Removal of phenylalanine from water with calcined CuZnAl-CO3 layered double hydroxides
来源期刊:中国有色金属学报(英文版)2012年第2期
论文作者:焦飞鹏 符招弟 帅丽 陈晓青
文章页码:476 - 482
关键词:CuZnAl-CO3-LDH;苯丙氨酸;吸附;机理
Key words:CuZnAl-CO3-LDH; phenylalanine; adsorption; mechanism
摘 要:
用水热法合成了Cu-Zn-Al-CO3双金属氢氧化物n(Cu)/n(Zn)=5,[n(Cu)+n(Zn)]/n(Al)≈2,简写为LDH),加热煅烧后得到焙烧型Cu-Zn-Al-CO3-LDH(CLDH),再采用批处理实验用其对模拟废水中的苯丙氨酸进行吸附。结果表明, CLDH对于废水中的苯丙氨酸是一种有效的吸附剂,其吸附效率明显高于同等条件下的LDH,当水相pH为6.7时,其吸附效率最高,吸附容量达到37.25 mg/g。该吸附过程符合Lagergren准一级动力学模型,同时其平衡吸附等温线能很好地符合Langmuir等温方程,通过对吸附热力学研究发现, 计算值为负, 计算值为正,这表明此类吸附本质上为吸热自发过程。吸附机理表明苯甲酸分子在层间是倾斜的,与羟基层形成了一个角度。
Abstract:
Cu-Zn-Al-CO3 layered double hydroxide (LDH), with a Cu to Zn mole ratio of 5:1 and a (Cu+Zn) to Al mole ratio of nearly 2, was prepared and its calcined product (CLDH) was obtained. Batch sorption studies were conducted to investigate removal of phenylalanine from water with CLDH. The results show that CLDH can be used as an effective adsorbent and its sorption capacity is higher than that of Mg-Al-CO3-LDH. The maximum adsorption was observed at pH 6.7. A maximum adsorption capacity is 37.25 mg/g. The adsorption processes follow the Lagergren’s first order kinetic model. The adsorption data are fitted well with the Langmuir isotherm equation. The thermodynamic parameters were calculated, and the negative and positive indicate that the adsorption processes are spontaneous endothermic in nature. The mechanism of adsorption also suggests that the benzoate molecules are tilted, forming an angle with the hydroxyl layers.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 476-482
JIAO Fei-peng1, FU Zhao-di2, SHUAI Li1, CHEN Xiao-qing1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Changsha Research Institute of Mining and Metallurgy CO., LTD. Changsha 410012, China
Received 5 January 2011; accepted 1 August 2011
Abstract: Cu-Zn-Al-CO3 layered double hydroxide (LDH), with a Cu to Zn mole ratio of 5:1 and a (Cu+Zn) to Al mole ratio of nearly 2, was prepared and its calcined product (CLDH) was obtained. Batch sorption studies were conducted to investigate removal of phenylalanine from water with CLDH. The results show that CLDH can be used as an effective adsorbent and its sorption capacity is higher than that of Mg-Al-CO3-LDH. The maximum adsorption was observed at pH 6.7. A maximum adsorption capacity is 37.25 mg/g. The adsorption processes follow the Lagergren’s first order kinetic model. The adsorption data are fitted well with the Langmuir isotherm equation. The thermodynamic parameters were calculated, and the negative ![]() and positive
and positive ![]() indicate that the adsorption processes are spontaneous endothermic in nature. The mechanism of adsorption also suggests that the benzoate molecules are tilted, forming an angle with the hydroxyl layers.
indicate that the adsorption processes are spontaneous endothermic in nature. The mechanism of adsorption also suggests that the benzoate molecules are tilted, forming an angle with the hydroxyl layers.
