J. Cent. South Univ. Technol. (2008) 15: 819-823
DOI: 10.1007/s11771-008-0151-3
Electrical conductivity of (Na3AlF6-40%K3AlF6)-AlF3-Al2O3 melts
HUANG You-guo(黄有国)1, 2, LAI Yan-qing(赖延清)1, TIAN Zhong-liang(田忠良)1,
LI Jie(李 劼)1, LIU Ye-xiang(刘业翔)1, LI Qing-yu(李庆余)2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Guangxi Normal University, Guilin 541004, China)
Abstract: The effects of contents of AlF3 and Al2O3, and temperature on electrical conductivity of (Na3AlF6-40%K3AlF6)- AlF3-Al2O3 were studied by continuously varying cell constant (CVCC) technique. The results show that the conductivities of melts increase with the increase of temperature, but by different extents. Every increasing 10 ℃ results in an increase of 1.85×10-2, 1.86×10-2, 1.89×10-2 and 2.20×10-2 S/cm in conductivity for the (Na3AlF6-40%K3AlF6)-AlF3 melts containing 0%, 20%, 24%, and 30% AlF3, respectively. An increase of every 10 ℃ in temperature results an increase about 1.89×10-2, 1.94×10-2, 1.95×10-2, 1.99×10-2 and 2.10×10-2 S/cm for (Na3AlF6-40%K3AlF6)-AlF3-Al2O3 melts containing 0%, 1%, 2%, 3% and 4% Al2O3, respectively. The activation energy of conductance was calculated based on Arrhenius equation. Every increasing 1% of AlF3 results in a decrease of 0.019 and 0.020 S/cm in conductivity for (Na3AlF6-40%K3AlF6)-AlF3 melts at 900 and 1 000 ℃, respectively. Every increase of 1% Al2O3 results in a decrease of 0.07 S/cm in conductivity for (Na3AlF6-40%K3AlF6)-AlF3-Al2O3 melts. The activation energy of conductance increases with the increase in content of AlF3 and Al2O3.
Key words: aluminum electrolysis; electrical conductivity; activation energy; additive; superheat
1 Introduction
Al2O3 can be dissolved well by elpasolite[1-4]. The problems of deposition of Al2O3 and cathodic crust are hopeful to be solved by elpasolite at low temperature electrolysis[5-6]. Meanwhile, the service condition of inert anode will be improved and important in energy saving during the process of aluminum electrolysis by the application of elpasolite. Therefore, the research on physicochemical properties of elpasolite or mixture of elpasolite and cryolite has attracted much attention[7-10].
In order to develop a new kind of aluminum electrolyte which has properties of lower crystallized temperature and higher solubility of Al2O3, and to improve service condition of inert anode, liquidus temperature of Na3AlF6-K3AlF6 mixture melts was studied systematically in Refs.[11-13]. However, 35%- 40% of the total energy consumption of electrolysis is in the voltage of bath during aluminum electrolysis[14]. Therefore, studying the relationship between electrical conductivity and its influencing factors, and adjusting the compositions of bath and technological parameter are important for the energy consumption in aluminum electrolysis[15-17]. Moreover, the study on conductance properties of bath will promote the research on structure of melts and migration character of ions under the effect of electric field[18].
However, most literatures concerned the system containing K3AlF6 or K3AlF6-Al2O3, and the study on the electrical conductivity of system containing mixture of K3AlF6 and Na3AlF6 was rarely. In this work, the electrical conductivity of the mixture (Na3AlF6- 40%K3AlF6)-AlF3-Al2O3 was studied by the continuously varying cell constant technique based on Refs.[19-20].
2 Experimental
Na3AlF6, K3AlF6 and Al2O3 were reagent grade. AlF3 was high pure reagent. In order to remove the water, all reagents were dried at 393 K for over 24 h in a vacuum drying box.
The schematic drawing of measurement assembly used in high temperature experiment is shown in Fig.1. A pyrolytic boron nitride (BN) tube was used as conductivity cell. Alternating current (AC) impedance was determined by an EG & G Model 273A type potentiostat and 5210 lock-in amplifier. The electrical conductivity of melts was measured by the continuously varying cell constant technique. The cross-sectional area of pyrolytic BN cell tube was determined by measuring the electrical conductivity of KCl aqueous. In this work, the AC amplitude was 5 mV; the frequency was varied from 1 to 50 kHz.
