
Hydriding and microstructure nanocrystallization of ZK60 Mg alloy by reaction milling in hydrogen
YUAN Yuan(袁 媛), WANG Heng(王 珩), HU Lian-xi(胡连喜), SUN Hong-fei(孙宏飞), FANG Wen-bin(房文斌)
School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China
Received 10 June 2009; accepted 15 August 2009
Abstract: The hydriding of as-cast Mg-5.5%Zn-0.6%Zr (ZK60 Mg) (mass fraction) alloy was achieved by room-temperature reaction milling in hydrogen, with the mechanical energy serving as the driving force for the process. The hydriding progress during milling was examined by hydrogen absorption measurement, and the microstructure change was characterized by X-ray diffraction analysis (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM), respectively. The results show that, by room-temperature reaction milling in hydrogen, the as-cast ZK60 Mg alloy can be fully hydrided to form a nanocrystalline MgH2 single-phase microstructure. In particular, the average grain size of the MgH2 phase obtained by room-temperature reaction milling in hydrogen for 16.2 h is about 8-10 nm, and the average particle size of the as-milled hydrided powders is 2-3 μm.
Key words: Mg alloy; reaction milling; hydriding; microstructure nanocrystallization
1 Introduction
Mg alloys are gaining increasing importance for structural applications including aerospace, automotive, materials handling, and portable electronic appliances [1-3]. However, conventional Mg alloys exhibit relatively poor mechanical strength. In order to exploit the potential of Mg alloys for high-performance structural applications, improvement of their mechanical strength is urgently demanded.
Grain refining is a general way to improve the mechanical strength of metallic materials. In most cases, the yield stress, σy, can be related to the grain size, d, by the Hall-Petch expression, σy=σ0+ky d-1/2, where σ0 and ky are positive constants for a specific material. For Mg alloys, the strengthening due to grain refining can be very tempting because of their high ky values[4-6]. For example, when the grain size is reduced to 100-200 nm, the Mg97Zn1Y2 (molar fraction, %) alloy presents a yield strength as high as 600 MPa[7-9].
Powder metallurgy (PM) is a quite potential way to prepare nanocrystalline or ultrafine grained bulk alloys. By this route, nanocrystalline alloy powders have to be used. For most Mg alloys, however, it is difficult to produce nanocrystalline alloy powders by rapid solidification or traditional mechanical milling due to their thermal/physical properties and limitations in thermodynamic aspects. Therefore, the search for ways of producing nanocrystalline Mg alloy powders is of great significance.
The process of hydrogenation, disproportionation, desorption, and recombination (HDDR) is very effective in grain refining. By this technique, Nd-Fe-B alloy powders with submicron crystallite grains can be produced directly from as-cast materials[10-11]. In particular, the incorporation of HDDR with mechanical milling gives rise to a new way to produce nanocrystalline Nd-Fe-B powders[13-14]. The key step of the latter new process is to produce nano-structured hydrided alloy powders by mechanically activated hydrogenation and disproportion via reaction milling in hydrogen[15], so that a final nanocrystalline microstructure is developed in the alloy after the subsequent desorption and recombination treatment. Since Mg can absorb hydrogen to form MgH2 and the reaction is reversible, this technique is believed to be also applicable to Mg alloys. In the present work, thehydriding and microstructure nanocrystallization of a Mg-5.5%Zn-0.6%Zr (ZK60 Mg) (mass fraction) alloy by reaction milling in hydrogen were investigated.
2 Experimental
The commercially available ZK60 Mg alloy was used as the starting material. Before it was used for reaction milling in hydrogen, the alloy was crushed into coarse powders by using a mechanical hammer. The hydrogenation of the alloy powders was performed by room-temperature reaction ball milling in hydrogen. The ball-mill used was a planetary type QM-DY4. During milling, the hydrogen pressure in the vial was kept above 0.5 MPa. The ball-to-powder mass ratio was 60?1, and the mill shaft rotation speed was 400 r/min.
The charging and discharging of the vials were performed in a glove box filled with pure argon. Before milling, the charged vial was evacuated and then filled with high-purity hydrogen until a pressure of about 0.6 MPa was achieved. This procedure was repeated for subsequent operations either to take sample powders or to re-fill hydrogen. During milling, the hydrogen pressure in the vial was in-situ monitored by a pressure gauge, and the hydrogen absorbed by the alloy was calculated referring to the vial pressure change.