Key words: Cu-Zn-Al-CO3-LDH; phenylalanine; adsorption; mechanism
1 Introduction
Phenylalanine (Phe) is an amino acid that is widely used in many fields, such as food, chemical, pharmaceutical, agricultural, medicine, and cosmetic industries [1]. To improve the efficiency of recovery, separation and purification of amino acids from wastewater coming from fermentations broths, several techniques have been employed [2-5]. In this way, many studies have been reported for adsorption of amino acids on various materials including activated carbon, silica, ion exchangers, alumina, and polymeric resins [6-10]. A class of anionic clays known as layered double hydroxides (LDHs) has been proved to be effective adsorbents for removal of a variety of anionic pollutants. The general formula of LDHs is [MII1-xMIIIx(OH)2](An-)x/n·mH2O, where MII is a divalent metal cation (Mg2+, Zn2+, Cu2+, etc), and MIII is a trivalent metal cation ( Al3+, Cr3+, Fe3+, etc) that occupies octahedral sites in the hydroxide layers, An- is a exchangeable interlayer anion, and x is defined as the ratio of MII to (MII+MIII) on which the layer charge will depend. Calcined layered double hydroxide (CLDH) is capable of recovering the LDH layered structure upon treatment with water or aqueous solution containing various anions via the so called “memory effect”, and has been demonstrated to be good ion exchangers/adsorbents for removal of toxic anions from contaminated water [11,12].
In this study, Cu-Zn-Al-CO3-LDH was synthesized, calcined, and then used for removal of Phe from water. The effects of several conditions on the removal process including the pH value of aqueous solution, the temperature, Phe-adsorbent contact time, and the Phe removal process have been investigated. A comparative experiment was conducted on Mg-Al- CO3-LDH. The equilibrium isotherm and kinetic model for the adsorption processes were studied to evaluate the viability and effectiveness of the process. The adsorption mechanism was also discussed with the supports of XRD, FT-IR results.
2 Experimental
2.1 Preparation of Cu-Zn-Al-CO3-CLDH
A solution containing Na2CO3 (0.09 mol/L) and NaOH (0.36 mol/L) was added to 500 mL distilled water. Another solution containing a mixture of metal salts of Cu(NO3)2·3H2O (0.10 mol/L), Zn(NO3)2·6H2O (0.02 mol/L) and Al(NO3)3·9H2O (0.06 mol/L) (Cu to Zn mole ratio of 5:1 and a Cu+Zn to Al mole ratio of nearly 2.0) was prepared, and it was added dropwise to the alkaline solution with continuous mechanical stirring. The pH was adjusted to 10 by the simultaneous addition of the mixture of Na2CO3 and NaOH. Once addition was completed, the slurry was mechanically stirred at room temperature for 1 h. The material obtained was submitted to hydrothermal reaction vessel treatment at 80 °C for 6 h. The slurry was centrifuged and washed several times with warm deionized water until neutral pH was reached [13]. CLDH was obtained by calcining LDH at 500 °C for 12 h.
2.2 Instrumentation
The structural characterizations were performed by X-ray diffraction (XRD) and IR spectroscopy. Powder XRD measurements were performed on a Rigaku Rint 6000 powder X-ray diffractometer, using Cu Kα radiation at 30 mA, 40 kV. The IR spectra were obtained on a NICOLET AVATAR 360 spectrophotometer by the standard KBr disk method. A temperature-controlled shaker bath with a temperature variation of ±0.5 °C was used for the equilibrium studies.
2.3 Reagents
Phe was used without further purification. Ninhydrin solution was obtained by dissolving 5.0 g Na2HPO4.12H2O, 3.0 g KH2PO4, 0.25 g ninhydrin and 0.15 g fructose in 50 mL volumetric flask (pH= 6.7). The stabilizer potassium iodinate was obtained by dissolving 1.0 g KIO3 in mixture of 300 mL distilled water and 200 mL 95% ethanol.
2.4 Batch adsorption experiments
The adsorption experiments were carried out in 100 mL stoppered conical flasks by mixing a 50.0 mL Phe solution with appropriate concentration and 0.10 g CLDH and shaking immediately in a constant temperature water bath. The mixtures were filtered at given time intervals. The experiments were carried out at different temperatures and repeated two times. The initial pH values of Phe working solution were adjusted by addition of HCl or NaOH solution.
2.5 Detection method
The concentration of Phe was detected by ninhydrin colorimetry on a UV-Vis spectrophotometer (UV-9600) by measuring absorbance at 569 nm. The supernatants were adjusted to 6.7 by addition of HCl or NaOH solution before detection. 2.0 mL Phe solution and 0.5 mL ninhydrin solution were mixed in a 25 mL color comparison tube. After heating for 16 min in boiling water and cooling for 15 min, 5.0 mL potassium iodinate was added to the mixture. The mixture was detected within 30 min.
