浇铝法制备铜铝复合铸锭时过渡层凝固过程与组织
来源期刊:中国有色金属学报(英文版)2016年第8期
论文作者:陈淑英 常国威 岳旭东 李青春
文章页码:2247 - 2256
关键词:铜包铝;浇铝法;过渡层;凝固过程;凝固组织
Key words:copper cladding aluminum; pouring aluminum method; transition layer; solidification process; solidification microstructure
摘 要:采用浇铝法制备铜铝复合铸锭,研究液体铝与固体铜复合过程中过渡层的凝固过程与组织变化规律,结果表明,铜铝复合铸锭中过渡层由α(Al)+α(Al)-CuAl2共晶、α(Al)-CuAl2共晶、CuAl2+α(Al)-CuAl2共晶和Cu9Al4四种组织组成,浇注温度、铜板外表面强制冷却方式以及浇注后到开始强制冷却时间不影响过渡层内组织种类,但过渡层内各种组织占过渡层厚度的比例随工艺参数而变化。过渡层顶部的纯铝最先开始凝固,而后α相以枝晶的方式、CuAl2相以平面晶或胞晶方式分别从过渡层的两侧向过渡层内生长。铜板外表面冷却强度越强,α枝晶越发达,CuAl2相越容易长成平面晶。
Abstract: The Cu-Al composite casts were prepared by the method of pouring molten aluminum. The solidification process and the microstructure of the transition layer were investigated during the recombination process of the liquid Al and the solid Cu. The results reveal that the microstructure of the transition layer in the Cu-Al composite cast consists of α(Al)+α(Al)-CuAl2 eutectic, α(Al)-CuAl2 eutectic, CuAl2+α(Al)-CuAl2 eutectic and Cu9Al4. Additionally, the pouring temperature, cooling mode of the Cu plate surface and start time of the forced cooling after pouring have no effect on the microstructure species. But the proportion of the various microstructures in the transition layer changes with the process parameters. The pure Al at the top of the transition layer starts to solidify first and then the α(Al) phase grows in a dendritic way, while the CuAl2 phase exhibits plane or cellular crystal growth from the two sides of the transition layer towards its interior. The stronger the cooling intensity of the Cu plate outer surface, the more developed the dendrite, and the easier it is for the CuAl2 phase to grow into a plane crystal.
Trans. Nonferrous Met. Soc. China 26(2016) 2247-2256
Shu-ying CHEN, Guo-wei CHANG, Xu-dong YUE, Qing-chun LI
School of Materials Science and Engineering, Liaoning University of Technology, Jinzhou 121001, China
Received 17 December 2015; accepted 4 May 2016
Abstract: The Cu-Al composite casts were prepared by the method of pouring molten aluminum. The solidification process and the microstructure of the transition layer were investigated during the recombination process of the liquid Al and the solid Cu. The results reveal that the microstructure of the transition layer in the Cu-Al composite cast consists of α(Al)+α(Al)-CuAl2 eutectic, α(Al)-CuAl2 eutectic, CuAl2+α(Al)-CuAl2 eutectic and Cu9Al4. Additionally, the pouring temperature, cooling mode of the Cu plate surface and start time of the forced cooling after pouring have no effect on the microstructure species. But the proportion of the various microstructures in the transition layer changes with the process parameters. The pure Al at the top of the transition layer starts to solidify first and then the α(Al) phase grows in a dendritic way, while the CuAl2 phase exhibits plane or cellular crystal growth from the two sides of the transition layer towards its interior. The stronger the cooling intensity of the Cu plate outer surface, the more developed the dendrite, and the easier it is for the CuAl2 phase to grow into a plane crystal.
Key words: copper cladding aluminum; pouring aluminum method; transition layer; solidification process; solidification microstructure
1 Introduction
The copper resources are scarce, and the high price limits its wide application in the communications and electric conduction industries. Copper cladding aluminum (CCA) composite as a perfect substitution for pure copper has attracted broad attention. Firstly, it was used as conductor material in Germany. Subsequently, Europe and America and other developed countries, and China began to study and apply the CCA composite one after another [1,2]. The available evidence shows that the microstructure and thickness of the transition layer play a decisive role in the bonding strength. Accordingly, the formation of the transition layer, the microstructure constitution in the layer and the mechanical properties of the transition layer has been investigated [3-7]. In particular, the influence rules of temperature, time, cooling ways, heat treatment and high magnetic field on the transition layer have been evaluated when different types of the methods to prepare copper cladding aluminum composites are used [8-13].