The progress in hydrogenation and the corresponding phase transition of the alloy were studied by XRD characterization of the as-milled sample powders obtained at different milling stages, and the change of phase crystallite size was estimated by calculations based on the XRD results. The morphology of the as-milled alloy powders was observed by SEM. The microstructure and grain size of the final fully-hydrogenated alloy powders was observed by TEM.
3 Results and discussion
3.1 Hydrogenation kinetics
Fig.1 shows the variation of the hydrogen content in the alloy with the milling time. It is seen that the hydrogenation of the alloy during milling can be divided into three stages, as shown byⅠ, Ⅱ, and Ⅲ, respectively. In stageⅠ, the alloy absorbed hydrogen very slowly. It was believed that stageⅠ was the incubation stage for the hydriding reaction of Mg to MgH2, during which surface and lattice defects were gradually developed in the powders with increasing milling time, and Mg(H) solid solution (α phase) formed as a result of H adsorption on the powder surface and further diffusion into the Mg lattice. In stage Ⅱ, the hydrogen absorption
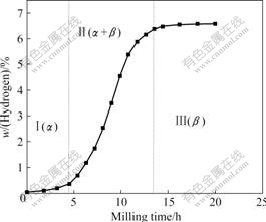
Fig.1 Variation of hydrogen content in alloy with milling time
kinetics was apparently accelerated, attributing to the initiation of the Mg hydriding reaction, that is, the transition of Mg(H) solid solution (α phase) to MgH2 (β phase), with the mechanical energy transferred by the ball collision serving as the activation energy. Therefore, in this stage, the hydrogen absorption kinetics at first followed a self-accelerating course up to the maximum absorbing rate corresponding to the slope of the quasi-rectilinear part of the curves, and then decelerated gradually, due to the fact that the fraction of the α phase remained became less and less with further increasing of the milling time, until the almost completion of the transition of α phase to β phase. In stage Ⅲ, since the hydriding reaction was almost completed and the fraction of the remained α phase was very low, the hydrogen absorption rate was correspondingly slow. Indeed, upon milling up to 16.2 h, the hydrogen content in the alloy was stabilized, suggesting that no more hydrogen could be absorbed during further milling. The hydrogen content upon saturation was found to be about 6.55% (mass fraction), which is a little lower than the theoretical hydrogen absorption capacity of the alloy (7.11%), calculated on the assumption that all the Mg in the alloy is transformed into the MgH2 phase. This can be attributed to the fact that actually the molar ratio of Mg to H at such local sites as grain boundaries and crystal defects is much lower than 1?2, the theoretical value for the MgH2 phase.
3.2 XRD analysis
Fig.2 shows the XRD patterns of the alloy powders milled in hydrogen for various times. The alloy milled for 3.2 h was well fitted with the Mg diffraction peaks. This suggested that, in the initial milling stage, hydrogen was absorbed by forming Mg(H) solid solution. By further milling in hydrogen for 7.2 h, MgH2 phase was
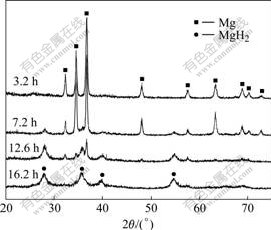
Fig.2 XRD patterns of ZK60 Mg alloy powders milled in hydrogen for various times
observed and the diffraction intensity of the Mg phase decreased correspondingly, suggesting that a portion of the Mg phase was hydrided to MgH2 by the mechanically driven solid-gas reaction Mg+H2→MgH2. With the
increase of the milling time, the fraction of MgH2 phase increased while that of Mg phase decreased correspondingly. Indeed, after milling for 16.2 h, the diffraction peaks of the Mg phase disappeared, and Mg in the alloy was fully hydrided to MgH2 phase. Therefore, XRD results confirmed that a full hydriding of Mg to MgH2 was achieved by 16.2 h of milling. Calculation based on the XRD patterns revealed that the average crystallite size of the MgH2 phase obtained by milling for 16.2 h was about 8 nm.