The removal efficiency was measured by the removal rate (r) of Phe:
![]() (1)
(1)
where ρ0 and ρe are the initial concentration and equilibrium concentration of the Phe in solution (mg/L), respectively.
3 Results and discussion
3.1 Characterization of materials
The LDH and CLDH after adsorbing Phe (PLDH) were characterized by XRD. Figure 1(a) shows the diffraction pattern of LDH. The XRD patterns present sharp, symmetric, strong lines at low 2θ. It is characteristic of well-crystallized product with layered structure of LDH at d003 (0.762 nm) and d006 (0.379 nm). Figure 1(b) shows the pattern of CLDH. The layered structure collapses due to the loss of interlayer water molecules, carbonate anions as carbon dioxide and surface hydroxyl groups. The characteristic reflections of a mixed oxides CuAl(O), CuO and ZnO appear at 2θ values of 31.6° and 36.8°, 35.4°, 38.7° and 32.4°, respectively. In Fig. 1(c) the diffraction pattern of PLDH is present. The reappearance of characteristic layered structure of LDHs indicates that the adsorption of Phe is by the ‘memory effect’ of CLDH. A new diffraction pattern appears at 5.35°. The increase in peak intensity and the reduction in peak width after intercalation of Phe indicate an increase in crystallization.
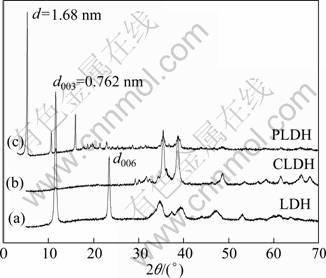
Fig. 1 XRD patterns of LDH (a), CLDH (b) and PLDH (c)
The IR spectrum of LDH (Fig. 2(a)) resembles those of other hydrotalcite-like phases [13]. Typical spectra are the strong broad absorbance bands between 3600 and 3200 cm-1 associated with the stretching mode of hydrogen-bonded hydroxyl groups from both the hydroxide layers and interlayer water. The strong asymmetric stretching mode of the carbonate ions is found at 1391 cm-1. The IR spectrum of Phe is shown in Fig. 2(c). A series of bands are recorded at 1074, 700, 1562 and 1625 cm-1 ascribed to vibration peaks of phenyl ring groups. The characteristic absorption peaks of carbonyl group are recorded at 1409, 1456 cm-1, and the bands due to NH3+ are recorded at 849, 3066 cm-1. The IR spectra of Phe (Fig. 2(b)) were preserved in PLDH, but the intensity decreases. The band due to NH4+ appears at 849 cm-1. The intensity of vibration peaks of aromatic ring recorded at 1076, 698, 1560 and 1619 cm-1 and carbonyl group recorded at 1456, 1409, 949 cm-1 decreased or disappeared in PLDH. It is shown that the Phe was adsorbed into interlayer and there is an interaction between Phe and LDH layers.
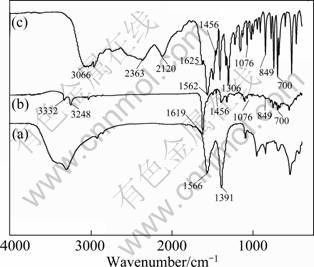
Fig. 2 IR spectra of LDH (a), PLDH(b) and Phe (c)
3.2 Effect of pH
Generally, pH is considered to be an important factor to control the adsorption at water–adsorbent interfaces [14]. So, the adsorption of Phe (0.1 g/L) on CLDH (0.1 g) was studied at different pH values ranging from 5 to 9.
The influence of pH on the Phe sorption is presented in Fig. 3. Near isoeletric point (pH=5.48), the decrease of the adsorption can be attributed to the zero net charge of Phe. On the other hand, for pH>7, carbonate contamination is likely to occur and may hinder the Phe adsorption due to the great affinity of LDH for carbonate anions [15]. The CLDH recorded the maximum adsorption at pH 6.7. Hence, the adsorption experiments were carried out at pH 6.7.
![]()
![]()
![]() (2)
(2)
where Ph represents phenyl ring.
