文章编号:1004-0609(2012)10-2875-07
用于室温相变储能材料的NaNO3-LiNO3-KNO3-H2O
体系相图预测
尹 霞1,陈启元1,曾德文1,杜 勇2
(1. 中南大学 化学化工学院,长沙 410083;
2. 中南大学 相图及材料设计与制造中心,长沙 410083)
摘 要:采用Pitzer-Simonson-Clegg模型对四元体系NaNO3-LiNO3-KNO3-H2O在273~358 K的溶解度相图进行预测,模型参数拟合于二元体系NaNO3-H2O、LiNO3-H2O、KNO3-H2O和三元体系NaNO3-LiNO3-H2O、NaNO3-KNO3-H2O、LiNO3-KNO3-H2O的水活度、渗透系数及溶解度数据,发现一个四元共晶点,该点组成为5.9% NaNO3、76.2% LiNO3·3H2O和17.9% KNO3(质量分数),相变温度为295.6 K。通过吸放热行为测试及DSC检测对共晶点组成材料的储热性能进行研究。结果表明:该材料在相变温度附近具有良好的储放热性能,且热焓很大,可作为潜在的室温相变储能材料。
关键词:NaNO3;LiNO3;KNO3;Pitzer-Simonson-Clegg 模型;相变储能材料;相图
中图分类号:O642.4 文献标志码:A
Phase diagram prediction of NaNO3-LiNO3-KNO3-H2O system as room temperature phase change materials
YIN Xia1, CHEN Qi-yuan1, ZENG De-wen1, DU Yong2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Science Center for Phase Diagram and Materials Design and Manufacture,
Central South University, Changsha 410083, China)
Abstract: A Pitzer-Simonson-Clegg model was applied to the predict phase diagram of the quaternary system NaNO3-LiNO3-KNO3-H2O from 273 K to 358 K, and the model parameters were fitted to the experimental water activity, osmotic coefficient and solubility of the binary systems NaNO3-H2O, LiNO3-H2O, KNO3-H2O and the ternary systems NaNO3-LiNO3-H2O, NaNO3-KNO3-H2O, LiNO3-KNO3-H2O. A quaternary eutectic point consisting of 5.9% NaNO3, 76.2% LiNO3·3H2O and 17.9% KNO3 was predicted, and the melting temperature is 295.6 K. The exothermal and endothermal behaviors of the predicted phase change material were measured and its DSC measurement was carried out. The experimental results show that the predicted material with larger melting heat has excellent heat storage and release ability, and it is expected to be used as the room temperature phase change material.
Key words: NaNO3; LiNO3; KNO3; Pitzer-Simonson-Clegg model; phase change material; phase diagram
基金项目:国家高技术研究发展计划资助项目(2012AA052503);国家自然科学基金资助项目(J0830415);湖南省自然科学基金资助项目(11JJ2011)
收稿日期:2011-09-20;修订日期:2012-02-20
通信作者:曾德文,教授,博士;电话:13618496806;E-mail: dewen_zeng@hotmail.com
目前,世界能源紧张日渐突出,新能源的开发成为目前国际研究的热点,其中相变储能材料由于其安全廉价及储能密度大等优点受到研究者的普遍关注。其作用原理是,当环境温度高于相变点时,储能材料通过融化自发从环境吸收热量,当环境温度低于相变点时,储能材料通过结冰向环境释放能量,以此调节环境温度。相变温度在室温范围(15~25 ℃)的相变材料因其可大量吸收储存低温热源的热,同时又可维持环境温度在人体感觉在较舒适的范围,在建筑调温方面有着广泛的应用前景。而目前国内外已知热容量大、相变温度在室温范围内的储能材料少之又少。本课题组在前期工作中[1-5]发现NaNO3-LiNO3-H2O和LiNO3- KNO3-H2O体系中存在共晶温度为298~301 K左右的相变材料,这些材料对调节室温来说,其相变温度依然稍稍偏高,更低一些的相变温度是人们所希望的。从相图基本原理来说,在NaNO3-LiNO3-KNO3-H2O四元体系中可能存在潜在的室温相变储能材料,其共晶温度将有可能落在室温范围内。但目前缺乏该四元体系的溶解度数据,无法判断其共晶点组成及相变温度。国际上寻找共晶点的常用方法是“配方法”,即向一种已知的熔盐水化物中添加另一种或几种无机物或它们的水化物,然后再实验测定该混合物的储放能性能。该方法的优点是研究方法相对简单易行,但要从众多的体系中用实验的方法找到理想的储能材料有如大海捞针,极为耗时耗财,而且所找的一般均为非共晶点材料。