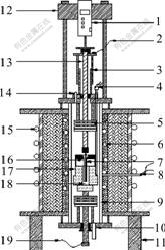
Fig.1 Schematic drawing of measurement of electrical conductivity for molten salts: 1—Motion sensor; 2—Leading wire of work electrode; 3—Motion setting; 4—Thermocouple for temperature measurement; 5—Corundun chip; 6—Heating wire; 7—Pt electrode; 8—Thermal couple of controller; 9—Al2O3 tube; 10—Gas inlet; 11—Base; 12—Bracket; 13—Electrode bar; 14—Gas outlet; 15—Cooling water duct; 16—Cell; 17—Graphite crucible; 18—Melts; 19—Leading wire of counter electrode
3 Results and discussion
3.1 Effect of temperature on electrical conductivity of melts
Fig.2 shows the electrical conductivity of the system (Na3AlF6-40%K3AlF6)-24%AlF3 and (Na3AlF6- 40%K3AlF6)-24%AlF3-Al2O3 as a function of tempera- ture, respectively. As shown in Fig.2, the electrical conductivity (s) is almost linear function of the temperature (t) and the variation trend for the two systems is similar. As shown in Fig.2(a), every increase of 10℃ temperature results in an increase about 1.85×10-2, 1.86×10-2, 1.89×10-2 and 2.20×10-2 S/cm in the electrical conductivity of the systems (Na3AlF6 + K3AlF6)-AlF3 containing 0, 20%, 24% and 30% AlF3, respectively. While, from Fig.2(b), every increase of 10 ℃ temperature results in an increase about 1.89×10-2, 1.94×10-2, 1.95×10-2, 1.99×10-2 and 2.10×10-2 S/cm in the electrical conductivity for melts with Al2O3 content of 0, 1%, 2%, 3% and 4%, respectively.
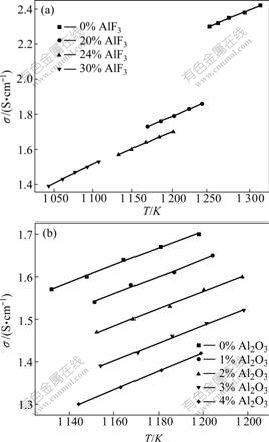
Fig.2 Electrical conductivity as function of bath temperature for different melts: Symbols—Experiment data in this work; Lines—Calculated values based on Arrhenius equation; (a) (Na3AlF6-40%K3AlF6)-AlF3 melt; (b) (Na3AlF6-40%K3AlF6)- 24%AlF3- Al2O3
Molten cryolitic fluoride is ionic conductor. The melts contain not only some simple ions, such as Na+, K+ and F-, but also some large volume complexes, such as AlF63-, AlF52- and AlF4-. The increase of temperature results in the increase of velocity of ions in melts, whichresults in the increase of electrical conductivity of melts. At the same time, the increase of temperature increases the ionization degree of complex ions, such as AlF63-, AlF52- and AlF4-. These complex ions partly decompose into small volume ion. Therefore, in unit volume, the number of ions involved in conductance increases. This is one of the reasons for the increase of electrical conductivity of melts. In microscopic analysis, only those ions with energy equal to or higher than activation energy take participate in the conductance of ions. The increase of temperature results in more ions to obtain sufficient kinetic energy, which means more ions involved in conductance. Finally, the electrical conductivity increases with the increase of temperature.
Quantitatively, the relationship between electrical conductivity and temperature is determined by the Arrhenius equation:
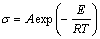 (1)
(1)
where s is the specific conductivity, S/cm; A is the pre-exponential related to chemical composition, S/cm; E is the activation energy of conductance, J/mol; T is the temperature of the melts, K; R is the universal gas constant and has the value of 8.314 J/( K·mol). By fitting the experimental electrical conductivities of different systems at different temperatures to Eqn.(1), A and E of different systems were obtained, respectively. The results are listed in Table 1 and Table 2, respectively.
