经氰化处理的黄铜矿和方铅矿的浮选行为与机理
来源期刊:中国有色金属学报(英文版)2016年第12期
论文作者:马艺闻 韩跃新 朱一民 李艳军 刘浩
文章页码:3245 - 3252
关键词:黄铜矿;方铅矿;氰化吸附;浮选;丁基黄药
Key words:chalcopyrite; galena; cyanide absorption; flotation; butyl xanthate
摘 要:研究了CN-与黄铜矿和方铅矿之间的吸附作用,然后在丁基黄药(BX)体系下研究了氰化吸附后的黄铜矿、方铅矿的浮选试验。结果表明,CN-与两种矿物表面存在化学吸附作用,并可用Langmuir等温模型近似描述。在pH值为6.5,丁基黄药用量为4.0 mg/L的适宜条件下,氰化后的黄铜矿和方铅矿的浮选回收率可分别达到82.1%和63.9%。尽管CN-降低了黄铜矿、方铅矿表面的接触角,但丁基黄药能够提高氰化矿物表面的疏水性。CN-对黄铜矿的抑制作用大于方铅矿。在pH值为4.2~8.4时,BX与氰化后的方铅矿表面的相互作用存在静电吸附;BX在氰化后的黄铜矿表面的吸附作用为化学吸附。
Abstract: Adsorbing tests between CN- and chalcopyrite or galena were conducted firstly, and then flotation tests of the two cyaniding minerals were investigated in butyl xanthate (BX) system. Results showed that the interaction between CN- and the two mineral surfaces were both chemical adsorption and can be described by the Langmuir adsorption isotherm model. In the optimum condition of pH 6.5 and 4.0 mg/L BX, the recovery of cyaniding chalcopyrite and galena reached 82.1% and 63.9%, respectively. BX improved the hydrophobicity of the surfaces of the two minerals, although CN- reduced the contact angle on the surface of minerals. The inhibitory effect of CN- on chalcopyrite far outweighed galena. Electrostatic adsorption exists in the interaction between BX and the surface of galena after cyanide treatment in the pH range of 4.2-8.4, while the interactions between BX and the surface of chalcopyrite after cyanide treatment is chemical adsorption.
Trans. Nonferrous Met. Soc. China 26(2016) 3245-3252
Yi-wen MA1,2, Yue-xin HAN1, Yi-min ZHU1, Yan-jun LI1, Hao LIU1
1. College of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China;
2. School of Mining Engineering, University of Science and Technology Liaoning, Anshan 114051, China
Received 4 January 2016; accepted 12 October 2016
Abstract: Adsorbing tests between CN- and chalcopyrite or galena were conducted firstly, and then flotation tests of the two cyaniding minerals were investigated in butyl xanthate (BX) system. Results showed that the interaction between CN- and the two mineral surfaces were both chemical adsorption and can be described by the Langmuir adsorption isotherm model. In the optimum condition of pH 6.5 and 4.0 mg/L BX, the recovery of cyaniding chalcopyrite and galena reached 82.1% and 63.9%, respectively. BX improved the hydrophobicity of the surfaces of the two minerals, although CN- reduced the contact angle on the surface of minerals. The inhibitory effect of CN- on chalcopyrite far outweighed galena. Electrostatic adsorption exists in the interaction between BX and the surface of galena after cyanide treatment in the pH range of 4.2-8.4, while the interactions between BX and the surface of chalcopyrite after cyanide treatment is chemical adsorption.
Key words: chalcopyrite; galena; cyanide absorption; flotation; butyl xanthate
1 Introduction
Gold cyanidation tailings are often associated with valuable minerals such as pyrite, chalcopyrite, galena, and sphalerite. The resource wastes and environmental pollution problems caused by the long-term accumulated gold cyanidation tailings, make the effective recycling of valuable minerals in the tailings significant. Due to very fine particle sizes by intense grinding and the existence of CN-, recovery of useful minerals by flotation from the cyanidation tailings is very difficult [1]. In order to recover the valuable elements, such as Cu, Pb, Zn, S and Fe, many research efforts were made on comprehensive utilization and recovery from gold cyanidation tailings. ZHU et al [2] showed that galena and sphalerite in cyanidation tailings were floated and recovered well by xanthate as a collector, where activated carbon was used to adsorb excess cyanide and residual flotation reagents in the process of gold cyanidation. LV et al [3] proved that sodium hypochlorite oxidized cyanide to cyanate, eliminating the negative effect of residual cyanide to the environment. In the meanwhile, sphalerite and pyrite were depressed by sodium hypochlorite enormously at pH>10.0, which benefited the recovery of chalcopyrite and galena. GAO et al [4] indicated that one-step chlorinating volatilization technology was suitable for eliminating Cu and As in the cyanidation slags.