本课题组以前的工作表明[1-6],应用热力学模型预测相图以期寻找温度适宜的相变点是一种行之有效的方法。通过对目前国际常用的几种热力学模型预测能力的比较研究[6],发现BET模型[7]适用于预测高溶解性盐水体系的热力学性质,Pitzer-Simonson-Clegg 模型(简称PSC 模型)[8-11]能比较准确地描述并预测浓度由低至高盐水体系的热力学性质。而在本研究的体系中既含有低温时溶解度不大的KNO3,又包括高溶解性的LiNO3,故选择PSC模型用于NaNO3-LiNO3- KNO3-H2O四元体系相图的预测。
1 模型方法
对于任意混合电解质溶液,CLEGG等[8]在1992年给出了溶剂水(W)、阳离子(M)和阴离子(X)活度因子(f)的PSC模型表达式,分别如下:
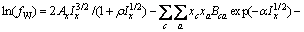
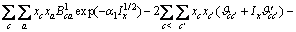
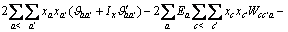
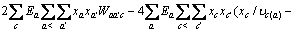
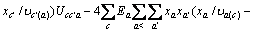
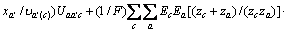
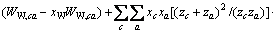

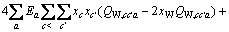
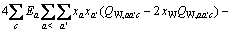
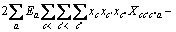
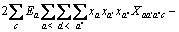
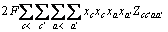 (1)
(1)
对于阳离子M:
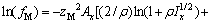
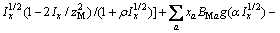

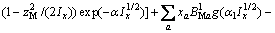
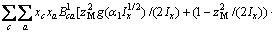
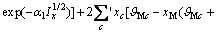
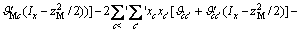
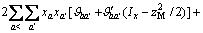
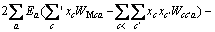
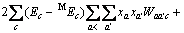
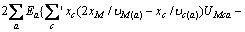
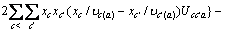
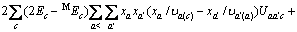
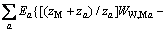

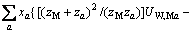
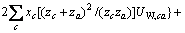
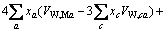
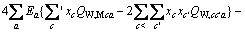
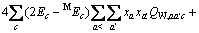
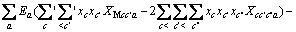
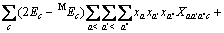
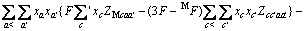
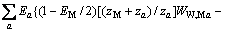
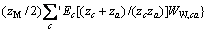 (2)
(2)
对于阴离子X:
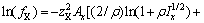
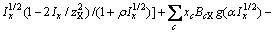

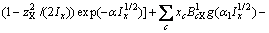
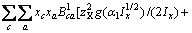
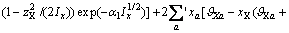
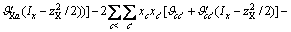

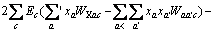
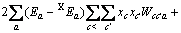
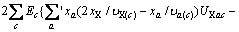
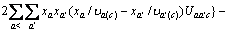
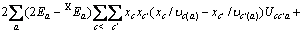
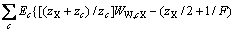
