (422), (511) and (440) plane diffractions, respectively. It indicates that all experimental reactions at different initial pH generate Fe3O4. Magnetite can be formed from ferrous salts according to the following reactions [19]:
(JCPDS, No. 19-0629) can be seen in all the XRD spectra, and its seven different characteristic reflections correspond to (111), (220), (311), (400), (422), (511) and (440) plane diffractions, respectively. It indicates that all experimental reactions at different initial pH generate Fe3O4. Magnetite can be formed from ferrous salts according to the following reactions [19]:
CaO+H2O→Ca(OH)2 (4)
FeSO4+Ca(OH)2+H2O→Fe(OH)2+CaSO4·2H2O (5)
Fe(OH)2+O2+H2O→Fe(OH)3 (6)
Fe(OH)3+Fe(OH)2→Fe3O4+H2O (7)
Meanwhile, gypsum (CaSO4·2H2O) is also observed in all the XRD spectra, indicating that magnetite is not completely separated from gypsum. Distinguishingly, goethite (FeOOH) is only observed when pH is 7 (Fig. 5(a)). It has been reported that Fe(OH)2 is easily oxidized to FeOOH at a low pH value [20]. The reaction is expressed by the following reaction equation:
Fe(OH)2+O2→FeOOH+H2O (8)
When the initial pH increases from 7 to 8.5, the residual rate of iron in reaction solution decreases sharply (Fig. 6). However, a slight downward trend is observed when the initial pH is greater than 8.5, which indicates that the iron in solution has been almost completely precipitated. With the increase of initial pH, the recovery rate of iron in magnetite product increases all the way, while the grade of iron achieves its maximum (56.11%) at a initial pH of 8 (Fig. 4(a)). Consequently, the magnetite product obtained at the initial pH of 8 is more Fe3O4 phase with a higher diffraction peak intensity of Fe3O4 (Fig. 5(b)). Taking into account all these parameters including iron grade, recovery rate of iron and residual rate of iron, the optimum initial pH is 8.5. The magnetite product obtained at this pH has a relatively high iron grade (45.98%) and a relatively low residual rate of iron (3.31%).
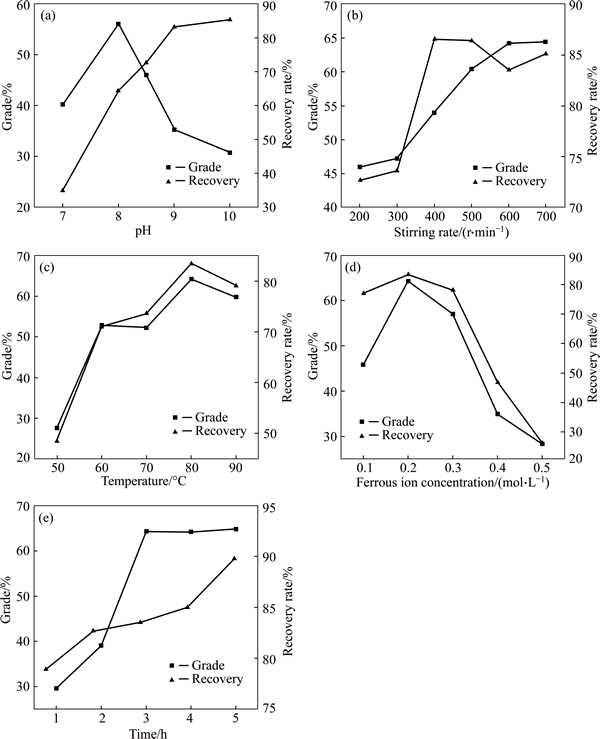
Fig. 4 Effects of initial pH (a), stirring rate (b), reaction temperature (c), ferrous ion concentration (d) and reaction time (e) on grade and recovery rate of iron in magnetite product
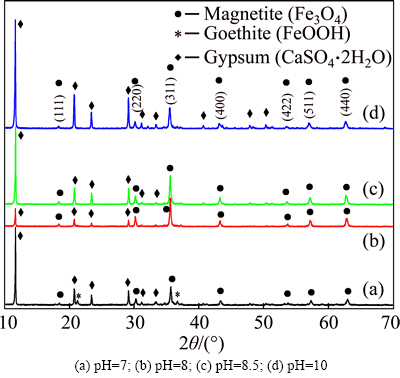
Fig. 5 XRD patterns of magnetite products obtained at different initial pH:
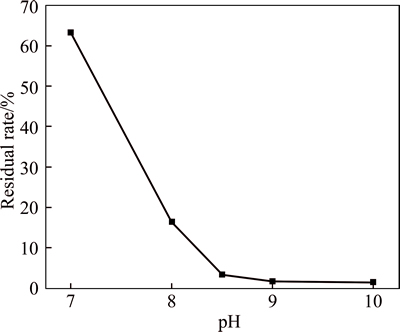
Fig. 6 Residual rate of iron in reaction solution at different initial pH
3.1.2 Effect of stirring rate on recovery of magnetite
To study the influence of stirring rate,a series of experiments were performed at different stirring rates ranging from 200 to 700 r/min under the optimal conditions for the other four experimental conditions: temperature 80 oC, initial pH 8.5, ferrous ion concentration 0.2mol/L, time 3h.