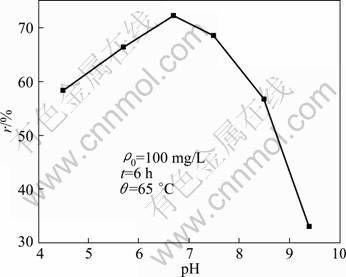
Fig. 3 Effect of pH on Phe retention by CLDH
3.3 Temperature effect
The effect was studied on suspensions of CLDH in Phe solutions (100 mg/L Phe concentration; 1.0 g/L solid/solution ratio) stirred at three constant temperatures (313, 338 and 353 K), respectively. Suspensions were centrifuged; the supernatants were adjusted to pH 6.7 and the Phe concentrations were determined as above.
As the adsorption process is strongly dependent upon temperature [16], the study of the temperature effect on Phe removal with CLDH enabled us to determine the thermodynamic parameters (![]() ,
, ![]() and
and ![]() ) of these reactions by using the following equations:
) of these reactions by using the following equations:
![]() (3)
(3)
![]() (4)
(4)
where R is the gas constant; T is the temperature, K; Kd is the distribution coefficient (amount of removed Phe per gram of material divided by its concentration in the liquid phase). The plot of lnKd against T-1 gives a straight line, and the slope and the intercept correspond to ![]() and
and ![]() , respectively.
, respectively.
The plot of lnKd vs T-1 for Phe removal with CLDH (Fig. 4) shows a negative slope. The thermodynamic parameters calculated from the values of the slopes and the intercepts are listed in Table 1. The negative value of ![]() and positive value of
and positive value of ![]() indicate that the adsorption processes are spontaneous and endothermic in nature.
indicate that the adsorption processes are spontaneous and endothermic in nature. ![]() can be used to describe the randomness at the solid–solution interface during the removal process. The positive value of
can be used to describe the randomness at the solid–solution interface during the removal process. The positive value of ![]() reflects the affinity of CLDH to the Phe ion in aqueous medium. The process may be masked by the increased intraparticle diffusion rate of Phe into the pores of LDH at higher temperatures that give rise to the observed increase in their adsorption.
reflects the affinity of CLDH to the Phe ion in aqueous medium. The process may be masked by the increased intraparticle diffusion rate of Phe into the pores of LDH at higher temperatures that give rise to the observed increase in their adsorption.
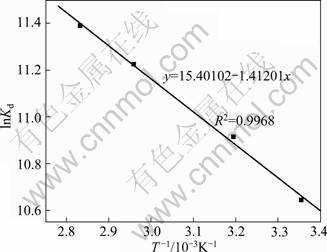
Fig. 4 Plot of lnKd vs T-1 for Phe removal with CLDH
Table 1 Thermodynamic parameters for Phe removal with CLDH

3.4 Effect of contact time
The amount of Phe adsorbed by CLDH as a function of contact time, using a constant adsorbent mass of 100 mg and initial concentration of 100 mg/L, is shown in Fig. 5. The kinetic curve of the Phe adsorption on the CLDH is achieved at 50 °C. It can be seen from Fig. 5 that the adsorption increases initially and then reaches the equilibrium state after a contact time of 10 h in the case of no change in the adsorbed amount. The similar phenomenon was observed by EI GAINI et al [14]. A comparative experiment was conducted on Mg-Al-CO3-LDH. The result showed that the Cu-Zn-Al-CO3-CLDH is a better adsorbent for removal of Phe than Mg-Al-CO3-LDH. From these experiments, we also find that Cu-Zn-Al-CO3 CLDH is a better choice for adsorption.
3.5 Adsorption kinetic
The sorption kinetic is an important aspect of processes for pollutants removal [17-21]. In order to investigate the adsorption mechanism of Phe on CLDH, the Lagergren’s first order kinetic model and the Ho’s pseudo-second-order model were applied to describing the experimental process.

Fig. 5 Curves of Phe removal with CLDH
The Lagergren’s first order kinetic model [21] is generally expressed in integrated form as follows:
![]() (5)
(5)
where k1 is the rate constant of adsorption, h-1; Qe and Qt (mg/g) are the adsorption capacities of the adsorbate at equilibrium and at time t (h), respectively. By plotting lg(Qe-Qt) against t, a linear graph can be obtained and the value of k1 and Qe can be calculated. The result is plotted in Fig. 6 and the calculated parameters are k1= 0.6138 h-1, Qe=36.37 mg/g.