The metallurgical bonding between copper and aluminum is the key technology in preparation process of copper cladding aluminum composite [14]. At present, all the preparation methods can be divided into two types according to the metallurgical bonding achieved before or after plastic processing. Achieving metallurgical bonding after plastic processing is that the copper is wrapped on the surface of the aluminum to realize the mechanical bonding and then the metallurgical bonding between the copper and aluminum is achieved by the atomic diffusion of copper and aluminum during the heat treatment. The rolling compound and tube-weld cladding technique belong to this type, and its disadvantage is that the heat treatment time is too long, the equipment is huge, the energy consuming is high and the bonding strength cannot be guaranteed [15,16]. Achieving metallurgical bonding before plastic processing is that the Cu-Al alloy or Al-Cu alloy is formed between the solid Cu and liquid Al after the contact of liquid Al with solid Cu, and the metallurgical bonding between Cu and Al is achieved after the solidification. The methods used are the continuous core-filling casting, horizontal core- filling continuous casting and pouring aluminum method [17,18]. The more advanced method to get the CCA profiles of high bonding strength and short technological process is the horizontal continuous casting [19]. But the disadvantages of the method of horizontal continuous casting are that the quality of the inner and outer surface of the copper tube billet (especially the outer surface) is not easy to guarantee, the process is complex, the equipment investment is large and the transition layer of Cu-Al composite is not even easily. The pouring aluminum method becomes the more practical method of preparing Cu-Al composite ingot for the easy processing and convenient operation. But the influences of the solidification process of the transition layer and technology parameters on the composite quality are not clear, which cause the difficult control of the composite quality. It is important to clarify the solidification process of the transition layer for controlling the tissue of the transition layer easily and improving the composite quality. So, the solidification process and the effects of related technological parameters on the composite quality are studied in this work when preparing the Cu-Al composite ingot by pouring aluminum method.
2 Experimental
The pure Al and pure Cu with 99.90% purity were chosen as the experimental materials. The Al ingot was placed into the clay graphite crucible, and the crucible was heated in the resistance furnace. Subsequently, the liquid Al was degassed and refined with C2Cl6 after the temperature of the liquid Al reached the set temperature (20 °C higher than the pouring temperature) and held for 20 min. When the temperature of the liquid Al dropped to 780 °C or 830 °C, the crucible containing the liquid Al was taken out and then poured into the experimental device shown in Fig. 1. Before pouring the liquid Al, the surface of the Cu plate with the dimensions of 80 mm × 80 mm × 6 mm was polished with sandpaper to remove the oxide skin. Next, the Cu plate was placed on the cooling device shown in Fig. 1. After that the sand mold with the cavity dimensions of 60 mm × 60 mm × 80 mm was placed on the Cu plate and sealed with the silicate bonded sand, and then argon was introduced into the casting mold to protect the internal surface of the Cu plate to prevent oxidation. The temperature of the liquid Al was maintained by spreading the insulation covering agent on the surface of the liquid after pouring. Then, the outside surface of the Cu plate was cooled by jet air, spraying or water injection, the average cooling rate of the internal interface of the Cu plate were 0.6, 1.8, and 4.2 °C/s, respectively (temperature range: 500-780 °C). The time from pouring to the beginning of forced cooling was 40, 60 or 110 s. The thermocouples were fixed to the longitudinal axis of the cavity, and their distances to the Cu plate were 0, 5, 10 and 20 mm, respectively. The nickel-chromium/nickel-silicon thermocouple wire with the diameter of 0.5 mm was used, and the error of calibrated thermocouple is 0.75%t, t is the measured temperature. The recording of the temperature-time curves for the four locations was performed by using a DMR2100 paperless recorder, as shown in Fig. 1. The metallographic samples were intercepted in the area of copper and aluminum composite, and they were etched with a solution containing 2.5 mL HNO3, 1.5 mL HCl, 1 mL HF, and 95 mL H2O after shining and polishing. The microstructure was observed with an Axiovert 200 MAT metallographic microscope and the energy spectrum analysis was proceeded by an SN-300 electron microscope.