The XRD patterns illustrated in Fig. 7 all have obvious diffraction peaks and match well with the standard Fe3O4 and CaSO4·2H2O reflections. With the increase of stirring rate, the diffraction peak intensity of Fe3O4 increases and that of CaSO4·2H2O decreases. The stronger the intensity of diffraction peak, the better the crystallization of particles. According to Fig. 7, when the stirring rate is 600r/min, the diffraction peak intensity of Fe3O4 is the highest, which indicates that the product at this stirring rate is more Fe3O4 phase with better degree of crystallization.
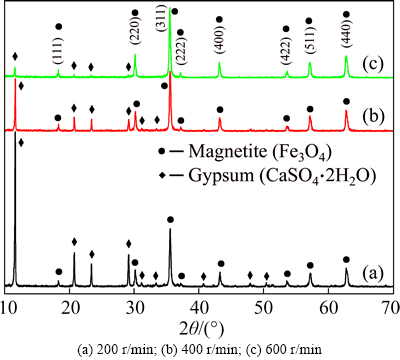
Fig. 7 XRD patterns of magnetite products obtained at different stirring rates:
While the stirring rate fluctuates from 200 to 400r/min, the recovery rate of iron in magnetite product increases sharply and reaches its peak (86.59%) at 400r/min, after that a slight downward trend is observed with a further increase of stirring rate (Fig. 4(b)). However, the iron grade increases all the way and reaches its peak (64.29%) at 600r/min, after that a nearly constant grade is observed when the stirring rate increases to 700r/min (Fig. 4(b)). Therefore, the optimal stirring rate is 600r/min, which is in agreement with the XRD results shown in Fig. 7. It can be inferred that the increase of stirring rate is favorable for recovery of magnetite. This might be because the increase of stirring rate is favorable for magnetic separation of magnetite and gypsum. With the increase of stirring rate, gypsum is thrown away from cylinder surface (Fig. 2) due to a greater centrifugal force. However, magnetite is attracted to the cylinder surface due to a strong enough magnetic force. For successful collection of magnetic particles by magnetic separator, the magnetic force attracting particles toward separation surface must dominate the fluid drag, gravitational, inertial and diffusion force as the particle suspension flows through the separator [21].
3.1.3 Effect of reaction temperature on recovery of magnetite
Reaction temperature is another important condition that shows an obvious influence on XRD pattern of the product, as shown in Fig. 8. The diffraction peak intensity of Fe3O4 enhances sharply with reaction temperature increasing between 50 and 80 oC whereas weakens a little at 90 oC. It can be inferred that high reaction temperature is benefit to prepare Fe3O4. At low temperature, Fe(II) can be oxidized to Fe(III) easily due to the low diffusion rate of Fe(II), so that the products are mainly FeOOH and Fe(OH)3 precipitates [20].