3.3 SEM and TEM observation
Fig.3 shows SEM micrographs of the alloy powders milled in hydrogen for various times. Before the MgH2 phase was formed, the effect of milling on particle size refining was not significant. Therefore, the powders after milling for 3.2 h were still rather coarse, with an average particle size of about 100 μm (Fig.3(a)). With the increase of the milling time, MgH2 phase was formed in the surface layer of Mg particles. Since MgH2 is intrinsically brittle, it is very easy to break down during
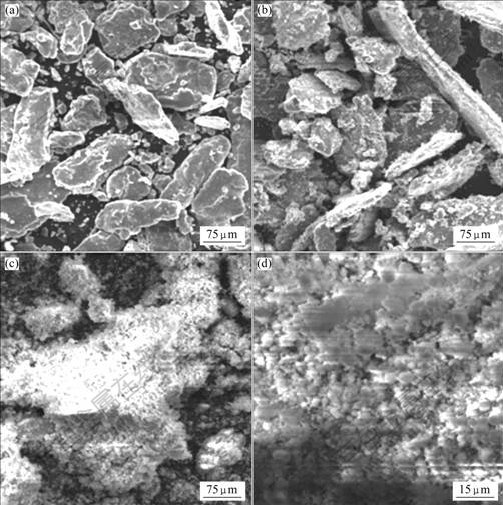
Fig.3 SEM micrographs of ZK60 Mg alloy powders milled in hydrogen for various times: (a) 3.2 h; (b) 7.2 h; (c) and (d) 16.2 h
milling. Therefore, very fine MgH2 particles were observed on the surfaces of the coarse original Mg particles (Fig.3(b)). That is to say, the hydriding of Mg to MgH2 helps to produce very fine powders. Upon milling in hydrogen for 16.2 h when the Mg phase was fully hydrided to MgH2, the average particle size was reduced to 2-3 μm, as shown in Figs.3(c) and (d), which is much finer than the average particle size of 15-20 μm of the AZ31 Mg alloy powders obtained by conventional mechanical milling in argon for 100 h[16]. This can be attributed to the intrinsic brittleness of the MgH2 phase formed during reaction milling.
Fig.4 shows a representative TEM image and the corresponding electron diffraction patterns of the fully hydrided alloy powders by reaction milling in hydrogen for 16.2 h. The TEM observation confirmed that the as-hydrided alloy powders were typically nano-structured, with the grain size being estimated to be 8-10 nm in average (Fig.4(a)). Compared with the average grain size of 85 nm of the AZ31 Mg alloy powders mechanically milled in argon for 100 h[16], the grain size of the as-hydrided ZK60 Mg alloy powders obtained in the present study is almost 10 times finer, suggesting that the mechanically driven hydriding reaction plays a key role in microstructure nanocrystallization. By electron diffraction analysis, MgH2 was identified to be the exclusive phase of the fully hydrided alloy (Fig.4(b)). Obviously, the TEM study was in agreement with the XRD results.
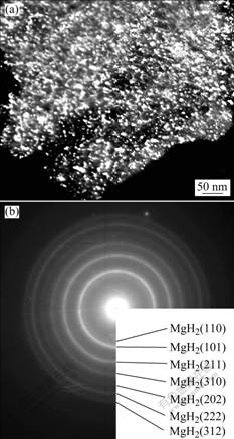
Fig.4 TEM image (a) and corresponding electron diffraction patterns (b) of fully hydrided ZK60 Mg alloy by reaction milling in hydrogen for 16.2 h
4 Conclusions
1) By room-temperature reaction milling in hydrogen, the as-cast ZK60 Mg alloy can be hydrided to MgH2 phase, with the hydrogen content absorbed upon full hydriding, i.e., hydrogen saturation, achieving about 6.55% (mass fraction).
2) The hydrogenation of the ZK60 Mg alloy during reaction milling shows a three-stage hydrogen absorption kinetics, which can be interpreted as the development of Mg(H) solid solution, the fast transformation of Mg(H) to MgH2, and the final hydriding to hydrogen saturation with the formation of the full MgH2 microstructure, respectively.
3) The as-hydrided ZK60 Mg alloy powders prepared via reaction milling in hydrogen are typically nano-structured, with a nanocrystalline MgH2 single-phase microstructure. In particular, the average grain size of the MgH2 phase obtained by reaction milling in hydrogen for 16.2 h is 8-10 nm, and the average particle size of the as-hydrided powders is 2-3 μm.