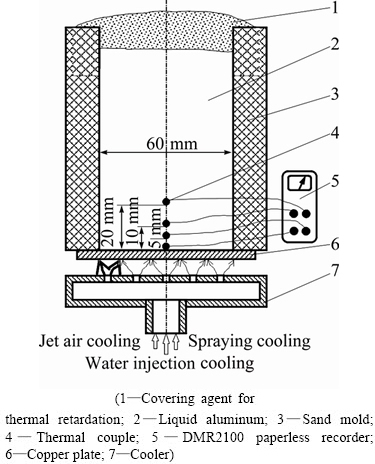
Fig. 1 Schematic plan of preparing Cu-Al composite cast using method of pouring molten aluminum
3 Results and discussion
3.1 Casting quality of Cu-Al composite cast
The photographs of the vertical section of the Cu-Al composite casts under different technological parameters are shown in Fig. 2. The pictures reveal that shrinkage cavities clearly appeared at the junction of the Cu and Al when the outside surface of the Cu plate was cooled by a air jet, and the longer the holding time, the more serious the phenomenon of shrinkage cavity formation. The Cu and Al metallurgical bonding was well achieved when the pouring temperature was 780 °C and the holding time was 40 s under spraying cooling and water injection cooling. The transition layer was present at the junction of the Cu and Al. Basically, the higher the temperature during pouring, the longer the holding time, the weaker the cooling intensity of the Cu plate outside surface, thus the thicker the transition layer.
3.2 Microstructures of transition layer
The samples were intercepted at the junction of the Cu-Al composite cast. The microstructures of the transition layers are shown in Figs. 3 and 4. Three kinds of microstructures were found in the transition layer, namely the hypoeutectic structure close to the pure Al, the eutectic structure in the middle of the transition layer, and the hypereutectic structure close to the pure Cu.
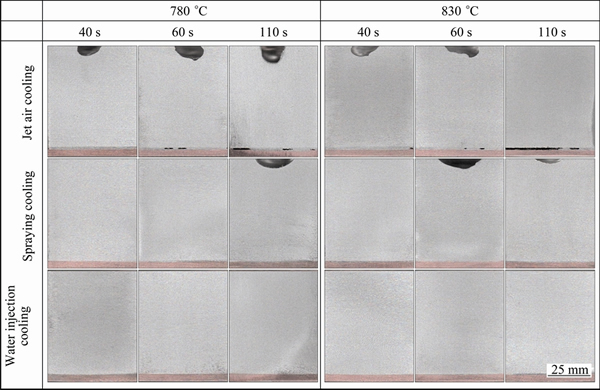
Fig. 2 Lengthwise section photographs of Cu-Al composite casts
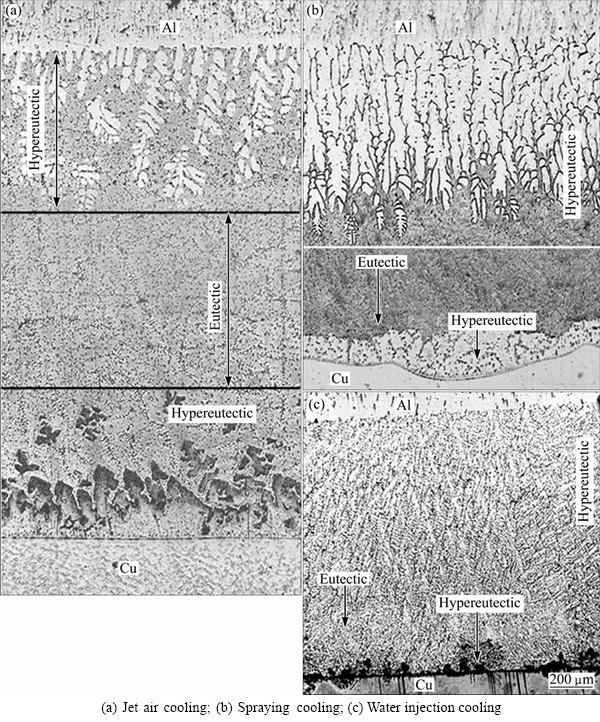
Fig. 3 Transition layers of Cu-Al composite casts when pouring at 780 °C and holding for 60 s
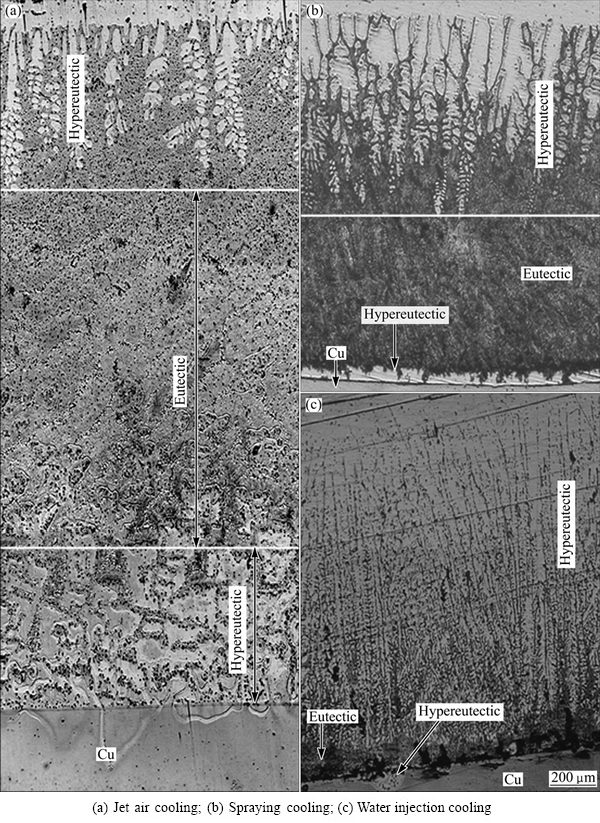
Fig. 4 Transition layers of Cu-Al composite casts when pouring at 830 °C and holding for 60 s
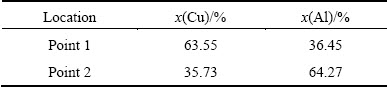
A scanning electron microscopy (SEM) image of the region adjacent to the pure Cu and the transition layer is shown in Fig. 5. A thin layer can clearly be seen between the pure Cu and the transition layer, indicated as γ in Fig. 5. EDS results of point 1 and point 2 shown in Fig. 5 are listed in Table 1, In addition, according to the research results reported by XIE et al [5], β phase and γ phase can be confirmed as CuAl2 and Cu9Al4, respectively. Thus, four kinds of microstructures are present in the transition layer of Cu-Al composite cast, namely the α(Al)+α(Al)-CuAl2 eutectic, the α(Al)- CuAl2 eutectic, the CuAl2+α(Al)-CuAl2 eutectic, and Cu9Al4.

Fig. 5 SEM image of adjacent region between pure Cu and transition layer