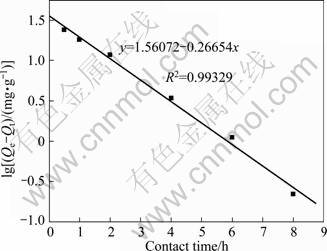
Fig. 6 First order plot for Phe removal with CLDH
Due to its good correlation with the experimental results, the more recent pseudo-second-order model has been extensively used by several researchers in the same field [21]. This model is expressed as
![]() (6)
(6)
where k2 is the rate constant of pseudo-second-order adsorption, g/(mg·h). By plotting t/Qt against t, a linear function can be obtained and the rate constant k2 as well as Qe also can be calculated. The result is plotted in Fig. 7 and the calculated parameters are k2=0.01916 g/(mg·h), Qe=43.01 mg/g, respectively.
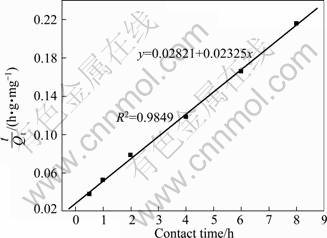
Fig. 7 Pseudo second order plot for Phe removal with CLDH
Good correlation is observed between experimental data and the Lagergren’s first order kinetic model with a determination coefficient value higher than 0.99. This indicates that the process of Phe removal is controlled by diffusion rather than by the rate of reaction of Phe with CLDH.
3.6 Adsorption isotherms
The equilibrium adsorption experimental data obtained in this study were analyzed using the commonly used Freundlich and Langmuir isotherm models. The Freundlich isotherm model is described by the following equation:
![]() (7)
(7)
where Qe is the equilibrium sorption concentration of solute per gram of adsorbent; mg/g; ρe is the equilibrium aqueous concentration of the solute, mg/L; KF and n are Freundlich constants which are related to adsorption capacity and intensity of adsorption.
The result is plotted in Fig. 8 and the calculated parameters are KF=12.74, n=2.90, R2=0.95828.
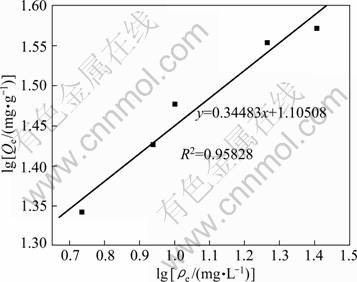
Fig. 8 Freundlich isotherm for Phe removal with CLDH
The Langmuir isotherms model is described by the following equation:
![]() (8)
(8)
where Qe is the amount of Phe removed per gram of adsorbent, mg/g; Qmax is the maximum sorption capacity, mg/g; ρe is the Phe concentration in the equilibrium solution, mg/L; KL is the Langmuir constant related to the adsorption energy, L/mg.
The result is plotted in Fig. 9 and the calculated parameters are Qmax=46.4 mg/g, KL=0.168 L/mg, R2= 0.99722.
The experimental result is well described by both models, but the Langmuir model remains better. It may be due to the homogenous distribution of active sites on the CLDH surface.
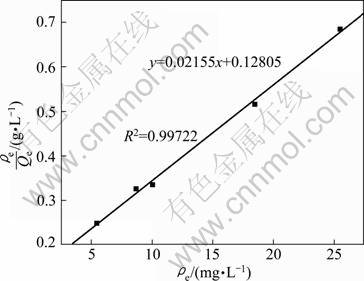
Fig. 9 Langmuir isotherm for Phe removal with CLDH
3.7 Mechanism of adsorption of Phe
Based on the results obtained by IR spectra and XRD, we can deduce that the adsorption of Phe is carried out by reconstruction of a matrix hydrotalcite intercalated by the Phe bounding in the interlayer spaces through electrostatic interactions and hydrogen bonding. The process takes place by reconstruction of oxide, with interrelation of hydroxyl anions, which are subsequently replaced by Phe via an ion-exchange process. The thickness of the LDHs layer is 0.48 nm, and the gallery height of [CuZnAl-CO3] after retention is 1.20 nm. The value of long axis of Phe anion (0.745 nm), calculated from the semi-empirical molecular orbital method of Gaussian 03 software, is well consisted with the gallery height of [CuZnAl-CO3] after the uptake of Phe anion, as shown in Fig. 10. NEXAFS studies by MOGGRIDGE et al [22] indicated a bilayer orientation, but the benzoate molecules are tilted, forming an angle of (35 ± 10)° with the hydroxyl layers.
