
Cr/nanodiamond composite plating with cobalt cation additive
Viet-Hue NGUYEN1, Thi-Nam HOANG1, Ngoc-Phong NGUYEN1, Sik-Chol KWON2, Man KIM2, Joo-Yul LEE2
1. Institute of Materials Science, 18 Hoang Quoc Viet, Hanoi, Vietnam;
2. Korea Institute of Materials Science, 66 Sangnam, Changwon, Korea
Received 18 June 2008; accepted 10 March 2009
Abstract: The effect of cationic additive on Cr/nanodiamond plating was studied. Chromium plating was performed in Sargent bath. Morphology of deposit was observed by scanning electron microscope(SEM); microhardness by hardness tester; wear rate by tribometer; amount of diamond in deposit by combustion method and passivity by potentiodynamic scan. Experimental results show that in the presence of cobalt cation, the amount of nanodiamond particle in the deposit is increased. With increasing diamond particle amount, the metallurgical, mechanical and electrochemical properties of Cr/nanodiamond deposit are improved. However, this improvement seems to be constrained. In the presence of 10 g/L of nanodiamond powder and 2.5 g/L of cobalt cation in the bath, the amount of diamond particle in deposit is increased by 4 times; and wear rate of Cr-Co/nanodiamond deposit is decreased by 2-3 times as compared with pure Cr/deposit. The passive current of Cr-Co/nanodiamond composite deposit is decreased from 18 to 8 μA. The morphology of Cr/nanodiamond is smooth remarkably in the presence of cobalt cation.
Key words: corrosion; wear rate; nano-powder; composite plating; microhardness
1 Introduction
It was conformed that the mechanical and corrosion properties of deposit of the Cr/nanodiamond composite plating were enhanced. The wear resistance of Cr/nanodiamond composite plating was increased by 2-3 times with inclusion of 0.01%-0.03% (mass fraction) of nanodiamond powder[1-4].
In the case of Ni/nanodiamond plating, using a surfactant containing an azobenzene moiety, as well as cobalt cation, the amount of nano-diamond included in nickel deposit was achieved to be 57.6% (volume fraction)[5-8].
The cerium cationic stimulator for chrome plating in the presence of 2 ?m SiC particle was investigated[9]. But so far, no information concerning additive for nanodiamond in hexa-chromium plating was presented.
In this work, cobalt cation as additive was selected to study Cr/nanodiamond composite plating in order to enhance the diamond amount codeposited in the plating, with the aim to improve the mechanical and corrosion properties. Inorganic compound was selected because chromic acid is a very strong oxidant.
2 Experimental
2.1 Sample preparation
The mirror polished AISI 1024 steel (composition: C 0.19-0.25, Mn 1.35-1.65, S 0.05 and P 0.04; mass fraction, %) and brass plates were selected as substrates. Pre-prepared steel and brass sheets were used. Samples were cut. After the stuck film was removed, they were degreased in alkaline soak solution (at 60 ℃), pickled in sulfuric acid (10%) and rinsed by double distilled water.
2.2 Plating bath
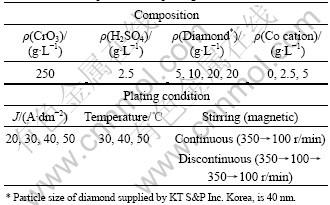
Bath composition and plating conditions are listed in Table 1.
Table 1 Bath composition and plating conditions
2.3 Wear test set-up
Wear resistance was measured on the tribometer (Korea). Testing parameters were revolutions of 1 000 and 1 500, speed of 200 r/min, load of 4.94 N, radius of track in disk of 10 mm and diameter of ruby ball of 3.14 mm. The width and height of abrasive slides were measured by surface profiler α-STEP TENCOR (Korea), then, conversed into wear volume. Wear rate was calculated by the following equation:
rw=VN/s (1)
where V is the wear volume (m3) (=wh?2πr); w and h are the width and height of abrasive slide (μm), respectively; r is the radius of track in the disk (mm); s is the total sliding distance (m) (=2πr?Re); Re is the revolution; and N is the load (N).
2.4 Diamond amount determination
The diamond amount in deposits was determined by combustion method (used LECO CS 600, USA). For this determination, the brass was selected as substrate. After plating, samples were immersed in 10% nitric acid, containing 30 g/L sodium chloride to dissolve the brass substrate. The remained Cr-deposit layer was washed in doubly distilled water several times, dried and pulverized by agate mortar to smooth pieces, then subjected to carbon analysis.
3 Results and discussion
3.1 Amount of diamond powder in deposit
The deposits, prepared from plating solutions containing 5-40 g/L diamond powder, amongst, one without and others with 1, 2.5, 5 g/L of cobalt cation, were subjected to diamond amount determination. One value was averaged from the three determinations. The mass of Cr-deposit powder sample was 1 g. Analysis result is shown in Table 2.
