用丝束电极研究NaNO2对钢铁防护性能的影响
王巧玲1, 2,黄桂芳3
(1. 湖南省环保局,湖南 长沙,410007;
2. 长沙环境保护职业技术学院,湖南 长沙,410004;
3. 湖南大学 物理与微电子科学学院,湖南 长沙,410082)
摘 要:采用丝束电极多电极电化学法研究钢铁在不同浓度的NaCl溶液中的电位变化,并分析NaNO2的加入对体系电位的影响和NaNO2对钢铁的防护作用。研究结果表明:钢铁在NaCl溶液中的电位分布在一定的电位区间,各丝电极的电位存在差异,各区域金属的腐蚀不均匀;随着溶液中NaCl含量从1%增大至5%,丝束电极的电极电位平均值从-0.43 V变为-0.54 V,电极电位负移,金属腐蚀倾向增大;由于NaNO2和钢铁电极表面的腐蚀产物Fe2+的相互作用并生成保护性的薄膜,在NaCl溶液中加入NaNO2将使体系电极电位明显正移;加入NaNO2后各丝电极电位的分布是随机的、不均匀的,且电位分布区间增大,表明所形成的保护性薄膜不均匀。
关键词:腐蚀;NaNO2;丝束电极
中图分类号:O646 文献标识码:A 文章编号:1672-7207(2008)02-0317-05
Effect of sodium nitrite on corrosion of mild steel by using wire beam electrode
WANG Qiao-ling1, 2, HUANG Gui-fang3
(1. Environmental Protection Bureau of Hunan Province, Changsha 410007, China;
2. Changsha Environmental Protection College, Changsha 410004, China;
3. College of Physics and Microelectronics Science, Hunan University, Changsha 410082, China)
Abstract: The potential distribution of steel in different contents of NaCl solution was investigated with wire-beam electrode, and the effect of NaNO2 on the potential distribution of steel and the protective behavior of NaNO2 was analyzed. The results show that the steel potential distributes in a certain range suggest the inhomogeneous of steel corrosion. The average potential shifts from -0.43 V to -0.54 V as the content of NaCl increases from 1% to 5%, which indicates that the steel tends to corrode as the NaCl content increases. With the addition of NaNO2, the potential of steel shifts to positive direction and leads to the formation of compact passivation film due to the interaction of Fe2+ and oxygen. The potential distributes randomly and the protective film is inhomogeneous.
Key words: corrosion; NaNO2; wire-beam electrode