Most research efforts were primarily focused on the flotation and recovery of sulfide minerals, and very few studies reported the flotation mechanism of sulfide minerals in the presence of CN-. PIAO et al [5] found that chalcopyrite was depressed slightly by O,O-bis(2,3- dihydroxypropyl) dithiophosphate (DHDTP) while galena was depressed strongly. Zeta potentia1 experiments indicated that DHDTP absorbed galena more efficiently than chalcopyrite, which was due to the electrostatic attraction between DHDTP and minerals. FU [6] reported that zinc sulphate and sodium sulphite could inhibit the flotation of sulfide minerals by Zn(OH)2 and Zn4(SO3)(OH)6·H2O generated on the surface of minerals. Kocabag and GULER [7] found that a critical cyanide concentration and pulp potential existed when cyanide was used as a depressant for chalcopyrite and pyrite, whereas with galena-chromate system no critical potential was observed. PIAO et al [8] indicated that sodium 2,3-dihydroxypropyl dithiocarbonate (SGX), could separate chalcopyrite from galena by depressing galena. HE et al [9] showed that in the presence of SGX, marmatite could be activated by Cu2+ and showed good flotability, while pyrite could not be activated and therefore showed poor flotability. Some researchers [10-14] discussed the mechanism of the interaction between CN- and sulfide mineral or metal surface from the point of view of molecular structure and quantum mechanics, which were less contacted with flotation and adsorption tests. WOODS [15] and BUSWELL et al [16], based on the electrochemical mechanism of the inhibition of sulfide mineral flotation, found that the oxidation of pyrite surface generated hydrophilic substances such as Fe(OH)3 and  was due to the decrease of surface oxidation potential in strong alkaline environment.
was due to the decrease of surface oxidation potential in strong alkaline environment.
Flotation behaviors of minerals and the interaction mechanisms between collector and the surface of cyaniding minerals play a significant role in the comprehensive utilization of gold cyanidation tailings. In this study, chalcopyrite and galena were firstly pretreated by CN- of which concentration was the same as that in gold cyanidation tailings, and the characteristic of cyanide adsorption was discussed. Secondly, flotation behaviors of chalcopyrite and galena after cyanidation were examined at different pulp pH values with BX as the collector, and the interaction mechanisms between the flotation reagents and the minerals surface were discussed based on the measurements of zeta potential and contact angle.
2 Experimental
2.1 Materials
Chalcopyrite and galena samples were obtained from Zhaoyuan, Shandong Province, China. After crushing and grinding, the size of the mineral samples was less than 0.045 mm, close to the actual size of gold tailings. Chemical compositions of the chalcopyrite sample were: 34.1% Cu, 29.5% Fe, 35.2% S, and 1.2% others. Chemical compositions of galena were 79.1% Pb, 16.5% S, and others 4.4%. Sodium cyanide used in the experiments was obtained from Shenyang Nonferrous Metal Research Institute, China. Industrial grade BX was used as the collector, and terpenic oil was used as frother, which were supplied by Tieling Flotation Reagents Factory, Liaoning, China. Analytical grade NaOH and HCl were employed to adjust the pH of the flotation system.
2.2 Cyanide adsorption process
Predetermined amounts of mineral samples were put into 150 mL NaCN solution (25.0 mg/L). Then, the suspensions were stirred using a magnetic stirrer (FuHua78-1, Made in China) at 2400 r/min for 5 min. The suspensions were subsequently filtered and dried naturally, to simulate the long-term accumulation of the gold cyanidation tailings.
2.3 Flotation test
The flotation tests were carried out in a micro-flotation cell with a 25 mL effective volume. The amount of cyaniding mineral samples used in each experiment was 2.0 g and the pulp density maintains at 10% with distilled water. The flotation time was 3 min and the stirring speed was 2400 r/min. NaOH and HCl solutions were used to adjust the pH of the flotation system. The floated and non-floated fractions were filtered, dried using a vacuum drying oven (DZF, Made in China) at 40 °C, and weighed for the recovery calculation.