Table 1 EDS results of point 1 and point 2
3.3 Influences of technological parameters on solidification microstructure
The experimental results show that, as shown in Figs. 3 and 4, when the pouring temperature was 780 °C and 830 °C, respectively, with a holding time of 40-110 s under the jet air cooling, spraying cooling or water injection cooling, the species of the microstructure in the transition layer of the Cu-Al composite cast did not change. However, the thickness of the four kinds of microstructures in the transition layer varied with the change of the technological parameters, as shown in Table 2, where hZ is the total thickness of the transition layer; hα is the thickness of the α(Al)+α(Al)-CuAl2 eutectic; hH is the thickness of the α(Al)-CuAl2 eutectic; hCu+ is the thickness of the CuAl2+ (Al)-CuAl2 eutectic; hβ is the thickness of the Cu9Al4.
The effects of the technological parameters on the radio of each microstructure thickness to the total thickness of the transition layer are shown in Fig. 6. Together, the results presented in Table 2 and Fig. 6 indicate the followings: 1) the stronger the cooling intensity of the outside surface of the Cu plate, the lower the pouring temperature, the shorter the holding time, thus the thinner the total thickness (hZ) of the transition layer, as shown in Table 2, and the higher the value of hα/hZ, as shown in Fig. 6(a); 2) the change laws of hCu+/hZ, hβ/hZ and hH/hZ are almost identical, explicitly, the stronger the cooling intensity of the outside surface of the Cu plate, the shorter the holding time, the lower the pouring temperature, thus the smaller the ratio of hCu+/hZ, hβ/ hZ and hH/hZ, as shown in Figs. 6(b)-(d).
3.4 Cooling curves
The cooling curves generated under different cooling methods are shown in Fig. 7 when the pouring temperature was 780 °C and the holding time was 60 s. A comparison of the two sets of cooling curves reveals that all the temperatures decreased below 660 °C and then remained unchanged within the area 20 mm away from the internal surface of the Cu plate at about 20 s after pouring. This indicates that the pure Al had started to solidify in this zone. The solidification time of the internal surface of the Cu plate was about 400 s under the jet air cooling, which suggests that this part was in a liquid or solid-liquid state for a long time in order to provide enough time for the dissolution of the solid Cu. The solidification quickly finished under the water injection cooling, so the dissolution of the solid Cu was also quickly ended.