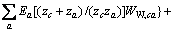
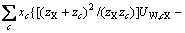

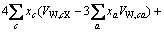
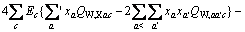
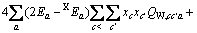
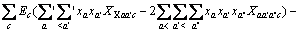
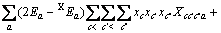
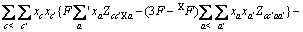
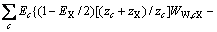
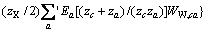 (3)
(3)
式中:Ax为Debye-Hückel参数;Ix是以摩尔分数为基础的离子强度;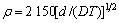 ,d、D分别是溶剂水的密度和介电常数;xi和zi分别是物种i的摩尔分数和i离子的电荷数;
,d、D分别是溶剂水的密度和介电常数;xi和zi分别是物种i的摩尔分数和i离子的电荷数;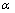 和
和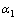 为常数,在本研究中,设定
为常数,在本研究中,设定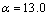 ,
, 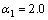 。其它参数的意义为文献[8]中公式(24)~(26)中存在的印刷错误,本文已在此更正。
。其它参数的意义为文献[8]中公式(24)~(26)中存在的印刷错误,本文已在此更正。
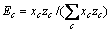
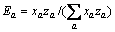
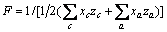
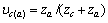
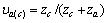
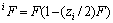
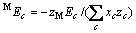 (c不包含M)
(c不包含M)
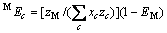 (c包含M)
(c包含M)
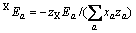 (a不包含X)
(a不包含X)
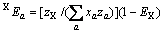 (a包含X)
(a包含X)
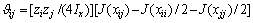
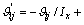
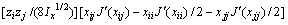
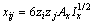
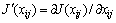
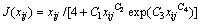
(C1=4.581, C2=-0.723 7, C3=-0.012, C4=0.528)
式中: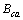 、
、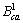 、
、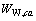 、
、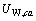 、
、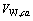 为二元参数,在本研究中,
为二元参数,在本研究中,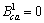 ,
,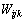 、
、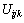 、
、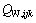 为三元粒子相互作用参数,在 此,忽略四元粒子相互作用参数
为三元粒子相互作用参数,在 此,忽略四元粒子相互作用参数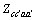 、
、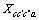 、
、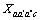 。
。
2 NaNO3-LiNO3-KNO3-H2O四元体系相图的计算
2.1 二元体系参数的确定
利用实验水的活度数据[3, 12-15]分别拟合NaNO3-H2O、LiNO3-H2O和KNO3-H2O体系的二元模型参数,其与温度的关系式见表1。
表1 二元PSC模型参数
Table 1 Binary PSC model parameters

为计算三元及四元体系的相图,需知道体系中各固相的溶度积常数。对某一固相MX·nH2O(s),其溶解平衡可表示为:MX·nH2O(s)=MX(aq)+nH2O(aq),达到平衡时,溶度积常数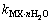 可表达为