2.4 Contact angle measurement
Contact angles of cyaniding mineral samples were measured using a contact angle instrument (XG-CAMB, Made in China). Cyanide mineral sample (2.0 g) was added to a 50 mL beaker with 20 mL distilled water. After that, the solution was stirred for 5 min with a magnetic stirring apparatus. Following that, the samples were filtered, dried, squashed, and then put onto the platform of the contact angle instrument. A certain dosage of distilled water was dropped one by one. Every sample was measured three times in different positions, and an average value was taken.
2.5 Zeta potential measurement
Zeta potentia1 of cyaniding mineral samples was measured using a zeta apparatus (Nano-ZS90, Made in England) by the measurements of electrophoresis. The samples were ground to smaller than 2 μm in the agate mortar. For each test, 30 mg of cyanidation mineral powder was added to a beaker with 50 mL distilled water. Then, the suspension was stirred for 3 min with a magnetic stirring apparatus. The pH of suspension was regulated with HCl and NaOH solutions to a desired value. After that the suspension was ready for the measurement. Every sample was measured three times, and an average value was taken.
3 Results and discussion
3.1 Cyanide adsorption
The adsorption of CN- on the surface of chalcopyrite and galena was investigated by the cyanide tests. According to the reaction mechanism of cyanide ions and silver ions in solution, CN- concentration in the solutions was determined using atomic absorption spectrophotometer with graphite furnace (AA-6300, Made in Japan) by means of silver cyanogen complexation. And then the adsorption quantity of CN- on mineral surface was obtained.
Considering that pH value is one of the important factors affecting adsorption properties, and it is easy for cyanide to hydrolyze and generate HCN in acidic conditions, the adsorption characteristics of CN- on the surface of chalcopyrite and galena discussed in pH range of 7.0-12.0 are shown in Fig. 1.
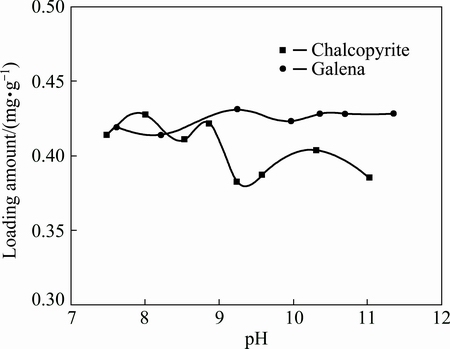
Fig. 1 Adsorption characteristics of CN- on surface of minerals versus pH
On the whole, the adsorption quantity of CN- on the surface of chalcopyrite decreased with the increase of pH value. At pH 8.0, the adsorption quantity reached the maximum value, and then appeared unsteadily in the pH range of 8.0-11.0. It was shown that, hydrophilic compounds generated on the surface of chalcopyrite by cyanide adsorption were not stable, and the inhibitory effect of CN- on chalcopyrite declined with the increasing pH value, relatively. The adsorption quantity of CN- on the surface of galena was less changed with the increase of pH value, indicating that adsorption of CN- on the surface of galena was almost not affected by pH value.
Adsorption isotherms of CN- on the surface of chalcopyrite and galena are shown in Fig. 2. The adsorption quantity of CN- on the surface of chalcopyrite and galena increased with the increase of CN- concentration. As seen from Fig. 2(a), adsorption quantity increased slowly, and then gradually stabilized, as a result of the copper cyanide complex ions that had a variety of balances, and gradually generated stable copper cyanide complex  [17]. Different from chalcopyrite, the adsorption quantity of CN- on the surface of galena leapt in the low concentration range (0-0.5 mg/L), because lead cyanide complex Pb(CN)+ generated was less stable. The inhibitory effect of CN- on chalcopyrite outweighed galena.
[17]. Different from chalcopyrite, the adsorption quantity of CN- on the surface of galena leapt in the low concentration range (0-0.5 mg/L), because lead cyanide complex Pb(CN)+ generated was less stable. The inhibitory effect of CN- on chalcopyrite outweighed galena.
Adsorption isotherms could approximate to L-type adsorption curve. According to the adsorption isotherms, the adsorption characteristics of CN- on the surface of chalcopyrite and galena could be described by the Langmuir adsorption isotherm model, as shown in Eq. (1):
 (1)
(1)
where c is the adsorption concentration of CN-; Г and Гs represent adsorption quantity and saturated adsorption quantity respectively; and b is a constant.