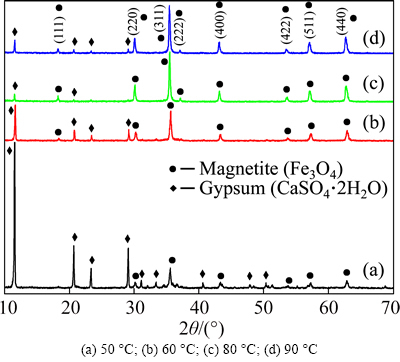
Fig. 8 XRD patterns of magnetite products obtained at different reaction temperatures:
It is shown in Fig. 4(c) that both the recovery rate and grade of iron in magnetite product increase significantly with the reaction temperature ranging from 50 to 80 oC and achieve their maximums (83.53% and 64.29%) at 80 °C, which is consistent with the results of XRD analysis shown in Fig. 8. However, both the recovery rate and grade decrease when the temperature increases from 80 to 90 °C. This might be due to the fact that a large amount of water loss caused by high temperature evaporation is unfavorable for magnetic separation of magnetite and gypsum in reaction solution. Therefore, the optimum reaction temperature is 80 °C.
3.1.4 Effect of ferrous ion concentration on recovery of magnetite
To study the effect of ferrous ion concentration, a series of experiments were performed with the concentration ranging from 0.1 to 0.5mol/L. According to Fig. 9, the diffraction peak intensity of Fe3O4 in product enhances with concentration increasing from 0.1 to 0.2mol/L whereas weakens at 0.5mol/L. Because it is easy for air to enter reaction solution at lower ferrous ion concentration, Fe(II) is oxidized to Fe(III) easily by rich air, which leads to over-oxidation of magnetite productdue to production of excess hematite(Fe2O3) [22]. When the ferrous ion concentration is too high, it is unfavorable for homogeneous distribution of air and magnetic separation of magnetite and gypsum in reaction solution, and induces heterogeneous nucleation of the precipitate due to partial over-oxidation or partial light oxidation and inclusion of magnetite and gypsum. Consequently, the product obtained at 0.2mol/L is more Fe3O4 phase with better degree of crystallization.
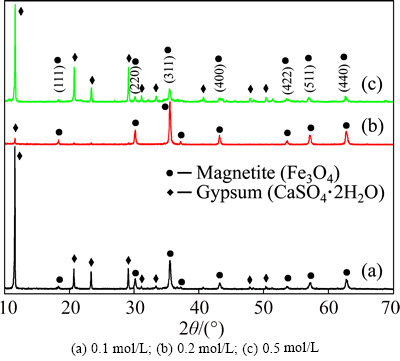
Fig. 9 XRD patterns of magnetite products obtained at different ferrous ion concentrations:
From Fig. 4(d), both the recovery rate and grade of iron in magnetite product achieve their maximum at a ferrous ion concentration of 0.2mol/L, and then decrease sharply with increasing the concentration from 0.2 to 0.5mol/L. The results are good agreement with the XRD results shown in Fig. 9. In conclusion, the optimal ferrous ion concentration is 0.2mol/L.
3.1.5 Effect of reaction time on recovery of magnetite
To study the effect of reaction time, a series of experiments were performed in different time durations ranging from 1 to 5h under the optimal experimental conditions: initial pH was 8.5, stirring rate was 600r/min, reaction temperature was 80 °C, and ferrous ion concentration was 0.2mol/L.