Table 2 Amount of diamond powder in deposit
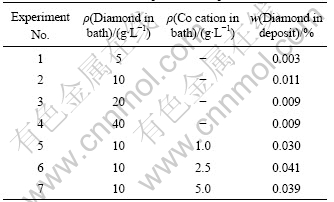
Without cobalt cation, the maximum amount of diamond powder in the deposit was detected at the diamond concentration of 10 g/L in the bath. Next experiment was performed with this diamond powder concentration in the presence of 1, 2.5, 5.0 g/L of cobalt cation. Results show that the amount of diamond particle in the deposit reaches a maximum at 10 g/L of diamond powder and 2.5 g/L of cobalt cation in the bath and is 4 times that without cobalt cation.
The rise in a certain limit of diamond amount in the deposit from hexa-chromium plating bath can be proposed that cobalt cation creates a more favorable condition for co-deposition of diamond particles than chromium cations. Furthermore, by conversion and electrostatic attraction, when searching and absorbing on the cathode surface, cobalt cation together with chromium can entrap diamond particles and embed them into growing plating layer. But concerning with poor affinity to inert particle and good leveling ability, chromium cations will inhibit somewhat the co-deposition of diamond particle. The balance of these effects tends to attain a maximum value (0.041%) of diamond powder in the deposit[11-13].
3.2 Morphology of deposit
Observed by scanning electron microscope(SEM, Jeol 35C, Japan), the micrographs of Cr/nano-diamond deposit with and without cobalt cation are shown in Fig.1.
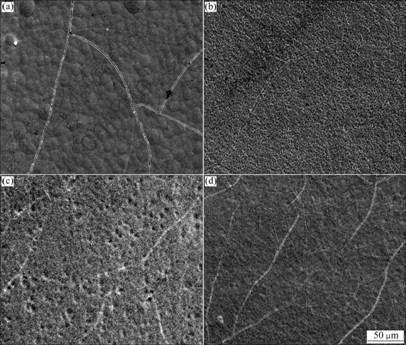
Fig.1 SEM surface morphologies of Cr and Cr/nanodiamond deposits without and with cobalt cation: (a) Cr; (b) Cr/nanodiamond; (c) Cr-Co; (d) Cr-Co/nanodiamond
It can be seen that the surface of chromium plating is lumpy with many knobs and racks. In the chromium plating with diamond powder, the surface becomes slightly rough, nodular, and it is not clear to see cracks. Surface of Cr-Co/nanodiamond plating is very smooth but there are still some cracks. However, the Cr-Co/ nanodiamond deposit seems very flat. Good geometric morphology of plating surface is concerned with the strength and perfection of the deposit microstructure.
3.3 Mechanical properties
3.3.1 Hardness of deposit
Microhardness was measured by microhardness tester (Matsuzawa, MAX70, Japan). One datum is averaged from three samples; and on each, three points were tested.
The results obtained on the Cr/nanodiamond plated samples with and without cobalt cation are shown in Table 3.
Table 3 Microhardness and wear rate of chromium deposit (Load: 0.49 N; Plating conditions: 50 ℃, 40 A/dm2, 90 min)

From Table 3, the hardness of composite deposit is always increased with the increase of the inclusion of diamond particle in the deposit. Hardness of Cr/nano- diamond plating is 20% and Cr-Co/nanodiamond is 23% higher than that of pure Cr plating. In the presence of cobalt cation, hardness is increased with increasing the amount of diamond particle in the deposit.
3.3.2 Wear resistance of deposit
Wear resistance of Cr/nano-diamond deposits plated with and without cobalt cation was measured by tribometer (Korea) by ball-on-disk sliding. Results are shown in Table 3.
It is confirmed that in the presence of cobalt cation, the wear rate of composite deposit is decreased. The wear rate of Cr/nanodiamond deposit is 1.95×10-16 m3/(N×min) and that of Cr-Co/nanodiamond composite is 1.23×10-16 m3/(N×min). Both are lower than that of pure Cr deposit (2.61×10-16m3/(N×min)). Lower wear rate can be attributed to the dispersion-strengthening effect with the incorporation of diamond particles.
3.4 Corrosion behavior of deposit
To evaluate the corrosion behavior of chromium deposit, the potentiodynamic polarization curves were recorded in 3.5% NaCl at ambient temperature[14] as shown in Fig.2 and data are listed in Table 4.