Fig. 2 Adsorption isotherms of CN- on surface of chalcopyrite (a) and galena (b)
Langmuir formula of CN- on the surface of minerals is shown in Fig. 3. The fitting degree of galena is higher. As calculation results shown, the adsorption quantity of CN- on the surface of chalcopyrite was 0.32 mg/g, and that on the surface of galena was 0.35 mg/g. The cyanide adsorption was a fast process, which can be regarded as a single molecular layer of chemical adsorption.
As seen from Fig. 2 and Fig. 3, the results indicated that interaction between CN- and the metal ions freed from the mineral surface was chemical adsorption, and generated a hydrophilic film in form of complex. The complex formation of chalcopyrite and CN- transformed to more stable form continuously, the inhibitory effect of CN- on chalcopyrite was strong, thus the inhibition of CN- on galena was less susceptible.
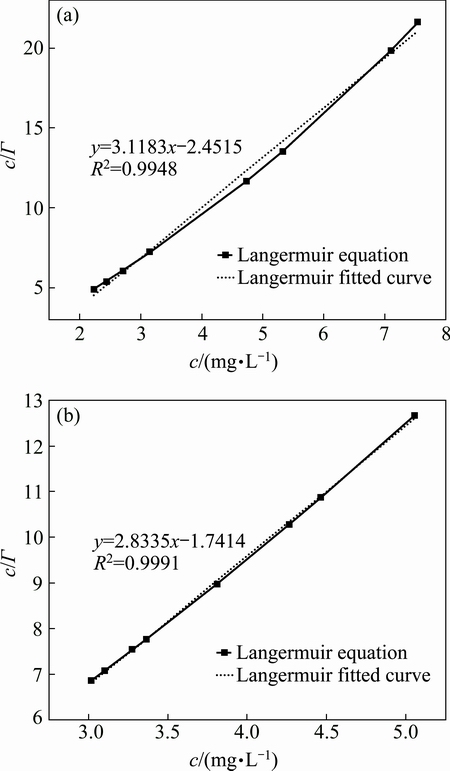
Fig. 3 Langmuir formula of CN- on surface of chalcopyrite (a) and galena (b)
3.2 Cyanidation mineral flotation
3.2.1 Effects of pH on flotation
The recovery of chalcopyrite and galena after cyanide treatment by BX (4.0 mg/L) as a function of pH is shown in Fig. 4. The recovery of chalcopyrite after cyanide treatment decreased slightly with the increase of pH value. At pH 6.0, the maximum recovery was 81.5%. The recovery of galena after cyanide treatment increased to a maximum value 67.8% at pH 7.0, after which it dropped to a minimum value of 52.0% at pH 10.0. The results indicated that the floatability of chalcopyrite after cyanide treatment was higher than that of galena after cyanide treatment in the whole pH range tested. The chalcopyrite after cyanide treatment would be flotation well below pH 7.0, while galena after cyanide treatment had better flotation results in the pH range of 6.5-9.0.
In summary, chalcopyrite and galena after cyanide treatment can be floated by BX as the collector, although they are depressed by the presence of CN-. This is mainly attributed to the competitive adsorption between BX and CN- on the mineral surfaces. Hydrophobic groups of BX are competitively adsorbed on the surfaces of chalcopyrite and galena after cyanidation to form a hydrophobic film, hindering the absorption of CN-, which even falls off. The chalcopyrite and galena in gold cyanidation tailings could be separated flotation and recycling in the different conditions of pH values.

Fig. 4 Effects of pH on flotation of chalcopyrite and galena after cyanide treatment by BX
3.2.2 Effects of collector concentration on flotation
The effects of BX concentration on flotation of chalcopyrite and galena after cyanide treatment were studied at a fixed pH 6.5. The recovery of chalcopyrite after cyanide treatment increased slightly with the increase of the BX concentration (Fig. 5). The recovery was above 70% in the whole range of concentration tested. The recovery of galena after cyanide treatment was significantly improved at the concentration of 4.0 mg/L. Although the recoveries of chalcopyrite and galena after cyanide treatment reached the maximum value at the concentration of 10.0 mg/L (2.5 times that of the concentration 4.0 mg/L), the concentration of 4.0 mg/L was considered the optimum reagent dosage with the consideration of the reagent cost. The recoveries of chalcopyrite and galena after cyanide treatment were about 82.1% and 63.9%, respectively, while the collector concentration was 4.0 mg/L. BX was the effective collector to select the chalcopyrite in gold cyanidation tailings.