The XRD patterns of magnetite products obtained at different reaction times shown in Fig. 10 all have obvious diffraction maximum. It matches well with the standard Fe3O4 and CaSO4·2H2O reflections. The diffraction peak intensity of Fe3O4 in product increases with increasing reaction time. Obviously, short oxidation time can not make Fe3+/Fe2+ ratio meet the requirement of Fe3O4 formula. When the reaction time is shorter than 3 h, it does not have enough time for the oxidation of Fe(II) and so Fe(II) would exist in the final products. After drying, Fe(II) turns into FeO and then is oxidized to Fe2O3, which makes the magnetite product impure with a lower Fe3O4 peak intensity (Fig. 10(a)). With the increase of reaction time, more Fe(II) can be oxidized to Fe(III), which makes the mole ratio of Fe3+/Fe2+ in product increase. When the ratio is about 2.0, the product is more Fe3O4 phase with a higher Fe3O4 peak intensity (Figs. 10(b) and (c)).
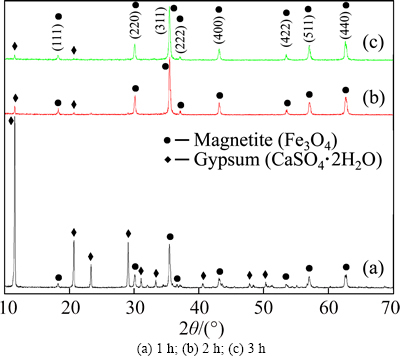
Fig. 10 XRD patterns of the magnetite products obtained at different reaction times:
From Fig. 4(e), it is found that with the increase of reaction time, the recovery rate of iron in magnetite product increases slowly from 78.89 to 89.79%. However, the grade of iron increases significantly and reaches its peak of 64.29% when the reaction time is maintained at 3 h, after which a constant grade is observed when the time increases to 5 h, which agrees well with the XRD results in Fig. 10. Considering the cost of production, 3 h for reaction time is better in this study.
3.2 Characterization of magnetite product and gypsum co-product
Characteristics of the magnetite product obtained under the optimum conditions were determined by XRD, FT-IR, SEM, LPSA, VSM, XRF and titration. The titration results show that the content of Fe3O4 in magnetite product is 88.78%. Figure 11(a) shows the XRD pattern of magnetite product, which indicates the highly crystalline character of magnetite and matches well with the standard Fe3O4 reflections. Figure 11(b) shows the FT-IR spectrum of magnetite product. As seen, a broad band exists at around 576.6cm-1, assignable to the Fe—O of the magnetite [23, 24]. The SEM image of magnetite product is shown in Fig. 11(c). From the image, one can find that spherical shaped Fe3O4 particles are obtained, which is consistent with the results by KULKARNI et al [25]. However, because of the large specific surface area (surface-to-volume ratio) and high surface energy, Fe3O4 particles are apt to aggregate during the process of filtration and drying [26]. Figure 11(d) shows the size distribution of magnetite product. It is observed that the size of the product is mainly between 0.35 and 10μm with a median particle size of 1.83μm.
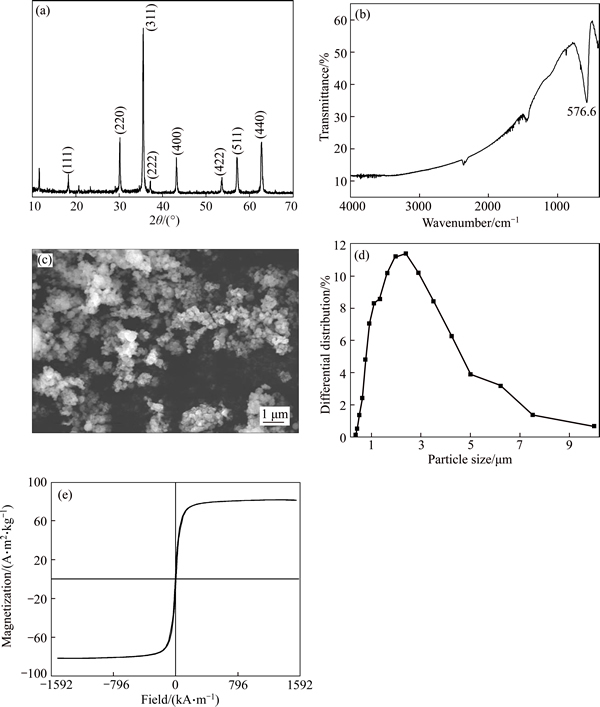
Fig. 11 XRD pattern (a), FT-IR spectrum (b), SEM image (c), size distribution (d) and magnetization hysteresis versus magnetic field curve (e) of magnetite product
The magnetization versus magnetic field curve of magnetite product is shown in Fig. 11(e). The magnetic hysteresis presents soft magnetic behavior with the highest saturation magnetization of 81.73A·m2/kg, the coercivity value of 5.79 kA/m, and the remanence value of 8.95A·m2/kg. This indicates that the Fe3O4 particles possess strong magnetic properties. To further understand the chemical composition of magnetite product, it was analyzed by XRF and the results are shown in Table 2. It is seen that Fe occupies 69.60% in product, and the major impurity elements are Ti, Ca, S, Mn and Mg.