Table 2 Structure thicknesses and ratios of structure thickness to total thickness of transition layer under different technological parameters
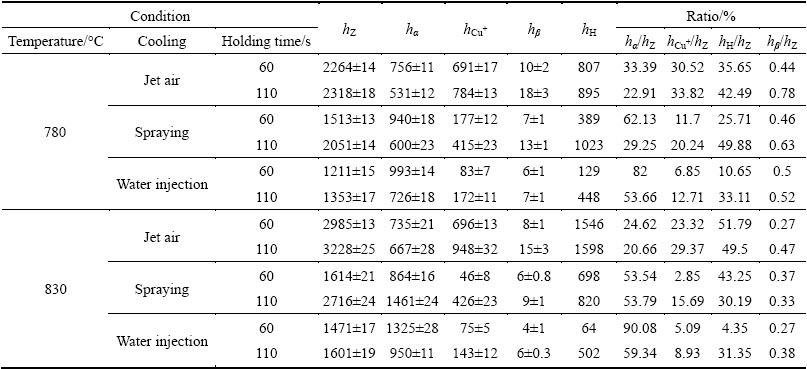
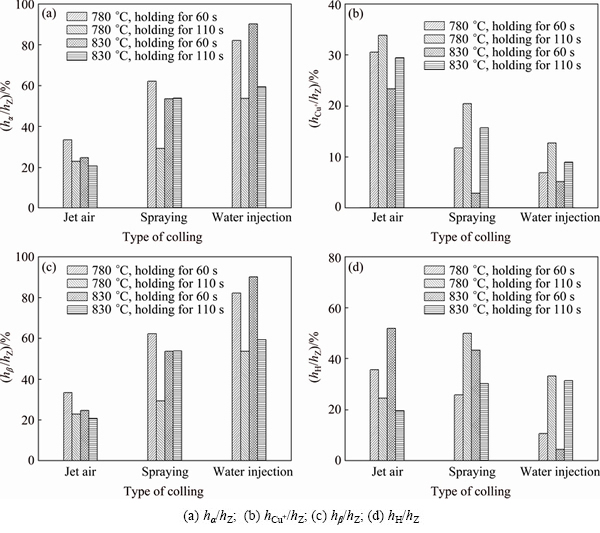
Fig. 6 Radio of each microstructure thickness to total thickness of transition layer under different technological parameters
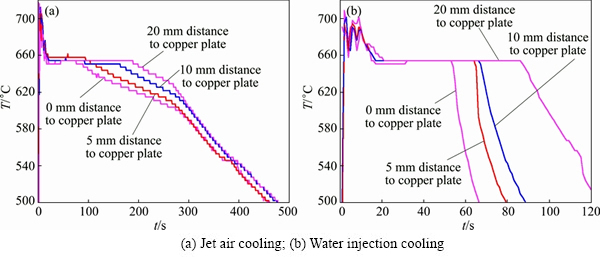
Fig. 7 Cooling curves of pouring at 780 °C and holding for 60 s
3.5 Content distribution of copper and aluminum in transition layer
The samples were taken when the pouring temperature was 780 °C, and the holding time was 60 s under the water injection cooling. The line scan results of Cu and Al in the transition layer are shown in Fig. 8(a) and the content of Cu is shown in Fig. 8(b). The Cu content of the melt close to the Cu plate was the highest and reached between 55%-60% (Fig. 8(b)). The Cu content quickly dropped below 50% within the area 10 μm away from the Cu plate. A comparison of the equilibrium phase diagram of Al-Cu alloy revealed that the alloy composition of the transition layer gradually changed from the hypereutectic Al-Cu alloy to pure Al. The gradient of the composition change decreased gradually.
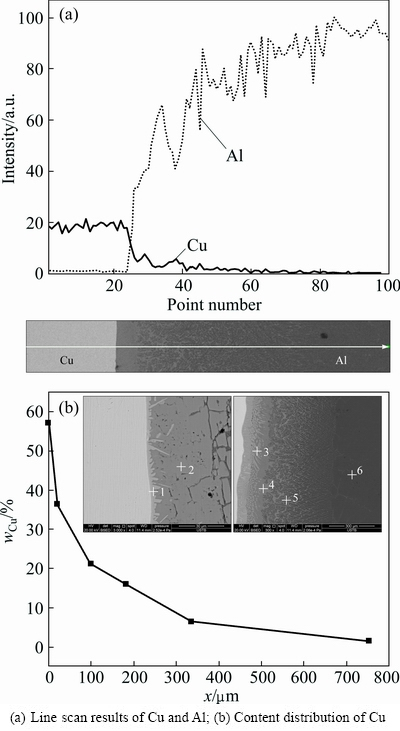
Fig. 8 EDS analysis of Cu and Al in transition layer
When the Cu-Al composite casts were fabricated using the method shown in Fig. 1, the diffusion of the Cu atoms from the solid Cu surface towards the liquid Al was very difficult due to the gravity hampering effect. Accordingly, the concentration gradient of Cu in the region close to the Cu plate reached 4%/μm, while that of the region close to the pure Al was only 0.004%/μm. In fact, the change law of Cu content in the transition layer favors the achievement of more hypoeutectic Al-Cu alloy and a small amount of hypereutectic organization.