Fig. 5 Effects of concentration of BX on flotation of chalcopyrite and galena after cyanide treatment at pH 6.5
3.3 Measurement of contact angle
Contact angle (CA) can be used to characterize the hydrophobicity and flotability of mineral surfaces. The increase of contact angle of the mineral surface indicates greater hydrophobicity, promoting the flotation process [18]. Contact angles on the surface of chalcopyrite and galena as a function of concentration of NaCN solutions are shown in Fig. 6.

Fig. 6 Effects of CN- concentration on contact angle of mineral surface
The original contact angles on the surface of chalcopyrite and galena surface were 59.8° and 79.8°. When NaCN solution (10.0 mg/L) was added, contact angle of chalcopyrite surface fell slightly to 58.4°, while contact angle of galena surface dropped remarkably to 66.2°. Then, contact angle of chalcopyrite surface jumped down, on the other hand, contact angle of galena surface decreased slowly with the increase of CN- concentration. When the concentration of sodium cyanide was greater than 150.0 mg/L, the contact angle of galena surface was reduced to 57.8° and stabilized, while the contact angle of chalcopyrite surface was reduced to less than 35.0°. The results illustrated that cyanide ions reduced the hydrophobicity of minerals surface, and enhanced the hydrophilicity, further indicated that the flotability of mineral was decreased, there existed inhibition effect of sodium cyanide on mineral flotation. And inhibitory effect of sodium cyanide on galena was weaker than on chalcopyrite, significantly.
Furthermore, a series of samples were prepared with BX as the collector at pH 6.5. Contact angle and flotation recovery as functions of concentration of BX on flotation of chalcopyrite and galena after cyanide treatment are shown in Fig. 7.

Fig. 7 Contact angle and flotation recovery as function of concentration of BX at pH 6.5
The contact angles on the surface of cyanide minerals were greatly increased at the moment of BX adding, and then the contact angle of cyaniding chalcopyrite surface increased significantly with the increase of the BX concentration, while the contact angle of cyanide galena surface increased slightly (Fig. 7). When the concentration was 4.0 mg/L, the contact angles of chalcopyrite and galena after cyanide treatment are 75.9° and 86.8°, respectively. The results demonstrated that the increasing trend of the contact angle of cyanide mineral surfaces was the same as the change trend of flotation recovery. BX could improve the floatability of chalcopyrite and galena after cyanide treatment, and flotation result of chalcopyrite after cyanide treatment is better. There exists competitive adsorption between BX and CN- on the surface of minerals. The chemical adsorption between xanthate generated by BX and mineral surface is stronger than that of CN-, and X- could activate metal ions on the surface of suppressed minerals.
Although the contact angle of cyaniding galena is larger than that of chalcopyrite after cyanide treatment, collecting effect of galena with BX is not as good as chalcopyrite, which seems to be contrary to theory of wettability. But the flotation process is a complex process, and adhesion of minerals on bubbles is another one of the key factors. Obviously, density of galena is greater than that of chalcopyrite, therefore galena particles are easier to fall off bubbles, which can affect the flotability of cyaniding galena. On the other hand, with the increase of BX concentration, xanthate of copper is generated gradually in the solution, and hydrophobic material S0 is generated on the surface of chalcopyrite after cyanide treatment, which makes the hydrophobicity of chalcopyrite after cyanide treatment enhanced and the flotability is improved [19].
3.4 Measurement of zeta potential
In order to further determine the difference of CN- inhibitory effect between chalcopyrite and galena, and to investigate the flotation mechanism of cyaniding chalcopyrite and galena with BX, zeta potential was measured to discuss electrical properties of the two mineral surfaces. The effects of CN- on zeta potential of the minerals versus pH are shown in Fig. 8. As a reference, the distribution map of CN- at different pH values is shown in Fig. 9, and the hydrolysis reactions of sodium cyanide are shown as Eqs. (2) and (3).