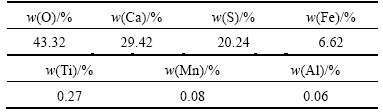
Characteristics of gypsum co-product obtained under the optimum conditions were determined by XRD, SEM and XRF. The XRD pattern of gypsum co-product is given in Fig. 12(a). The position and relative intensity of diffraction peaks in the XRD pattern matches well with the standard of CaSO4·2H2O reflections, indicating that the obtained CaSO4·2H2O particles are highly crystallized. Figure 12(b) shows the SEM image of gypsum co-product. It is evident that crystalline aggregates of different sizes are obtained in blocky or irregular shape. The chemical composition of gypsum co-product was analyzed by XRF and the results are given in Table 3. It indicates that calcium occupies 29.42% in product, and the major impurity elements are Fe and Ti.
Table 2 Chemical composition of magnetite product
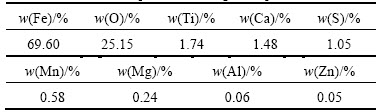
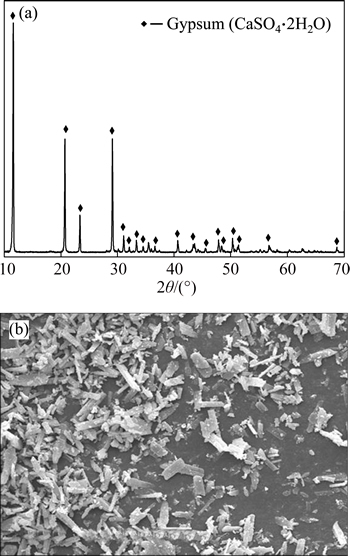
Fig. 12 XRD pattern (a) and SEM image (b) of gypsum co-product
Table 3 Chemical composition of gypsum co-product
4 Conclusions
1) As a waste slag from TiO2 industry, ferrous sulphate was successfully used to recover magnetite particles by a simple, efficient, economical and environment-friendly co-precipitation method with calcium hydroxide as the precipitant. The optimum technological parameters are shown below: initial pH 8.5, stirring rate 600r/min, reaction temperature 80 °C, ferrous ion concentration is 0.2mol/L, reaction time 3h and the air flow rate 1L/min.
2) XRD pattern and FT-IR spectrum of the obtained magnetite product match well with the standard data of magnetite Fe3O4 (JCPDS, No. 19-0629). Titration analysis shows that the purity of Fe3O4 particles is 88.78%, and XRF analysis indicates the presence of trace impurities (Ti, Ca, S, Mn and Mg). According to SEM image and laser particle size analysis, the obtained spherical magnetite particles are apt to aggregate with a median particle size of 1.83 μm. The magnetic hysteresis curve of magnetite product indicates its soft magnetic property with a saturation magnetization of 81.73A·m2/kg. In addition, a highly crystallized gypsum co-product is obtained in blocky or irregular shape. Predictably, this study would provide a great opportunity for manufacturing of low-cost Fe3O4 particles for magnetic seeding flocculation in water treatment field.