可表达为
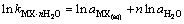
其中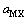 和
和 分别为盐MX和水的活度。结合各体系的二元模型参数和二元溶解度数据[16-18],计算出NaNO3-LiNO3-KNO3-H2O四元体系中各固相在不同温度下的
分别为盐MX和水的活度。结合各体系的二元模型参数和二元溶解度数据[16-18],计算出NaNO3-LiNO3-KNO3-H2O四元体系中各固相在不同温度下的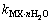 ,并将其与温度的关系式拟合为:
,并将其与温度的关系式拟合为: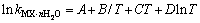 ,结果见表2。
,结果见表2。
表2 NaNO3-LiNO3-KNO3-H2O体系中固相的ln k与温度(T)的关系
Table 2 Relationship between parameters ln k of solid phases in system NaNO3-LiNO3-KNO3-H2O and temperature
的关系.jpg)
2.2 三元体系相图的计算
以前的研究[3]表明,若仅用二元模型参数,PSC模型不能准确计算相关三元体系的相图。故此分别用NaNO3-LiNO3-H2O、NaNO3-KNO3-H2O和LiNO3- KNO3-H2O体系的溶解度及水活度数据拟合这三个体系的三元离子相互作用参数(见表3),并结合表1~3中所给参数计算了3个体系的完整相图(见图1~3),发现计算值与实验值一致。
表3 三元PSC模型参数
Table 3 Ternary PSC model parameters

2.3 NaNO3-LiNO3-KNO3-H2O四元体系相图及相变材料的预测
结合表1~3的数据,对四元体系NaNO3-LiNO3- KNO3-H2O在273 K~358 K的溶解度相图进行预测(见图4),并找到一个熔点为295.6 K的四元共晶点(图4中的E点),其组成按质量分数为NaNO3: 5.9%,LiNO3·3H2O: 76.2%,KNO3: 17.9%。
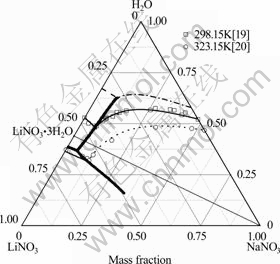
图1 NaNO3-LiNO3-H2O体系相图的计算值与实验值[19-20]比较
Fig. 1 Comparison of calculated (lines) and experimental (symbols)[19-20] phase diagram of NaNO3-LiNO3-H2O system ( : Predicted polytherm; ?: Calculated isotherm at 298.15 K; ……: Calculated isotherm at 323.15 K; ----: Calculated isotherm at 273.15 K)
: Predicted polytherm; ?: Calculated isotherm at 298.15 K; ……: Calculated isotherm at 323.15 K; ----: Calculated isotherm at 273.15 K)
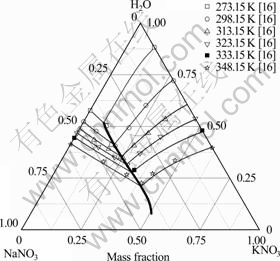
图2 NaNO3-KNO3-H2O体系相图的计算值与实验值[16]比较
Fig. 2 Comparison of calculated (lines) and experimental (symbols)[16] phase diagram of NaNO3-KNO3-H2O system ( : Predicted polytherm; ?: Calculated isotherms)
: Predicted polytherm; ?: Calculated isotherms)
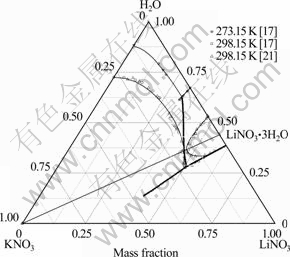
图3 LiNO3-KNO3-H2O体系相图的计算值与实验值[17, 21]比较
Fig. 3 Comparison of calculated (lines) and experimental (symbols)[17, 21] phase diagram of LiNO3-KNO3-H2O system ( : Predicted polytherm; ?: Calculated isotherms)
: Predicted polytherm; ?: Calculated isotherms)

图4 NaNO3- LiNO3-KNO3-H2O体系的溶解度相图
Fig. 4 Solubility phase diagram of NaNO3-LiNO3- KNO3-H2O system ( : Predicted eutectic line; ----: Predicted isotherms; ●(E): Predicted eutectic point)
: Predicted eutectic line; ----: Predicted isotherms; ●(E): Predicted eutectic point)
3 储能材料吸放热性能检测
3.1 实验部分