金属腐蚀是影响国民经济发展的一个很重要问题。研究金属的腐蚀规律和防护对策,从而降低和抑制金属腐蚀具有重大的社会经济意义。缓蚀剂法是在腐蚀介质中添加能降低腐蚀速率的物质(称缓蚀剂),从而达到抑制或降低金属腐蚀的目的。由于化学缓蚀剂法较为简便,缓蚀效果优良,且成本低廉而得到广泛应用。通常在中性介质和碱性介质中使用的无机缓蚀剂有NaNO2,它们与金属离子作用生成难溶盐覆盖于金属表面,成为具有保护性的薄膜,阻滞了阳极反应,从而降低了金属的腐蚀速率。
根据现代电化学理论,常温下金属的腐蚀是一个电化学过程。因此,电化学分析方法可应用于金属腐蚀规律和腐蚀机制的研究。在20世纪六七十年代,研究者从电化学入手来研究金属的腐蚀和防护问题,取得了很大进展[1-10]。S. T. Arab等[7]采用极化和交流阻抗测试Fe78B13Si9钢在含10% CH3OH和0.2 mol/L Na2SO4 溶液中的腐蚀和缓蚀效率,发现铁基金属的腐蚀主要由电荷转移过程控制。J. Wloka等[8]通过测量开路电位分析AA7010样品的腐蚀行为,发现由于镁的选择性溶解导致开路电位显著增大;陈有业等[9]采用电化学方法来评价防锈油对金属的防护性能比传统测试方法简单、快速,且数据可靠。目前,电化学方法正广泛应用于金属腐蚀机理、腐蚀速度的测定和腐蚀控制等多个方面。尽管电化学方法已成为研究金属腐蚀及防护问题的有力手段,但由于金属腐蚀,特别是在有保护膜情形下的金属腐蚀是随机的、不均匀腐蚀,测试的重现性和可靠性有待于提高。自20世纪90年代以来,研究者针对金属腐蚀的随机不均匀性,采用丝束电极多电极体系进行电化学测试,表明采用多电极体系统计分析的方法可明显提高测试重现性和可靠 性[11-12]。丝束电极已广泛应用于金属的局部腐蚀,缓蚀剂的缓蚀效果以及有保护膜的金属体系的腐蚀研 究[13-16]。在此,本文作者采用多电极电化学法分析NaNO2对金属的防护作用,直观地探测液膜局部区域的电化学参数分布,研究液膜下金属的腐蚀情况,分析NaCl和NaNO2浓度对金属腐蚀的影响。
1 实 验
丝束电极是将一定数目的铁丝均匀排列,用环氧树脂浇封固化。丝束电极的工作表面依次用粗砂布、粗砂纸、细砂纸至5号金相砂纸打磨至镜面,用无水乙醇、丙酮依次清洗、吹干后待用。将丝束电极放入NaCl以及NaCl和NaNO2的混合溶液中,以饱和甘汞电极为参比电极,测试每一丝电极的电位及其随时间的变化。测试电路如图1所示。
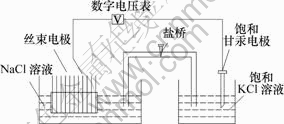
图1 电位测试装置
Fig.1 Schematic arrangement of potential measurement
2 结果与讨论
2.1 NaCl浓度对丝束电极在溶液中电位的影响
丝束电极在1%和5% NaCl溶液中的电位分布如图2所示,其中X对应于丝电极的位置(以下同)。

(a) 1% NaCl; (b) 5% NaCl
图2 丝束电极在1% 和5% NaCl溶液中的电位分布
Fig.2 Potential distributions of wire beam electrode in 1% and 5% NaCl solution
从图2可以看出,不同丝电极的腐蚀电位是随机的,有些地方电位较负,有些地方电位较正。说明丝束电极间存在电化学差异,液膜下存在腐蚀微电池,电极在NaCl溶液中的腐蚀是不均匀的。丝束电极的腐蚀电位在一定程度上反映了液膜下基底金属的腐蚀倾向。电位高表明金属具有较高的热力学稳定性,金属受到保护,反之,金属有发生腐蚀的可能。所以,从丝束电极的腐蚀电位分布及其变化,可以判别金属的腐蚀趋势。从图2还可知,丝束电极在1% NaCl溶液中的电位分布在-0.6~-0.4 V之间,其中大部分分布于-0.4~-0.5 V范围,当NaCl溶液浓度为5%时,丝束电极的电位在-0.7~-0.4 V范围内均有分布,大部分集中在-0.5~-0.6 V电位区间,腐蚀倾向增大。即随着NaCl溶液浓度的增大,体系的电极电位负移,说明氯离子浓度增大使钢的腐蚀增大。
在中性NaCl溶液中,腐蚀电池的阳极发生铁溶解反应:Fe-2e→Fe2+;