References
[1] GAZQUEZ M J, BOLIVAR J P, GARCIA-TENORIO R, VACA F. Physicochemical characterization of raw materials and co-products from the titanium dioxide industry [J]. Journal of Hazardous Materials, 2009, 166(2, 3): 1429-1440.
[2] EEC. Council directive 92/112/EEC of 15 December 1992 on procedures for harmonizing the programmes for the reduction and eventual elimination of pollution caused by waste from the titanium dioxide industry [S].
[3] TAVANI E L, LACOUR N A. Making of iron(III) tanning salts from a waste of the titanium recovery by the sulphate process [J]. Materials Chemistry and Physics, 2001, 72(3): 380-386.
[4] GUSKOS N, PAPADOPOULOS G J, LIKODIMOS V, PATAPIS S, YARMIS D, PRZEPIERA A, PRZEPIERA K, MAJSZCZYK J, TYPEK J, WABIA M, AIDINIS K, DRAZEK Z. Photoacoustic, EPR and electrical conductivity investigations of three synthetic mineral pigments: Hematite, goethite and magnetite [J]. Materials Research Bulletin, 2002, 37(6): 1051-1061.
[5] LIU Zhao-cheng, ZHENG Ya-jie. Micaceous iron oxide prepared from pyrite cinders by hydrothermal method [J]. Journal of Central South University, 2011, 18(1): 89-95.
[6] ZHENG Ya-jie, LIU Zhao-cheng. Preparation of monodispersed micaceous iron oxide pigment from pyrite cinders [J]. Powder Technology, 2011, 207(1-3): 335-342.
[7] ZHENG Ya-jie, GONG Zhu-qing, CHEN bai-zhen, LIU Li-hua. Preparation of solid polyferric sulfate from pyrite cinders and its structure feature [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(3): 690-694.
[8] LAKSHMANAN R, RAJARAO-KUTTUVAL G. Effective water content reduction in sewage wastewater sludge using magnetic nanoparticles [J]. Bioresource Technology, 2014, 153: 333-339.
[9] JIANG Chen, WANG Ren, MA Wei. The effect of magnetic nanoparticles on Microcystis aeruginosa removal by a composite coagulant [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 369(1-3): 260-267.
[10] LI Yi-ran, WANG Jun, ZHAO Ying, LUAN Zhao-kun. Research on magnetic seeding flocculation for arsenic removal by superconducting magnetic separation [J]. Separation and Purification Technology, 2010, 73(2): 264-270.
[11] WEI Xin-chao, VIADERO-JR. R C. Synthesis of magnetite nanoparticles with ferric iron recovered from acid mine drainage: Implications for environmental engineering [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 294(1-3): 280-286.
[12] SHEN La-zhen, QIAO Yong-sheng, GUO Yong, MENG Shuang-ming, YANG Guo-chen, WU Mei-xia, ZHAO Jiang-guo. Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles [J]. Ceramics International, 2014, 40(1, Part B): 1519-1524.
[13] NABIYOUNI G, JULAEE M, GHANBARI D, ALIABADI P C, SAFAIE N. Room temperature synthesis and magnetic property studies of Fe3O4 nanoparticles prepared by a simple precipitation method [J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 599-603.
[14] LIN Chia-chang, HO Jui-min, WU Min-shan. Continuous preparation of Fe3O4 nanoparticles using a rotating packed bed: Dependence of size and magnetic property on temperature [J]. Powder Technology, 2015, 274: 441-445.
[15] ZHANG Dong-en, TONG Zhi-wei, LI Shan-zhong, ZHANG Xiao-bo, YING Ai-ling. Fabrication and characterization of hollow Fe3O4 nanospheres in a microemulsion [J]. Materials Letters, 2008, 62(24): 4053-4055.