CN-+H2O HCN+OH- (2)
HCN+OH- (2)
HCN CN-+H+ (3)
CN-+H+ (3)

Fig. 8 Effects of CN- on zeta potential of minerals surface
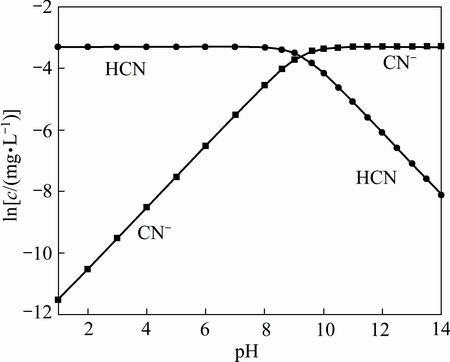
Fig. 9 lg c-pH curves of components in 25.0 mg/L CN- solution
The point of zero charge (PZC) of chalcopyrite moved negatively from pH 5.7 to 4.2 with the presence of CN-. It indicated that freed CN- from the solution was adsorbed on the surface of chalcopyrite, which brought negative charges to the mineral particles, therefore hydrophilicity of mineral surface was enhanced. What is more, CN- ions were adsorbed on the surface diffusion layer of chalcopyrite by electrostatic adsorption when pH value was less than 4.2. While in the pH range of 4.2-5.7, zeta potential of chalcopyrite moved from positive to negative, which reveals that CN- had already penetrated into the inner of the electric double layers on the surface of chalcopyrite, and it was characterized by chemical adsorption. In the pH range of 6.7-9.2, surface charge of cyaniding chalcopyrite was reduced, because CN- ions were combined with H+ ions to generate HCN gradually with pH increasing, thus HCN was the mainly existence form below pH 9.2 (Fig. 9). Above pH 9.2, negative charge on the surface of cyaniding chalcopyrite was increased by adsorption of CN- in solutions. The results indicated that CN- adsorption on the surface of chalcopyrite could be chemical adsorption with weak electrostatic adsorption, the inhibitory effect of CN- on chalcopyrite was strong.
The zeta potential of galena remained negatively with no obvious change in the whole pH range, whether CN- existed or not. The results indicated that CN- adsorption on the surface of galena was chemical adsorption, and the inhibitory effect of CN- on galena was weaker.
In flotation solution, BX may exist in a variety of forms, such as X- (C4H9OCS2-) and HX (C4H9OCS2H), the reactions are shown in Eqs. (4) and (5), and the distribution map of BX solution at different pH values is shown in Fig. 10.
C4H9OCS2-+H2O OH-+C4H9OCS2H (4)
OH-+C4H9OCS2H (4)
C4H9OCS2H C4H9OCS2-+H+ (5)
C4H9OCS2-+H+ (5)
The effects of BX on zeta potential and flotation recovery of chalcopyrite after cyanide treatment versus pH are shown in Fig. 11.

Fig. 10 lgc-pH curve of components in 4.0 mg/L BX solution
Zeta potential of cyaniding chalcopyrite surface moved to the negative direction gradually after adding BX (Fig. 11), and the point of zero charge of cyaniding chalcopyrite surface shifted to pH 3.6. When pH was less than 3.6, cyaniding chalcopyrite surface was positively charged, and BX was mainly existed as HX (Fig. 10), which was adsorbed on the surface diffusion layer of cyaniding chalcopyrite by electrostatic adsorption interactions. While in the pH range of 3.6-4.7, zeta potential of chalcopyrite after cyanide treatment moved from positive to negative, which reveals that X- had already penetrated into the inner of the electric double layer on the surface of chalcopyrite after cyanide treatment, and it was characterized by chemical adsorption. BX was mainly existed as X- above pH 5.0 (Fig. 10), therefore, HX gradually converted to X- above pH 4.7 (Fig. 11). When the pH range was 6.2-8.9, zeta potential of cyaniding chalcopyrite moved negatively, while flotation recovery was depressed, indicating that absorption of X- was reduced; however, the hydrophobic film formed on the surface of cyaniding chalcopyrite, hindering the absorption of CN-, then made flotation recovery increase, thus improving flotability and the flotation performance. And the interaction between BX and cyaniding chalcopyrite surface was chemically bonded adsorption, as the result of electrochemical reaction of BX that occurred in alkaline condition [20]. Negative charge on the surface of cyaniding chalcopyrite was decreased above pH 8.9, while flotation recovery was depressed. It is suggested that absorption of X- was weaker than CN-, the suitable condition of cyaniding chalcopyrite flotation was below pH 8.9.

Fig. 11 Zeta potential and flotation recovery of cyaniding chalcopyrite as function of pH value
The effects of BX on zeta potential and flotation recovery of galena after cyanide treatment versus pH are shown in Fig. 12.