对预测的四元共晶点KNO3-LiNO3·3H2O- NaNO3(图4中的E点)的融化与结晶行为进行测定,实验装置图在前期工作[1]中已有介绍。首先按E点的比例配置50克储能材料样品,然后将封装了样品的100 mL试管固定在1 L左右的空烧杯内,再把烧杯放入恒温水浴槽(控温精度± 0.1 K),并将一支与计算机相连的温度传感器插入样品中,在线监测并记录样品的温度变化情况。测定熔化或结晶行为前,分别将水浴温度控制在高于或低于熔点温度5~7 K,待水浴的温度恒定后,再将装有样品的试管放入空烧杯中,检测样品的吸热和放热曲线。
用分析纯试剂按图4中E点组成进行配样,采用差示扫描量热仪(DSC Q10, TA公司生产)对样品的热焓及熔点进行测试。取15 mg样品置于带盖样品盘中,以10 K/min的速度从263 K升温至323 K。
3.2 实验结果与讨论
储能材料(Phase change material, PCM)的融化结晶行为曲线如图5。一般而 言,储能材料的升降温曲线与环境温度曲线之间面积即为储能材料吸放热能力,且结晶时的相变温度均稍低于熔化时的相变温度。由图5可见,该材料在292.4 K附近有一段明显的降温平台,表明材料在这一温度下凝固并向环境释放热量;在295 K附近有一明显的升温平台,这是由材料从环境吸收热量而融化导致。
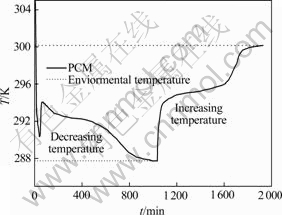
图5 NaNO3-LiNO3·3H2O-KNO3储能材料的吸放热行为
Fig. 5 Releasing and storage heat behavior of PCM of NaNO3-LiNO3·3H2O-KNO3
由储能材料的DSC测定结果(图6)可以看出,测定的熔点为295 K,偏离预测值0.6 K,热焓为200.1 J/g。
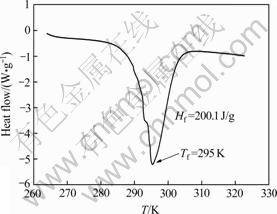
图6 NaNO3-LiNO3·3H2O-KNO3储能材料的DSC曲线
Fig. 6 DSC curves of PCM of NaNO3-LiNO3·3H2O-KNO3
实验结果充分说明本文作者用PSC模型预测到的四元共晶点KNO3-LiNO3·3H2O-NaNO3,其熔点正确,且储能效果甚佳(热焓很大),可望用作潜在的室温相变储能材料。
4 结论
1) 利用PSC模型对三元体系NaNO3-LiNO3-H2O、NaNO3-KNO3-H2O、LiNO3-KNO3-H2O的相图进行计算和预测,二元和三元模型参数通过拟合相关实验数据(如水活度、渗透系数或溶解度)得到。
2) 结合二元和三元PSC模型参数,对四元体系NaNO3-LiNO3-KNO3-H2O的溶解度相图进行预测,并找到一个熔点为295.6 K的共晶点NaNO3(5.9%)- LiNO3·3H2O(76.2%)-KNO3(17.9%)。
3) 对上述熔点为295.6 K共晶点组成进行融化结晶行为及DSC测试,研究结果表明该材料从低温热源储热性能优异,是潜在的室温相变储能材料。
REFERENCES
[1] LI B H, ZENG D W, YIN X, CHEN Q Y. Theoretical prediction and experimental determination of room-temperature phase change materials using hydrated salts as agents[J]. Journal of Thermal Analysis and Calorimetry, 2010, 100: 685-693.
[2] ZENG D W, LIU H X, CHEN Q Y. Simulation and prediction of solubility phase diagram for the separation of MgCl2 from LiCl brine using HCl as a salting-out agent[J]. Hydrometallurgy, 2007, 89: 21-31.
[3] ZENG D W, WU Z D, YAO Y, HAN H J. Isopiestic determination of water activity on the system LiNO3+KNO3+ H2O at 273.1 and 298.1 K[J]. Journal of Solution Chemistry, 2010, 39: 1360-1376.
[4] ZENG D W, VOIGT W. Phase diagram calculation of molten salt hydrates using the modified BET equation[J]. Computer Coupling of Phase Diagrams and Thermochemistry, 2003, 27: 243-251.
[5] YIN X, CHEN Q Y, ZENG D W, WANG W L. Phase diagram of the system KNO3 + LiNO3 + Mg(NO3)2 + H2O[J]. Computer Coupling of Phase Diagrams and Thermochemistry, 2011, 35: 463-472.
[6] 尹 霞, 李琴香, 曾德文, 万艳鹏. 高溶解性盐水体系热力学模型预测能力的比较研究Ⅰ: 二元体系[J]. 化学学报, 2008, 66(15): 1815-1826.
YIN Xia, LI Qin-xiang, ZENG De-wen, WAN Yan-peng. Comparison of thermodynamic models in high-solubility salt+H2O systems Ⅰ: Binary systems[J]. Aata Chimica Sinica, 2008, 66(15): 1815-1826.