3.6 Discussion
3.6.1 Solidification process of transition layer
The cooling curve shown in Fig. 7 can be used to confirm that the chilling effect of the Cu plate on the liquid Al does not form the solid Al on the surface of the solid Cu after the liquid Al is poured into the casting mold shown in Fig. 1. In fact, the liquid Al makes contact with the solid Cu for a long time after pouring. During such time, the Cu atoms on the surface of the solid Cu constantly dissolve into the liquid Al to form Al-Cu alloy melt (so-termed transition layer) close to the Cu plate. Additional, as shown in Fig. 8, the content of Cu decreases gradually from the bottom to the top. At the same time, the heat dissipation rate of the Cu plate surface is quick, and the temperature in the liquid Al gradually increases from the bottom to the top. The temperature gradient is very small because of the natural cooling. When the temperature at the top of the transition layer drops to 660 °C, the liquid Al begins to solidify. As the liquidus temperature of the Al-Cu alloy melt is low, the pure Al at the top of the transition layer rather than the transition layer solidifies first. The Cu content of the Al-Cu alloy melt close to the Cu plate is the highest and its liquidus temperature is also lower, so this region still remains in the liquid state after the top region of the transition layer begins to solidify. The Cu atoms on the Cu plate surface continues to dissolve into the melt, the process is depicted in Fig. 9(a).
When the temperature continues to decrease or starts to cool the outside surface of the Cu plate, the transition layer begins to solidify and the region at the bottom of the transition layer grows upward to form α(Al)+α(Al)-CuAl2 eutectic. The region at the top of the transition layer grows downward to form the CuAl2+α(Al)-CuAl2 eutectic, while the pure aluminum grows upward, the process is displayed in Fig. 9(b). Finally, the α(Al)-CuAl2 eutectic is formed in the middle of the transition layer.
The Al atoms diffuse into the solid Cu to form the Cu9Al4 during the process of dissolution of solid Cu. The Al content at the bottom of the transition layer in the microstructure is higher, thus the Al atoms will continue to diffuse into the solid Cu and increase the number of Cu9Al4. Although the Cu9Al4 phase does not belong to the solidification microstructure, it is formed during the solidification process of the transition layer. Accordingly, it is also incorporated in the solidification process.
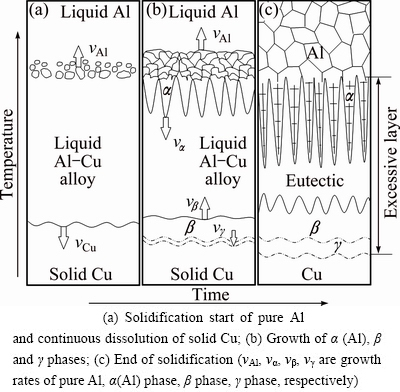
Fig. 9 Schematic plans of solid Cu dissolution and solidification process of transition layer
3.6.2 Influence mechanism of technological parameters on microstructures
The higher the pouring temperature, the higher the position of start solidification of pure Al, and the longer the contact time between liquid Al and solid Cu, thus the more the dissolved solid Cu, and the thicker the transition layer of Al-Cu alloy. Hence, the height location of the transition layer is mainly controlled by the pouring temperature. The influences of the cooling mode of the external surface of the Cu plate and the holding time on the microstructures of the transition layer are shown in Fig. 10.
The stronger the cooling intensity of the Cu plate external surface, the higher the temperature gradient in the front of the solid-liquid interface at the bottom of the transition layer, thus the easier it is for the primary β phase to grow into a plane or cellular crystal, and the thinner the thickness of the CuAl2+α(Al)-CuAl2 eutectic.
Simultaneously, as shown in Fig. 10(a), the higher the negative temperature gradient in the front of the solid-liquid interface at the top of the transition layer, the faster the growth rate of the primary α(Al) phase , the easier it is to grow into a dendrite, the thicker the thickness of the α(Al)+α(Al)-CuAl2 eutectic.