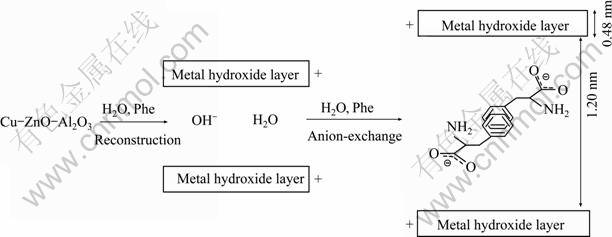
Fig. 10 Schematic illustration of Phe intercalation into layer
4 Conclusions
1) It was confirmed that CLDH might be a potential material for the removal of Phe from aqueous solution. The sorption was found to be a pH dependent and the maximum adsorption was observed at pH 6.7. A maximum adsorption capacity is 37.25 mg/g.
2) The kinetic and sorption data fit well the Lagergren’s first order kinetic model and the Langmuir model respectively with good values of the determination coefficient. The negative value of ![]() and the positive value of
and the positive value of ![]() indicate that the adsorption processes are spontaneous endothermic in nature.
indicate that the adsorption processes are spontaneous endothermic in nature.
3) The mechanism of adsorption also suggests that the benzoate molecules are tilted, forming an angle with the hydroxyl layers.
References
[1] SILV?RIO F, DOSREIS M J, TRONTON J, VLIM J B. Adsorption of phenylalanine on layered double hydroxides: Effect of temperature and ionic strength [J]. Journal Materials Science, 2008, 43: 434-439.
[2] HONG S U, BRUENING M L. Separation of amino acid mixtures using multilayer polyelectrolyte nanofiltration membranes [J]. Journal Membrane Science, 2006, 280: 1-5.
[3] HAN M H, YUN Y S. Mechanistic understanding and performance enhancement of biosorption of reactive dyestuffs by the waste biomass generated from amino acid fermentation process [J]. Biochemical Engineering Journal, 2007, 36: 2-7.
[4] CHAO Y M, LIANG T M. A feasibility study of industrial wastewater recovery using electrodialysis reversal [J]. Desalination, 2008, 221: 433-439.
[5] OSHIMA T, SAISHO R, OHE K, BABA Y, OHTO K. Adsorption of amino acid derivatives on calixarene carboxylic acid impregnated resins [J]. Reacive & Functional Polymers, 2009, 69: 105-110.
[6] NAMASIVAYAM C, DINESH KUMAR M, SELVI K, ASHRUFFUNISSA BEGUM R, VANATHI T, YAMUNA R T. Waste' coirpith—A potential biomass for the treatment of dyeing wastewaters [J]. Biomass Bioenergy 2001, 21: 477-483.
[7] ORTHMAN J, ZHU H Y, LU G Q. Use of anion clay hydrotalcite to remove coloured organics from aqueous solutions [J]. Separation Science Technology, 2003, 31: 53-59.
[8] POLLARD S J T, FOWLER G D, SOLLARS C J, PERRY R. Low-cost adsorbents for waste and wastewater treatment: A review [J]. Science Total Environment, 1992, 116: 31-52.
[9] QI J, ZHI L, GUO Y, XU H. Adsorption of phenolic compounds on micro- and mesoporous rice husk-based active carbons [J]. Materials Chemistry Physics, 2004, 87: 96-101.
[10] TIZAOUI C, SLATER M J. The design of an industrial waste-water treatment process using adsorbed ozone on silica gel [J]. Process Safety Environmental Protection, 2003, 81: 107-113.
[11] GOH K H, LIM T T, DONG Z L. Application of layered double hydroxides for removal of oxyanions: A review [J]. Water Research, 2008, 42: 1343-1368.