阴极发生氧去极化反应:O2 +2H2O+4e→4OH-。
阴阳极反应生成的Fe2+和OH-,在电极表面相遇结合成Fe(OH)2,在电极表面附近氧的氧化作用下生成Fe2O3或Fe3O4,电极表面生成的不可溶物Fe(OH)2,Fe2O3和Fe3O4阻隔了腐蚀介质和金属基体的接触,从而抑制了腐蚀介质对金属的腐蚀。由于Cl-是活性很强的阴离子,Cl-对金属的腐蚀作用机理可认为Cl-破坏钢表面的钝化膜,使钢发生局部腐蚀。作用机理如下:Cl-能吸附在钝化膜上并渗透到钢表面,造成钝化膜局部破坏和基体金属局部腐蚀。由于Cl-局部破坏钢表面的钝化膜,露出铁基体,这些铁基体与钝化膜完好部分存在电位差构成“腐蚀电池”。铁基体作为阳极,钝化膜完好部分作为阴极。腐蚀电池作用的结果使钢铁电极表面产生点蚀。形成大阴极-小阳极结构,点蚀发展十分迅速。同时,阳极反应过程产生的Fe2+也可能与Cl-结合生成可溶性的FeC12,或活性很强的Cl-置换钝化膜中的氢氧根离子生成可溶性FeC12,FeC12在向内扩散时遇到OH-,生成Fe(OH)2,又进一步氧化成铁的氧化物,从而加速阳极过程。
丝束电极是一个多电极体系,可用其统计结果进行表征。图3所示为丝束电极在不同NaCl溶液中的电位平均值。

图3 丝束电极的电位平均值随NaCl溶液浓度的变化
Fig.3 Dependence of average potential of wire-beam electrode on content of NaCl solution
由图3可见,Cl-浓度越高,电位越负,特别是当NaCl溶液浓度低于1%时,随着NaCl溶液浓度的增大,电极电位急剧负移;而当NaCl溶液浓度大于1%时,继续增大NaCl溶液的浓度,电位变化趋势减缓。这可归于Cl-的导电作用。Cl-能否引起钢腐蚀取决于游离态的Cl-浓度与其表面孔隙液中OH-浓度的比值。若钢周围OH-浓度高(即pH值高),则钝化占优势;若局部C1-浓度高,则去钝化占优势。当中性NaCl溶液浓度高于1%时,金属腐蚀占优势。
2.2 NaNO2的加入对金属在NaCl溶液腐蚀的影响
NaNO2是一种有效的无机缓蚀剂,通过在一定浓度的NaCl溶液中加入一定量的NaNO2,来探讨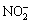 对金属腐蚀的影响。图4所示为丝束电极在 5% NaCl和添加不同含量的NaNO2溶液中的电位分布。
对金属腐蚀的影响。图4所示为丝束电极在 5% NaCl和添加不同含量的NaNO2溶液中的电位分布。
由图4(a)可以看出,在没有加NaNO2的NaCl溶液中,各丝电极的电位分布集中于-0.5~-0.6 V。与没有加NaNO2的NaCl溶液相比,添加NaNO2后体系的腐蚀电位分布范围增大,各丝电极的电位是随机、不均匀分布的,且体系电位明显正移。随着NaCl溶液中NaNO2浓度的增加,丝束电极的主要电位分布区间由NaCl溶液中的-0.5~-0.6 V逐步移至-0.2~-0.3 V和-0.2~-0.1 V(见图4(b)~4(d))。说明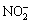 在电极表面吸附,阻滞了阳极反应的进行,同时与金属电极作用,在电极表面形成钝化膜,改变了电极的表面状态,导致电极电位正移,丝束电极不同区域钝化膜的形成是不均匀的、随机的。溶液中存在的Cl-和NaNO2对钝化膜的形成起相反作用,即Cl-对钝化膜的破坏作用以及NaNO2促进氧的还原和对电极表面形成致密钝化膜的促进作用并存,因而电位在一定范围内分布。
在电极表面吸附,阻滞了阳极反应的进行,同时与金属电极作用,在电极表面形成钝化膜,改变了电极的表面状态,导致电极电位正移,丝束电极不同区域钝化膜的形成是不均匀的、随机的。溶液中存在的Cl-和NaNO2对钝化膜的形成起相反作用,即Cl-对钝化膜的破坏作用以及NaNO2促进氧的还原和对电极表面形成致密钝化膜的促进作用并存,因而电位在一定范围内分布。