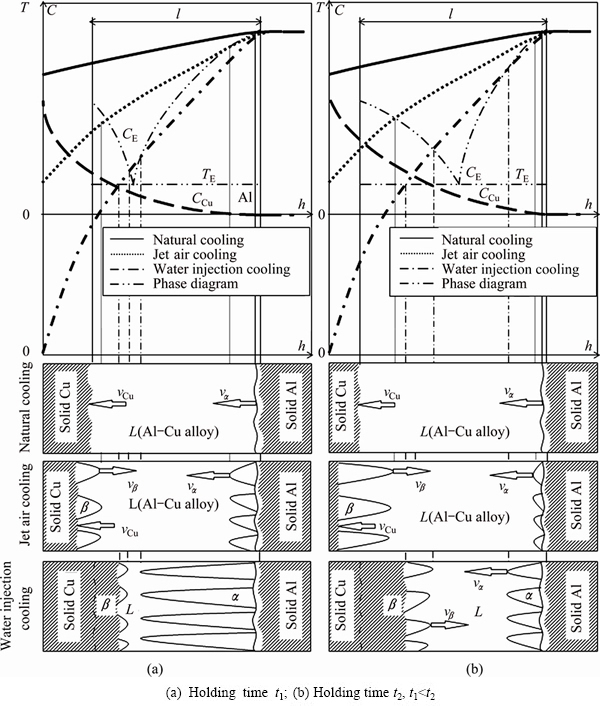
Fig. 10 Schematic plans of influences of technological parameters on microstructures of transition layer The analysis above, together with the results shown in Fig. 7, indicate that the starting solidification time of the pure Al is not associated with the holding time. The longer the holing time, the more the dissolved Cu which increases the thickness of the transition layer. The smaller the concentration gradient of Cu in the transition layer, the thicker the thickness of the CuAl2+α(Al)- CuAl2 eutectic, the thinner the thickness of the α(Al)+ α(Al)-CuAl2 eutectic. This is shown in Fig. 10(b) and Fig. 6. 4 Conclusions 1) When preparing Cu-Al composite casts by the method of pouring molten aluminum, the transition layer is composed of the α(Al)+α(Al)-CuAl2 eutectic, the α(Al)-CuAl2 eutectic, the CuAl2+α(Al)-CuAl2 eutectic and the Cu9Al4. The pouring temperature, the external surface cooling mode of the Cu plate and the starting time of forced cooling after pouring have no effect on their microstructures. Nevertheless, But the proportions of the four kinds of microstructures to the total thickness of the transition layer change with the technological parameters. 2) The stronger the external surface cooling intensity, the lower the pouring temperature, the shorter the holding time, thus the thinner the thickness of the transition layer, and the larger the proportion of the α(Al)+α(Al)-CuAl2 eutectic and the smaller the proportions of the α(Al)-CuAl2 eutectic and the Cu9A4. 3) The pure Al at the top of the transition layer starts to solidify first, then the α phases grow in a dendritic way and the CuAl2 phases grow in a plane or cellular crystal way from the two sides of the transition layer towards its interior. The stronger the external surface cooling intensity of the Cu plate is, the more developed the dendrites are, the easier it is for the CuAl2 phases to grow into a plane crystal. References [1] GIBSON A. Emerging applications for copper-clad steel and aluminum wire [J]. Wire Journal International, 2008, 41(2): 142-148. [2] KO D C, LEE S K, KIMC B M. Evaluation of copper coating ratio in steel/copper clad wire drawing [J]. Journal of Materials Processing Technology, 2007, 186(1-3): 22-26. [3] HUG E, BELLIDO N. Brittleness study of intermetallic (Cu, Al) layers in copper-clad aluminium thin wires [J]. Materials Science and Engineering A, 2011, 528(s22-23): 7103-7106. [4] MOHEBBI M S, AKBARZADEH A. Fabrication of copper/ aluminum composite tubes by spin-bonding process: experiments and modeling [J]. Int J Adv Manuf Technol, 2011, 54(9-12): 1043-1055. [5] XIE W, YAMAGUCHI T, NISHIO K. Formation of intermetallic phases on the bond interface of aluminum clad copper [J]. J Japan Inst Metals, 2011, 75(3): 166-172. (in Japanese) [6] PAUL H, SKA L L K, PRAZMOWSKI M. Microstructure and phase constitution near the interface of explosively welded aluminum/ copper plates [J]. Metallurgical and Materials Transations A, 2013, 44(8): 3836-3851. [7] LI Xiao-bing, ZU Guo-yin, WANG Ping. Microstructural development and its effects on mechanical properties of Al/Cu laminated composite [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 36-45. [8] XU B, TONG W P, LIU C Z, ZHANG H, ZUO L, HE J C. Effect of high magnetic field on growth behavior of compound layers during reactive diffusion between solid Cu and liquid Al [J]. J Mater Sci Technol, 2011, 27(9): 856-860. [9] MEGURO K, M O, KAJIHARA M. Growth behavior of compounds due to solid-state reactive diffusion between Cu and Al [J]. J Mater Sci, 2012, 47(12): 4955-4964. [10] KIM I K, SUN I H. Effect of heat treatment on the bending behavior of tri-layered Cu/Al/Cu composite plates [J]. Materials and Design, 2013, 47(9): 590-598. [11] USCINOWICZ R. Experimental identification of yield surface of Al-Cu bimetallic sheet [J]. Composites: Part B, 2013, 55(12): 96-108. [12] MEHR V Y, TOROGHINEJAD M R, REZAEIAN A. The effects of oxide film and annealing treatment on the bond strength of Al-Cu strips in cold roll bonding process [J]. Materials and Design, 2014, 53(1): 174-181. [13] JUNG J M, KIM J G, LATYPOV M I, KIM H S. Effect of the interfacial condition on the microtexture near the interface of Al/Cu composites during multi-pass caliber rolling [J]. Materials and Design, 2015, 82: 28-36. [14] SU Y J, LIU X H, HUANG H Y, LIU X F, XIE J X. Interfacial microstructure and bonding strength of copper cladding aluminum rods fabricated by horizontal core-filling continuous casting [J]. Metallurgical and Materials Transations A, 2011, 42(13): 4088-4099. [15] SHENG L Y, YANG F, XI T F, LAI C, YE H Q. Influence of heat treatment on interface of Cu/Al bimetal composite fabricated by cold rolling [J]. Composites: Part B, 2011, 42(6): 1468-1473. [16] LIN Z C, HUANG T G. Hot rolling of an aluminum-copper sandwich flat strip with the three-dimensional finite element method [J]. Journal of Material Processing Technology, 2000, 99(1-3): 154-168. [17] HE Liang, XUE Zhi-yong, WU Chun-jing, LIU Qing, WU Yuan. Research on continuous core-lling casting forming process of copper-clad aluminum bimetal composite material [J]. Acta Metall Sin, 2010, 23(3): 206-214. [18] LUO Jun-ting, ZHAO Shuang-jing, ZHANG Chun-xiang. Microstructure of aluminum/copper clad composite fabricated by casting-cold extrusion forming [J]. J Cent South Univ Technol, 2011, 18(4): 1013-1017. [19] SU Y J, LIU X H, HUANG H Y, LIU X F, XIE J X. Effects of processing parameters on the fabrication of copper cladding aluminum rods by horizontal core-filling continuous casting [J]. Metallurgical and Materials Transations B, 2011, 42(1): 104-113. 陈淑英,常国威,岳旭东,李青春 辽宁工业大学 材料科学与工程学院,锦州 121001 摘 要:采用浇铝法制备铜铝复合铸锭,研究液体铝与固体铜复合过程中过渡层的凝固过程与组织变化规律,结果表明,铜铝复合铸锭中过渡层由α(Al)+α(Al)-CuAl2共晶、α(Al)-CuAl2共晶、CuAl2+α(Al)-CuAl2共晶和Cu9Al4四种组织组成,浇注温度、铜板外表面强制冷却方式以及浇注后到开始强制冷却时间不影响过渡层内组织种类,但过渡层内各种组织占过渡层厚度的比例随工艺参数而变化。过渡层顶部的纯铝最先开始凝固,而后α相以枝晶的方式、CuAl2相以平面晶或胞晶方式分别从过渡层的两侧向过渡层内生长。铜板外表面冷却强度越强,α枝晶越发达,CuAl2相越容易长成平面晶。 关键词:铜包铝;浇铝法;过渡层;凝固过程;凝固组织 (Edited by Yun-bin HE) Foundation item: Project (LJQ2014062) supported by the Outstanding Young Scholars in Colleges and Universities of Liaoning Province, China Corresponding author: Shu-ying CHEN; Tel: +86-416-4199650; E-mail: csy_lg@126.com DOI: 10.1016/S1003-6326(16)64343-1浇铝法制备铜铝复合铸锭时过渡层凝固过程与组织