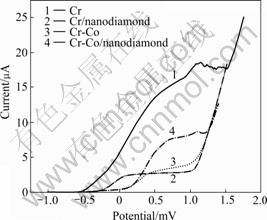
Fig.2 Potentiodynamic polarization curves of chromium and Cr/nanodiamond composite plating with and without cobalt cation in 3.5% NaCl
Table 4 Electrochemical data of chromium deposit in 3.5% NaCl (Plating conditions: 50 A/dm2, 50 ℃, 120 min)

It is obvious that the passivity of Cr-Co/ nanodiamond plating behaves better than Cr plating. The passive current of former is 2.5 μA and that of latter is 18 μA. Passive current density of Cr-Co/nanodiamond plating is slight higher than that of Cr/nanodiamond plating, which may be caused by the inhomogeneous structure of the former.
4 Conclusions
1) Cobalt cationic additive created a stimulation effect in Cr/nanodiamond plating process.
2) In the presence of cobalt cation, the amount of diamond particle in the deposit from hexa-chromium bath was increased, but tended to attain a maximum value at diamond powder concentration of 10 g/L and cobalt cation of 2.5 g/L.
3) With increasing the amount of diamond particle indeposited in the plating, the mechanical and corrosion properties of Cr/nanodiamond plating were improved; hardness was increased by 23%; wear rate was decreased twice; however, the passivity of Cr-Co/nanodiamond plating was somewhat worsened.
Acknowledgements
This project was funded by KIMM’ Project and KOFST (Brain Pool Program). The Authors would like to thank Dr. J. J. Rha, Dr. K. H. Lee, and others for assistance during this project.
References
[1] NGUYEN V H, HOANG T N, KWON S C, LEE J Y. Study on the properties of chromium matrix composite plated with nanosized diamond powders [J]. Advanced Materials Research, 2007, 26/28: 1361-1364.
[2] MANDICH N V, DENNIS J K. Codeposition of nanodiamonds with chromium [J]. Metal Finishing, 2001, 99(6): 117-119.
[3] BURKAT G K, FUJIMURA T, DILATOR V Y, ROLLOVER E A, VERETENNIKOVA M V. Obtaining of wear resistance chrome coatings with nanodiamonds of different origins [J]. Sverchtverdye Materials, 2000, 1: 84-95. (in Russian)
[4] VINOKUROV E G, ARSENKIN A M, GRIGOROVICH K V, BONDAR V V. Electrodeposition and physico-mechanical properties of chromium coatings modified with disperse particles [J]. Protection of Metals, 2006, 42(3): 290-294.
[5] NABEEN K S, MASUKO M. Effect of particle size on the co-deposition of diamond with nickel in the presence of a redox-active surfactant and mechanical property of the coatings [J]. Diamond and Related Materials, 2006, 15: 1570-1575.
[6] NABEEN K S, TETSUO S. Composite plating using an electro-active surfactant—A new approach to incorporate high amount of ceramic particles into a metallic matrix [J]. Journal of the Surface Finishing Society of Japan, 2006, 57: 489-495.
[7] WANG L P, GAO Y, LIU H W, XUE Q J, XU T. Effect of bivalent Co ion on the co-deposition of nickel and nano-diamond particles [J]. Surface and Coating Technology, 2005, 191: 1-6.
[8] SHI L, SUN C F, GAO P, ZHOU F, LIU W M. Mechanical properties and wear and corrosion resistance of electrodeposited Ni-Co/SiC nanocomposite coating [J]. Applied Surface Science, 2006, 252: 3591-3599.
[9] BRECH O, ESKIN S, ZAHAVI J. Effect of additives on electrodeposition of composite chromium coatings [J]. Plating and Surface Finishing, 1994, 81(3): 62-64.
[10] GUGLIELMI N. Kinetics of the deposition of inert particles from electrolytic baths [J]. J Electrochemical Society, 1972, 119(8): 1009-1012.
[11] HOVESTAD A, JANSSEN L J J. Electrochemical codeposition of inert particles in a metallic matrix [J]. Journal of Applied Electro- chemistry, 1995, 25: 519-527.
[12] MATSUBARA H. Codeposition behavior of nanodiamond with electrolessly plated nickel films [J]. Journal of the Surface Finishing Society of Japan, 2006, 57(7): 484-488.
[13] CHAO I, VEREECKEN P M, CAMMARATA R C, SEARSON P C. Kinetics of particles codeposition of nanocomposite [J]. J Electrochemical Society, 2002, 149(11): C610-C614.
[14] NGUYEN V H, NGO T A T, NGUYEN N P, KWON S C, LEE J Y. An application of electrochemical method for studying nano- composite plating [J]. Metals and Materials International, 2006, 12: 493-498.
Corresponding author: Viet-Hue NGUYEN; Tel: +84-04-3756-2738; Fax: +84-04-3758-3037; E-mail: ngviet_hue@yahoo.com
DOI: 10.1016/S1003-6326(08)60389-1
(Edited by YANG Bing)