电极在添加不同浓度的NaNO2溶液中的平均电位变化如图5所示。由图5可见,随着NaCl溶液中NaNO2浓度的增加,平均电位正移。处于-0.2~-0.3 V电位区间,说明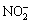 具有抑制电极发生腐蚀的作用,在电极表面形成致密的钝化膜,抑制了金属的阳极溶解。NaNO2是阳极型缓蚀剂,在NaCl溶液中加入一定量的NaNO2,由于它能促进氧的还原,促使电极表面形成致密的钝化膜或修复Cl- 对钝化膜的破坏,从而有效地抑制金属的阳极溶解。随着NaNO2浓度增大,电极表面区域参与钝化成膜和修复钝化膜的几率增大,致密钝化膜的形成使电位正移。
具有抑制电极发生腐蚀的作用,在电极表面形成致密的钝化膜,抑制了金属的阳极溶解。NaNO2是阳极型缓蚀剂,在NaCl溶液中加入一定量的NaNO2,由于它能促进氧的还原,促使电极表面形成致密的钝化膜或修复Cl- 对钝化膜的破坏,从而有效地抑制金属的阳极溶解。随着NaNO2浓度增大,电极表面区域参与钝化成膜和修复钝化膜的几率增大,致密钝化膜的形成使电位正移。
通过实验发现,随着丝束电极在溶液中浸泡时间的延长,某些丝电极的电极电位正移,这可归于NaNO2对钝化膜的修复作用。腐蚀电位平均值随时间的变化规律如图6所示。由图6可见,随着时间的推

(a) 5% NaCl;(b) 5% NaCl+1% NaNO2溶液;(c) 5% NaCl+3% NaNO2溶液;(d) 5% NaCl+5% NaNO2 溶液
图4 丝束电极浸入5% NaCl和不同含量NaNO2溶液中的电位分布
Fig.4 Potential distributions of wire-beam electrode in 5% NaCl and different contents of NaNO2 solution

图5 丝束电极的电位平均值随NaNO2溶液浓度的变化
Fig.5 Dependence of average potential of wire-beam electrode on the content of NaNO2 solution

1—5% NaCl+1% NaNO2; 2—5% NaCl+3% NaNO2;
3—5% NaCl+5% NaNO2
图6 加入不同浓度NaNO2的平均电位随浸泡时间的变化曲线
Fig.6 Dependence of average potential of wire-beam electrode on time with different contents of NaNO2
移,腐蚀电位负移,说明电极发生腐蚀的倾向增 大。随着氧气的渗透以及氯离子对氧化膜的破坏,电极发生腐蚀,形成腐蚀原电池,进而加速电极腐蚀过程。同时,由于溶液中氯离子对钝化膜的局部破坏作用和NaNO2对钝化膜的修复作用并存,单个丝电极的电位随浸泡时间的延长可能出现波动。由图6还可以看出,在浸泡初期,NaNO2浓度对电极电位的分布影响较大,但若浸泡时间加长,则其浓度对电极电位分布的影响减小。
3 结 论
a. 用丝束电极研究钢铁在NaCl溶液中的腐蚀,电位测试结果表明,金属在NaCl溶液中的电位分布在一定区间内,表明金属的腐蚀是不均匀的,电极与电极之间存在腐蚀微电池。
b. 在中性NaCl溶液中,金属表面由于溶液中氧的作用生成钝化膜,由于Cl-对铁基体表面钝化膜的破坏,局部发生腐蚀,阴极区受到保护,阳极区发生腐蚀。Cl-浓度的增大加速了金属铁的腐蚀,腐蚀电位负移。
c. 在NaCl溶液中加入一定量的NaNO2,由于它能促进氧的还原,从而在电极表面形成致密的钝化膜,抑制金属的阳极溶解。随着NaNO2浓度增大,修复钝化膜的几率增大,基底金属腐蚀倾向降低,从而有效地防止基底金属腐蚀。
参考文献:
[1] Mills D J. DC electrical measurements to asses anticorrosive coatings[J]. JOCCA, 1986, 69(8): 203-208.
[2] 谭 胜, 付洪瑞, 姚素薇, 等. 用电化学方法评价油膜防锈性能[J]. 军械工程学院学报, 2001, 13(2): 29-32.