Cyaniding galena surface was negatively charged and never reached the isoelectric point in the whole pH range tested by BX (Fig. 12). The zeta potential of cyaniding galena surfaces became more negative below pH 4.2, while BX mainly existed as X-. When the pH range was 4.2-8.4, the zeta potential of cyaniding galena surface increased after adding BX, while little increase trend existed in the flotation recovery. This illustrated that BX was adsorbed on the surface diffusion layer of galena after cyanide treatment by electrostatic adsorption interactions and formed a hydrophobic film on the surface of cyaniding galena, thus its hydrophobicity and flotability were improved. Considering the zeta potential of cyaniding, galena surface was essentially unchanged above pH 8.4, and the flotation recovery changed significantly above pH 10, indicating that BX would lose activity basically above pH 10, while a hydrophobic membrane was generated by lead xanthate on the surface of galena after cyanide treatment, which cut off internal charge transfer through the electric double layer, and then hydrophobicity was increased. There was no inhibitory effect of CN- on galena in strong alkaline condition.
In summary, there was no electrostatic absorption in the interaction between CN- and chalcopyrite after cyanide treatment within the whole pH range tested; however, electrostatic absorption existed in the interaction between BX and the surface of galena after cyanide treatment in the pH range of 4.2-8.4.

Fig. 12 Zeta potential and flotation recovery of cyaniding galena as function of pH value
4 Conclusions
1) The adsorption characteristics of CN- on the surface of chalcopyrite and galena could be according with the Langmuir adsorption isotherm model, which can be regarded as a single molecular layer of chemical adsorption. The greater the concentration of CN-, the lower the flotability of chalcopyrite. The inhibitory effect of CN- on chalcopyrite was far outweighed galena.
2) BX improved the flotability of chalcopyrite and galena after cyanide treatment. In the optimum conditions of pH 6.5 and 4.0 mg/L BX, the recovery of cyanide chalcopyrite reached 82.1%, and the recovery of cyanide galena was 63.9%. The chalcopyrite in the gold cyanidation tailings can be recycled effectively by BX, and the cyaniding chalcopyrite and galena could be separated flotation at different pH values.
3) Contact angle measurements illustrated that BX increased the contact angle of cyanide chalcopyrite surface to 75.9°, and that of cyanide galena surface was improved to 86.8°. Despite that CN- reduced the hydrophobicity on the surface of chalcopyrite and galena, and BX improved the contact angle of the cyanide mineral surfaces, the chemical adsorption of xanthate generated by BX on the mineral surface was stronger than that of CN-.
4) Zeta potential measurements indicated that electrostatic absorption existed in the interaction between BX and the surface of galena after cyanide treatment in the pH range of 4.2-8.4, while the interaction between BX and the surface of chalcopyrite after cyanide treatment is chemical adsorption. The chemical absorption of X- on the surface of chalcopyrite is weaker than CN- below pH 8.9.
References
[1] GAO Jun-feng, LI Xiao-bo. Utilization of cyanided tailings from gold ore dressing plant [J]. Minerals Engineering, 2005, 3(4): 38-39.
[2] ZHU Lei, KANG Guang-feng, LI Shu-fen, CHU Xian-feng, WU Xiang-yang. Research on multi-element resources of utilizing cyaniding tailings [J]. Environmental Science and Technology, 2010, 23(2): 5-11.
[3] LV Cui-cui, DING Jian, QIAN Peng, LI Qing-chun, YE Shu-feng, CHEN Ye-fa. Comprehensive recovery of metals from cyanidation tailing [J]. Minerals Engineering, 2015, 70: 141-147.
[4] GAO Yuan, WANG Ji-min, WU Hao, LIU Tian-ping. Study on comprehensive utilization of cyanidation slags [J]. Materials Research and Application, 2010, 4(2): 156-160.
[5] PIAO Zheng-jie, WEI De-zhou, LIU Zhi-lin, LIU Wen-gang, GAO Shu-ling, LI Ming-yang. Selective depression of galena and chalcopyrite by O, O-bis (2, 3-dihydroxypropyl) dithiophosphate [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3063-3067.
[6] FU Dan. The study of flotation and surface adsorption mechanism of copper-lead-zinc sulphide ore [D]. Ganzhou:Jiangxi University of Science and Technology, 2007. (in Chinese)
[7] Kocabag D, Guler T. Two-liquid flotation of sulphides: An electrochemical approach [J]. Minerals Engineering, 2007, 20: 1246-1254.