[7] STOKE R H, ROBINSON R A. Ioinc hydration and activity in electrolyte solutions[J]. Journal of the American Chemical Society, 1948, 70: 1870-1878.
[8] CLEGG S L, PITZER K S, BRIMBLECOMBE P. Thermodynamics of multicomponent, miscible, ionic solutions. Mixtures including unsymmetrical electrolytes[J]. Journal of Physical Chemistry, 1992, 96: 9470-9479.
[9] CLEGG S L, PITZER K S. Thermodynamics of multicomponent, miscible, ionic solutions: Generalized equations for symmetrical electrolytes[J]. Journal of Physical Chemistry, 1992, 96: 3513-3520.
[10] SIMONSON J M, PITZER K S. Thermodynamics of multicomponent, miscible, ionic systems: The system LiNO3-KNO3-H2O[J]. Journal of Physical Chemistry, 1986, 90: 3009-3013.
[11] PITZER K S, SIMONSON J M. Thermodynamics of multicomponent, miscible, ionic systems: Theory and equations[J]. Journal of Physical Chemistry, 1986, 90: 3005-3009.
[12] PEARCE J N, HOPSON H. The vapor pressures of aqueous solutions of sodium nitrate and potassium thiocyanate[J]. Journal of physical chemistry, 1937, 41: 535-538.
[13] BOBMANN E, RICHTER J, STARK A. Experimental results and aspects of analytical treatment of vapor pressure measurements in hydrated melts at elevated temperatures[J]. Berichte der Bunsen-Gesellschaft Physical Chemistry, 1993, 97: 240-245.
[14] BRAUNSTEIN H, BRAUNSTEIN J. Isopiestic studies of very concentrated aqueous electrolyte solutions of LiCl, LiBr, LiNO3, Ca(NO3)2, LiNO3+KNO3, LiNO3+CsNO3, and Ca(NO3)2+CsNO3 at 100 to 150 ℃[J]. Journal of Chemical Thermodynamics, 1971, 3: 419-431.
[15] BARRY J C, RICHTER J, STICH E. Vapour pressures and ionic activity coefficients in the system KNO3+H2O from dilute solutions to fused salts at 425 K, 452 K and 492 K[J]. Berichte der Bunsen-Gesellschaft Physical Chemistry, 1988, 92: 1118-1122.
[16] LINKE W F, SEIDELL A. Solubilities: inorganic and metal-organic compounds[M]. 4th ed. Washington DC: American Chemical Society; 1965.
[17] 尹 霞, 吴玉双, 李琴香, 曾德文. LiNO3-KNO3-H2O三元体系相平衡研究[J]. 无机化学学报, 2010, 26(1): 45-48.
YIN Xia, WU Yu-shuang, LI Qin-xiang, ZENG De-wen. Phase equilibrium of LiNO3-KNO3-H2O system[J]. Chinese Journal Inorganic Chemistry, 2010, 26(1): 45-48.
[18] ZENG D W, MING J W, VOIGT W. Thermodynamic study of the system (LiCl + LiNO3+H2O)[J]. Journal of Chemical Thermodynamics, 2008, 40: 232-239.
[19] KURTOVA L V, BOLSHAKOVA L P, PLYUSHCHEV V E. Equilibriums in the system LiNO3-NaNO3-H2O at 25 ℃[J]. Zhurnal Neorganicheskoi Khimii, 1963, 8: 1993-1994.
[20] ANDRONOVA N P. Sodium nitrate-lithium nitrate-water system at 50.deg[J]. Uchenye Zapiski-Yaroslavskii Gosudarstvennyi Pedagogicheskii Institut imeni K. D. Ushinskogo, 1972, 103: 50-51.
[21] SILVKO T A, SHAKHNO I V, PLYUSHCHEV V E. Solubility Li+, K+‖NO3-, CO32- -H2O at 25.deg[J]. Zhurnal Neorganicheskoi Khimii, 1968, 13: 2020-2026.
(编辑 李艳红)