TAN Sheng, FU Hong-rui, YAO Su-wei, et al. Estimating rust preventive ability of oil coating by means of electrochemical method[J]. Journal of Ordnance Engineering College, 2001, 13(2): 29-32.
[3] Walter G W. A critical review of d.c. electrochemical tests for painted metals[J]. Corrosion Science, 1986, 26(1): 39-47.
[4] Walter G W. A review of impedance plot methods used for corrosion performance analysis of painted metals[J]. Corrosion Science, 1986, 26(9): 681-703.
[5] El-Feki A A, Walter G W. Corrosion rate measurements under conditions of mixed charge transfer plus diffusion control including the cathodic metal ion deposition partial reaction[J].Corrosion Science, 2000, 42(6): 1055-1070.
[6] Tan C G, Jiang W J, Zhang Z C, et al. The effect of Ti-addition on the corrosion behavior of the partially crystallized Ni-based bulk metallic glasses[J]. Materials Chemistry and Physics, 2008, 18(1): 29-32.
[7] Arab S T, Emran K M. Structure effect of some thiosemicarbazone derivatives on the corrosion inhibition of Fe78B13Si9 glassy alloy in Na2SO4 solution[J]. Materials Letters, 2008, 62(6/7): 1022-1032.
[8] Wloka J, Bürklin G, Virtanen S. Influence of second phase particles on initial electrochemical properties of AA7010-T76[J]. Electrochimica Acta, 2007, 53(4): 2055-2059.
[9] 陈有业, 姚素薇, 张卫国, 等. 防锈油性能的快速评价方法研究[J]. 天津大学学报: 自然科学版, 2002, 35(1): 87-90.
CHEN You-ye, YAO Su-wei, ZHANG Wei guo, et al. Research on the methods of rapidly evaluating the property of rust preventive oil[J]. Journal of Tianjin University: Science and Technology, 2002, 35(1): 87-90.
[10] Irena N A. Electrochemical studies of carbon steel coated with inhibiting oil films containing nitrated petroleum products[J]. Corrosion Science, 1981, 21(1): 41-48.
[11] Tan Y J. The effects of inhomogeneity in organic coatings on electrochemical measurements using a wire beam electrode: Part Ⅰ[J]. Prog Org Coat, 1991, 19(1): 89-94.
[12] Tan Y J. The effects of inhomogeneity in organic coatings on electrochemical measurements using a wire beam electrode: Part Ⅱ[J]. Prog Org Coat, 1991, 19(3): 257-263.
[13] Wu C L, Zhou X J, Tan Y J. A study on the electrochemical inhomogeneity of organic coatings[J]. Prog Org Coat, 1995, 25(4): 379-389.
[14] Zhong Q D. Study of corrosion behavior of mild steel and copper in thin film salt solution using the wire beam electrode[J]. Corrosion Science, 2002, 44(5): 909-916.
[15] 黄桂芳, 吴翠兰, 靳九成. 油膜下局部腐蚀的探讨[J]. 腐蚀科学与防护技术, 2000, 12(1): 30-31.
HUANG Gui-fang, WU Cui-lan, JIN Jiu-cheng. Study on localized corrosion of metal under oil film[J]. Corrosion Science and Technology Protection, 2000, 12(1): 30-31.
[16] 李国希, 王 丹, 朱日龙. 用丝束电极研究亚硝酸钠对钢铁缝隙腐蚀的影响[J]. 湖南大学学报: 自然科学版, 2005, 32(6): 91-93.
LI Guo-xi, WANG Dan, ZHU Ri-long. Effect of sodium nitrite on crevice corrosion of mild steel using wire beam electrode[J]. Journal of Hunan University: Natural Science, 2005, 32(6): 91-93.
收稿日期:2008-01-09;修回日期:2008-03-12
基金项目:湖南省科技厅资助项目(2007CK3057;2007FJ4136);长沙环境保护职业技术学院资助项目(NYC0705);湘财建指资助项目([2007]185号)
通信作者:王巧玲(1971-),女,河南开封人,副教授,从事化学化工、环境工程研究;电话:0731-7277296;E-mail: bin1000@163.com