Table 1 Pre-exponential (A) and activation energy (E) of conductance of (Na3AlF6-40%K3AlF6)- AlF3 melt

Table 2 Pre-exponential (A) and activation energy (E) of conductance of (Na3AlF6-40%K3AlF6)- 24%AlF3-Al2O3 melt

Based on Eqn.(2), the calculated values of electrical conductivity are shown in Fig.2 (line).
3.2 Effects of AlF3 content on electrical conductivity
The influence of AlF3 content on the electrical conductivity of melts (Na3AlF6-40%K3AlF6) is shown in Fig.3. As shown in Fig.3, the electrical conductivity of melts decreases with increasing content of AlF3. Every increase of 1% AlF3 in the AlF3 content range of 0-30% results in a decrease about 0.02 S/cm in the electrical conductivity for the melts (Na3AlF6-40%K3AlF6)-AlF3 at 1 273 K. While every increase of 1% AlF3 results in a decrease about 0.019 S/cm in the electrical conductivity at 1 173 K. This effect is probably due to the reaction of AlF3 with the free F- under formation of large volume complexes, such as AlF52- and AlF4-, after the addition of AlF3 into the cryoltie mixture. The molar fractions of AlF52- and AlF4- increase with the increase of the content of AlF3[21], which increases the interaction to Na+ and hinders the migration of Na+. Therefore, the increase in AlF3 content results in the decrease of the electrical conductivity.

Fig.3 Electrical conductivities of cryolitic (Na3AlF6- 40%K3AlF6)-AlF3 mixtures at different temperatures
Moreover, as shown in Fig.3, the electrical conductivity of system (Na3AlF6-40%K3AlF6)-AlF3 is lower than that of system Na3AlF6-AlF3. The main reasonis that though both of Na and K are alkali, the radius of K+ is longer than that of Na+. Therefore, the rate of migration of K+ is slower than that of Na+ in the electric field. Na3AlF6 has better conductance performance than K3AlF6 in the same condition (the electrical conductivity of Na3AlF6 is 0.55 S/cm higher than that of K3AlF6). Thus, the electrical conductivity decreases after the addition of the elpasolite into cryolite.
Activation energy of conductance is related to the energy-barrier through which ions transport, and the higher the activation energy of conductance, the less the electrical conductivity. Activation energies of conductance are 10.5, 12.5, 13.2, and 14.4 kJ/mol for system containing 0%, 20%, 24%, and 30% AlF3 respectively. Activation energy of conductance increases with increasing AlF3 content. The experimental electrical conductivity decreases with increasing AlF3 content and activation energy of conductance.
3.3 Effect of Al2O3 content on electrical conductivity of melts
Fig.4 shows the electrical conductivity of system (Na3AlF6-40%K3AlF6)-24%AlF3 as a function of Al2O3 content. As shown in Fig.4, the electrical conductivity of (Na3AlF6-40%K3AlF6)-24%AlF3 decreases with increasing content of Al2O3 at different temperatures. Every increase of 1% Al2O3 results in a decrease about 0.07 S/cm in the electrical conductivity in the temperature range of 1 123-1 223 K. Considering the influence of Al2O3 on liquidus temperature of bath, the electrical conductivity decreases further at the same superheat after the effect of temperature is removed. The electrical conductivity of (KF-AlF3-Al2O3) (cryotile ratio (CR)=1.3) melts was determined by KRYUKOVSKY et al[8]. The results show that every increase 1% of Al2O3 content results in a decrease about 0.025 S/cm in the electrical conductivity. The electrical conductivity of (KF-AlF3-Al2O3) (CR=1.2) melts was measured by HIVES and THONSTAD[7]. However, the results show that every increasing 1% of Al2O3 content results in a decrease about 0.010 S/cm in the electrical conductivity, which is lower than that in this work.

Fig.4 Electrical conductivity as function of Al2O3 content for cryolite mixture
Firstly, Al2O3 is decomposed, which releases O2- after adding Al2O3 into cryolite. Because the radius of O2- is close to that of F-, F- in AlF63- complex ion will be substituted by O2-. The aluminum-oxygen-fluorine complex (such as Al2OF62- and Al2O2F42-) comes into being. These complex ions are similar to AlF52- and AlF4- with large volume and hinder the migration of Na+. Thus, the electrical conductivity of melts decreases after the addition of Al2O3.