[16] ALBORNOZ C, JACOBO S E. Preparation of a biocompatible magnetic film from an aqueous ferrofluid [J]. Journal of Magnetism and Magnetic Materials, 2006, 305(1): 12-15.
[17] MAO Bao-dong, KANG Zhen-hui, WANG En-bo, LIAN Suo-yuan, GAO Lei, TIAN Chun-gui, WANG Chun-lei. Synthesis of magnetite octahedrons from iron powders through a mild hydrothermal method [J]. Materials Research Bulletin, 2006, 41(12): 2226-2231.
[18] VALENZUELA R, FUENTES M C, PARRA C, BAEZA J, DURAN N, SHARMA S K, KNOBEL M, FREER J. Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method [J]. Journal of Alloys and Compounds, 2009, 488(1): 227-231.
[19]  A, LAGZDINA S, JUHNEVICA I, JAKOVLEVS D, MAIORV M. Precipitation synthesis of magnetite Fe3O4 nanoflakes [J]. Ceramics International, 2014, 40(7, Part B): 11437-11440.
A, LAGZDINA S, JUHNEVICA I, JAKOVLEVS D, MAIORV M. Precipitation synthesis of magnetite Fe3O4 nanoflakes [J]. Ceramics International, 2014, 40(7, Part B): 11437-11440.
[20] SHEN La-zhen, QIAO Yong-sheng, GUO Yong, TAN Jun-ru. Preparation and formation mechanism of nano-iron oxide black pigment from blast furnace flue dust [J]. Ceramics International, 2013, 39(1): 737-744.
[21] AMBASHTA R D,  M. Water purification using magnetic assistance: A review [J]. Journal of Hazardous Materials, 2010, 180(1-3): 38-49.
M. Water purification using magnetic assistance: A review [J]. Journal of Hazardous Materials, 2010, 180(1-3): 38-49.
[22] SHEN La-zhen, QIAO Yong-sheng, GUO Yong, TAN Jun-ru. Preparation of nanometer-sized black iron oxide pigment by recycling of blast furnace flue dust [J]. Journal of Hazardous Materials, 2010, 177(1-3): 495-500.
[23] ZHANG Wen-xue, LU Bin, TANG Hui-hui, ZHAO Jing-xiang, CAI Qing-hai. Reclamation of acid pickling waste: A facile route for preparation of single-phase Fe3O4 nanoparticle [J]. Journal of Magnetism and Magnetic Materials, 2015, 381: 401-404.
[24] CHEN Ru-fen, SONG Shan-shan, WEI Yu. Study on the formation and phase transformation of 1D nanostructured Fe3O4 particles under an external magnetic field [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 395: 137-144.
[25] KULKARNI S A, SAWADH P S, PALEI P K, KOKATE K K. Effect of synthesis route on the structural, optical and magnetic properties of Fe3O4 nanoparticles [J]. Ceramics International, 2014, 40(1, Part B): 1945-1949.
[26] HONG R Y, LI J H, LI H Z, DING J, ZHENG Y, WEI D G. Synthesis of Fe3O4 nanoparticles without inert gas protection used as precursors of magnetic fluids [J]. Journal of Magnetism and Magnetic Materials, 2008, 320(9): 1605-1614.
(Edited by YANG Hua)
Cite this article as: YU Wang, PENG Ying-lin, ZHENG Ya-jie. Recovery of magnetite from FeSO4·7H2O waste slag by co-precipitation method with calcium hydroxide as precipitant [J]. Journal of Central South University, 2017, 24(1): 62-70. DOI: 10.1007/s11771-017-3409-9.
Foundation item: Project(2013A090100013) supported by the Special Project on the Integration of Industry, Education and Research of Guangdong Province, China; Project(201407300993) supported by the High-Tech Research and Development Program of Xinjiang Uygur Autonomous Region, China
Received date: 2016-02-22; Accepted date: 2016-05-27
Corresponding author: ZHENG Ya-jie, Professor, PhD; Tel: +86-731-88836285; E-mail: zyj@csu.edu.cn