[8] PIAO Zheng-jie, WEI De-zhou, LIU Zhi-lin. Influence of sodium 2, 3-dihydroxypropyl dithiocarbonate on floatability of chalcopyrite and galena [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 3343-3347.
[9] HE Ming-fei, QIN Wen-qing, LI Wei-zhong, ZENG Ke. Pyrite depression in marmatite flotation by sodium glycerine-xanthate [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1161-1165.
[10] Chen Ye, Chen Jian-hua. The first-principle study of the effect of lattice impurity on adsorption of CN on sphalerite surface [J]. Minerals Engineering, 2010, 23(5): 676-684.
[11] Kuznetsov A M, Maslii A N, Shapnik M S. Molecular-continuum model for the cyanide ion adsorption from aqueous solutions on copper metals [J]. Russian Journal of Electrochemistry, 2000, 36(12): 1309-1313.
[12] Polcik M, Kittel M, Hoeft J T, Terborg R, Toomes R L, Woodruff D P. Adsorption geometry of CN on Cu(111) and Cu(111)/O [J]. Surface Science, 2004, 563(6): 159-168.
[13] JIN Jia-qi, Miller J D, Dang L X, Wick C D. Effect of surface oxidation on interfacial water structure at a pyrite (100) surface as studied by molecular dynamics simulation [J]. International Journal of Mineral Processing, 2015, 139: 64-76.
[14] ZHAO Cui-hua, CHEN Jian-hua, WU Bo-zeng, LONG Xian-hao. Density functional theory study on natural hydrophobicity of sulfide surfaces [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 491-498.
[15] WOODS R. Electrochemical potential controlling flotation [J]. International Journal of Mineral Processing, 2003, 72: 151-162.
[16] BUSWELL A M, BRADSHAW D J, HARRIS P J, EKMEKCI Z. The use of electrochemical measurements in the flotation of a platinum group minerals (PGM) bearing ore [J]. Minerals Engineering, 2002, l5 (6): 395-404.
[17] BACHILLER D, TORRE M, RENDUELES M. Cyanide recovery by ion exchange from gold ore waste effluents containing copper [J]. Minerals Engineering, 2004, 17: 767-774.
[18] Yan Xiao-ci, LUO Ming-dao. Interface chemistry [M]. Beijing: Chemical Industry Press, 2005. (in Chinese)
[19] BULATOVIC S M. Handbook of flotation reagents [M]. WEI Ming-an, tras. Beijing: Chemical Industry Press, 2014. (in Chinese)
[20] QIN Wen-qing, YAO Guo-cheng, GU Guo-hua, QIU Guan-zhou, WANG Dian-zuo. Electrochemistry of sulfide minerals and its floatability [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(10): 2669-2677. (in Chinese).
马艺闻1,2,韩跃新1,朱一民1,李艳军1,刘 浩1
1. 东北大学 资源与土木工程学院,沈阳 110819;2. 辽宁科技大学 矿业工程学院,鞍山 114051
摘 要:研究了CN-与黄铜矿和方铅矿之间的吸附作用,然后在丁基黄药(BX)体系下研究了氰化吸附后的黄铜矿、方铅矿的浮选试验。结果表明,CN-与两种矿物表面存在化学吸附作用,并可用Langmuir等温模型近似描述。在pH值为6.5,丁基黄药用量为4.0 mg/L的适宜条件下,氰化后的黄铜矿和方铅矿的浮选回收率可分别达到82.1%和63.9%。尽管CN-降低了黄铜矿、方铅矿表面的接触角,但丁基黄药能够提高氰化矿物表面的疏水性。CN-对黄铜矿的抑制作用大于方铅矿。在pH值为4.2~8.4时,BX与氰化后的方铅矿表面的相互作用存在静电吸附;BX在氰化后的黄铜矿表面的吸附作用为化学吸附。
关键词:黄铜矿;方铅矿;氰化吸附;浮选;丁基黄药
(Edited by Wei-ping CHEN)
Foundation item: Project (2012BAB08B03) supported by the National Key Technologies R&D Program of China
Corresponding author: Yue-xin HAN; Tel: +86-24-83688920; E-mail: dongdafulong@mail.neu.edu.cn
DOI: 10.1016/S1003-6326(16)64457-6