The activation energies of conductance of (Na3AlF6- 40%K3AlF6)-24%AlF3-Al2O3 melt are 14.1, 14.9, 16.0 and 17.7 kJ/mol for systems containing 1%, 2%, 3%, and 4% Al2O3, respectively. The activation energy of conductance increases with the increase of Al2O3 content, which means that the migration of the current of carrying species is blocked by Al2OF62- and Al2O2F42-.
4 Conclusions
1) The electrical conductivity is almost a linear function of the temperature. The conductive capacity of melts increases with the increase of the temperature, but by different extents. Every increase of 10 K temperature results in an increase about 1.85×10-2, 1.86×10-2, 1.89×10-2 and 2.2×10-2 S/cm in the electrical conductivity for the melts with AlF3 content of 0%, 20%, 24%, and 30%, respectively. Every increase 10 K in temperature results in an increase about 1.89×10-2, 1.94×10-2, 1.95×10-2, 1.99×10-2 and 2.10×10-2 S/cm for the melts containing 0%, 1%, 2%, 3% and 4% Al2O3, respectively. Based on Arrhenius equation, the activation energies of conductance are calculated for both (Na3AlF6-40%K3AlF6)-AlF3 melt and (Na3AlF6-40% K3AlF6)-24%AlF3-Al2O3 melt.
2) For the system (Na3AlF6-40%K3AlF6)-AlF3, the electrical conductivity decreases with the increase of AlF3 content. Every increase of 1% AlF3 results in a decrease about 0.019 and 0.02 S/cm in the electrical conductivity with the AlF3 content from 0 to 30% at 1 173 and 1 273 K, respectively. The activation energy of conductance of (Na3AlF6-40%K3AlF6)-AlF3 melt increases with the increase of AlF3 content.
3) The electrical conductivity decreases with the increase of Al2O3 content for the system (Na3AlF6- 40%K3AlF6)-24%AlF3 at different temperatures. Every increase of 1% Al2O3 results in a decrease about 0.07 S/cm in the electrical conductivity with the Al2O3 content ranging from 0 to 4% within the temperature range of 1 123-1 223 K. The activation energy of conductance of (Na3AlF6-40%K3AlF6)-24%AlF3-Al2O3 melt increases with the increase of Al2O3 content.
References
[1] JIANHONG Y, GRACZYK D G, WUNSCH C, HRYN J N. Alumina solubility in KF-AlF3-based low-temperature electrolyte system [C]// WONBONG C. Light Metals. Orlando: TMS, 2007: 537-541.
[2] REDKIN A, TKATCHEVA O, ZAIKOV Y, ZAIKOV Y, APISAROV A. Modeling of cryolite-alumina melts properties and experimental investigation of low melting electrolytes [C]// SORLIE M. Light Metals. Orlando: TMS, 2007: 513-517.
[3] YANG J, HRYN J N, KRUMDICK G K. Aluminum electrolysis tests with inert anodes in KF-AlF3-based electrolytes [C]// GALLOWAY T. Light Metals. San Antonio: TMS, 2006: 421-424.
[4] YANG J, HRYN J N, DAVIS B R, ROY A, KRUMDICK G K, POMYKALA J J. New opportunities for aluminum electrolysis with metal anodes in a low temperature electrolyte system [C]// TABEREAUX A T. Light Metals. Carlotte: TMS, 2004: 321-326.
[5] REN Bi-jun, SHI Zhong-ning, LIU Shi-ying, QIU Zhu-xian. Deterioration mechanism of cathode in 300 kA prebaked anode aluminum reduction cells [J]. Journal of Northeastern University: Natural Science, 2007, 28(6): 843-846. (in Chinese)
[6] LIU Shi-ying, SHI Zhong-ning, QIU Zhu-xian, REN Bi-jun, CAO Quan-hong. Sludge formation and analysis in aluminium reduction cells [J]. Light Metals, 2006(7): 34-36. (in Chinese)
[7] HIVES J, THONSTAD J. Electrical conductivity of low-melting electrolytes for aluminium smelting [J]. Electrochimica Acta, 2004, 49(28): 5111-5114.