[12] LI F, DUAN X. Applications of layered double hydroxides [J]. Structure and Bonding, 2006, 119: 193-223.
[13] BUSCA G, COSTANTINO U, MARMOTTINI F, MONTANARI T, PATRONO P, PINZARI F, RAMIS G. Methanol steam reforming over ex-hydrotalcite Cu-Zn-Al catalysts [J]. Applied Catalysis A: General, 2006, 310: 70-78.
[14] EI GAINI L, LAKRAMI M, SEBBAR E, MEGHEA A, BAKASSE M. Removal of indigo carmine dye from water to Mg-Al-CO3-calcined layered double hydroxides [J]. Journal Hazardous Materials, 2009, 161(2-3): 627-632.
[15] INACIO J, TAVIOT-GUEHO C, FORANO C, BESSE J P. Adsorption of MCPA pesticide by MgAl-layered double hydroxides [J]. Applied Clay Science, 2001, 18: 255-264.
[16] DAS J, SAIRAM PAREA B, BALIARSINGH N, PARIDA K M. Calcined Mg-Fe-CO3-LDH as an adsorbent for the removal of selenite [J]. Journal of Colloid and Interface Science, 2007, 316: 216-223.
[17] BOURAADA M, LAFJAH M, OUALI M S, de MENORVAL L C. Basic dye removal from aqueous solutions by dodecylsulfate-and dodecyl benzene sulfonate-intercalated hydrotalcite [J]. Journal Hazardous Materials, 2008, 153: 911-918.
[18] MCKAY G. The adsorption of basic dye onto silica from aqueous solution-solid diffusion model [J]. Chemical Engineering Science, 1984, 39(1): 129-138.
[19] HO Y S, MCKAY G. The kinetics of sorption of divalent metal ions onto sphgnum moss peat [J]. Water Research, 2000, 34: 735-742.
[20] LAZARIDIS N K, ASOUHIDOU D D. Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg-Al-CO3 hydrotalcite [J]. Water Research, 2003, 37: 2875-2882.
[21] HO Y S, MCKAY G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34: 451-465.
[22] MOGGRIDGE G D, PARENT P, TOURILLON G. A NEXAFS study of the orientation of benzoate intercalated into a layer double hydroxide [J]. Clays Clay Minerals, 1994,42: 462-472.
焦飞鹏1, 符招弟2, 帅 丽1, 陈晓青1
1. 中南大学 化学化工学院,长沙 410083;
2. 长沙矿冶研究院有限责任公司,长沙 410012
摘 要:用水热法合成了Cu-Zn-Al-CO3双金属氢氧化物n(Cu)/n(Zn)=5,[n(Cu)+n(Zn)]/n(Al)≈2,简写为LDH),加热煅烧后得到焙烧型Cu-Zn-Al-CO3-LDH(CLDH),再采用批处理实验用其对模拟废水中的苯丙氨酸进行吸附。结果表明, CLDH对于废水中的苯丙氨酸是一种有效的吸附剂,其吸附效率明显高于同等条件下的LDH,当水相pH为6.7时,其吸附效率最高,吸附容量达到37.25 mg/g。该吸附过程符合Lagergren准一级动力学模型,同时其平衡吸附等温线能很好地符合Langmuir等温方程,通过对吸附热力学研究发现,![]() 计算值为负,
计算值为负,![]() 计算值为正,这表明此类吸附本质上为吸热自发过程。吸附机理表明苯甲酸分子在层间是倾斜的,与羟基层形成了一个角度。
计算值为正,这表明此类吸附本质上为吸热自发过程。吸附机理表明苯甲酸分子在层间是倾斜的,与羟基层形成了一个角度。
关键词:Cu-Zn-Al-CO3-LDH;苯丙氨酸;吸附;机理
(Edited by LI Xiang-qun)
Foundation item: Project (21176263) supported by the National Natural Science Foundation of China; Project (2009RS3039) supported by Hunan Provincial Postdoctoral Special Foundation of China; Project (09JJ3026) supported by Hunan Provincial Natural Science Foundation of China
Corresponding author: CHEN Xiao-qing; Tel: +86-731-88830833; Fax: +86-731-88830833; E-mail: chenxqcsu@163.com
DOI: 10.1016/S1003-6326(11)61201-6