[8] KRYUKOVSKY V A, FROLOV A V, TKATCHEVA O Y, REDKIN A A, ZAIKOV Y P, KHOKHLOV V A, APISAROV A P. Electrical conductivity of low melting cryolite melts [C]// GALLOWAY T J. Light Metals. San Antonio: TMS, 2006: 409-413.
[9] WANG J, LAI Y, TIAN Z, LI J, LIU Y. Investigation of 5Cu-(10NiO-NiFe2O4) inert anode corrosion during low-temperature aluminum electrolysis [C]// SORLIE M. Light Metals. Orlando: TMS, 2007: 525-530.
[10] ZAIKOV Y, CHUIKIN A, REDKIN A, KHRAMOV A, SHUROV N, CHEMEZOV O, KRYUKOVSKII V. Interaction of heat resistance concrete with low melting electrolyte KF-AlF3(CR=1.3) [C]// SORLIE M. Light Metals. Orlando: TMS, 2007: 369-372.
[11] WANG Jia-wei, LAI Yan-qing, TIAN Zhong-liang, LIU Ye-xiang. Effect of electrolysis superheat degree on anticorrosion performance of 5Cu/(10NiO-NiFe2O4) cermet inert anode [J]. Journal of Central South University of Technology, 2007, 14(6): 768-772.
[12] LAI Yan-qing, TIAN Zhong-liang, LI Jie, YE Shao-long, LI Xian-zheng, LIU Ye-xiang. Results from 100 h electrolysis testing of NiFe2O4 based cermet as inert anode in aluminum reduction [J]. Trans Nonferrous Met Soc China, 2006, 16(4): 970-974.
[13] WANG J, LAI Y, TIAN Z, LI J, LIU Y. Temperature of primary crystallization in party of system Na3AlF6-K3AlF6-AlF3 [C]// DEYOUNG D. Light Metals. New Orleans: TMS, 2008: 513-518.
[14] QIU Zhu-xian. Aluminum smelting in pre-baked cell [M]. Beijing: Metallurgical Industry Press, 2005: 286. (in Chinese)
[15] KEMPKES M, MEIER W. New concept for a green anode plant [C]// DEYOUNG D. Light Metals. New Orleans: TMS, 2008: 919-922.
[16] TANDON S C, PRASAD R N. Energy saving in hindalco’s aluminium smelter [C]// KVANDE H. Light Metals. San Francisco: TMS, 2005: 303-309.
[17] LU H, YU L. Technique and mechanism of aluminum floating electrolysis in molten heavy Na3AlF6-AlF3-BaF2-CaF2 bath system [C]// CREPEAU D P. Light Metals. San Diego: TMS, 2003: 351-356.
[18] WOJAKOWSKA A, PLINSKA S, JOSIAK J, KRZYZAK E. Electrical conductivity of molten cobalt dibromide + potassium bromide mixtures [J]. Journal of Chemical and Engineering Data, 2006, 51(4): 1256-1260.
[19] KIM K B, SADOWAY D R. Electrical conductivity measurements of molten alkaline-earth fluorides [J]. Journal of the Electrochemical Society, 1992, 139(4): 1027-1033.
[20] WANG X, PETERSON R D, TABEREAUX A T. Electrical conductivity of cryolitic melts [C]// CUGSHALL E R. Light Metals. San Diego: TMS, 1992: 481-488.
[21] GILBERT B, ROBERT E, TIXHON E, OLSEN J E, OSTVOLD T. Acid-base properties of cryolite based melts with, CaF2, MgF2 and Al2O3 additions: A comparison between Raman and vapour pressure measurements [C]// EVANS J W. Light Metals. Las Vegas: TMS, 1995: 181-194.
Foundation item: Project(2005CB623703) supported by the Major State Basic Research and Development Program of China; Project(2008AA030503) supported by the National High-Tech Research and Development Program of China; Project(GUIKEJI 0639032) supported by Applied Basic Research in Guangxi Province, China
Received date: 2008-05-14; Accepted date: 2008-07-25
Corresponding author: HUANG You-guo, Doctoral candidate; Tel: +86-731-8830474; E-mail: huangyg72@163.com
(Edited by YANG Hua)