References
[1] HU Lian-xi, WU Yang, YUAN Yuan, WANG Hong. Microstructure nanocrystallization of a Mg-3wt.%Al-1wt.%Zn alloy by mechanically assisted hydriding-dehydriding [J]. Materials Letters, 2008, 62(17/18): 2984-2987.
[2] YU Kun, RUI Shou-tai, WANG Xiao-yan, WANG Ri-chu, LI Wen-xian. Texture evolution of extruded AZ31 magnesium alloy sheets [J]. Trans Nonferrous Met Soc China, 2009, 19(3): 511-516.
[3] VALLE J A, PE?ALBA F, RUANO O A. Optimization of the microstructure for improving superplastic forming in magnesium alloys [J]. Mater Sci Eng A, 2007, 467(1/2): 165-171.
[4] GAN W M, ZHENG M Y, CHANG H, WANG X J, QIAO X G, WU K, SCHWEBKE B, BROKMEIER H G. Microstructure and tensile property of the ECAPed pure magnesium [J]. Journal of Alloys and Compounds, 2009, 470(1/2): 256-262.
[5] CHEN Zhen-hua, YAN Hong-ge, CHEN Ji-hua, QUAN Ya-jie, WANG Hui-min, CHEN Ding. Magnesium alloy [M]. Beijing: Chemical Industry Press, 2004. (in Chinese)
[6] CHANG T C, WANG J Y, O C M, LEE S. Grain refining of magnesium alloy AZ31 by rolling [J]. Journal of Materials Processing Technology, 2003, 140(1/3): 588-591.
[7] INOUE A, KAWAMURA Y, MATSUSHITA M, HAYASHI K, KOIKE J. Novel hexagonal structure and ultrahigh strength of magnesium solid solution in the Mg-Zn-Y system [J]. Journal of Materials Research, 2001, 16(7): 1894-1900.
[8] ABE E, KAWAMURA Y, HAYASHI K, INOUE A. Long-period ordered structure in a high-strength nanocrystalline Mg-1 at% Zn-2 at% Y alloy studied by atomic-resolution Z-contrast STEM [J]. Acta Materialia, 2002, 50(15): 3845-3857.
[9] NISHIDA M, KAWAMURA Y, YAMAMURO T. Formation process of unique microstructure in rapidly solidified Mg97Zn1Y2 alloy [J]. Mater Sci Eng A, 2004, 375/377: 1217-1223.
[10] HARRIS I R, MCGUINESS P J. Hydrogen: Its use in the processing of NdFeB-type magnets [J]. Journal of the Less Common Metals, 1991, 172/174(3): 1273-1284.
[11] RAGG O M, KEEGAN G, NAGEL H, HARRIS I R. The HD and HDDR processes in the production of Nd-Fe-B permanent magnets [J]. International Journal of Hydrogen Energy, 1997, 22(2/3): 333-342.
[12] SHI Gang, HU Lian-xi, GUO Bin, SUN Xiu-dong, WANG Er-de. Investigation of hard magnetic behaviors for Nd-Fe-B based magnets prepared by hydrogen decrepitation technique [J]. Materials Science Forum, 2005, 475/479: 2185-2188.
[13] SHI Gang, HU Lian-xi, WANG Er-de. Preparation, microstructure, and magnetic properties of a nanocrystalline Nd12Fe82B6 alloy by HDDR combined with mechanical milling [J]. Journal of Magnetism and Magnetic Materials , 2006, 301(2): 319-324.
[14] HU Lian-xi, WANG Er-de, GUO Bin, SHI Gang. Microstructure and magnetic properties of Nd2Fe14B/α-Fe nanocomposite prepared by HDDR combined with mechanical milling [J]. Materials Science Forum, 2007, 534/536: 1349-1352.
[15] HU Lian-xi, SHI Gang, WANG Er-de. Mechanically activated disproportionation of NdFeB alloy by ball milling in hydrogen [J]. Trans Nonferrous Met Soc China, 2003, 13(5): 1070-1074.
[16] WANG Heng, HU Lian-xi, CHEN Xian-jue, WANG Er-de. Preparation of nanocrystalline magnesium alloy powders by high-energy ball milling [J]. Powder Metallurgy Technology, 2008, 26(6): 403-406. (in Chinese)
(Edited by LI Xiang-qun)
Foundation item: Project(50574034) supported by the National Natural Science Foundation of China; Project(20060213016) supported by Doctoral Education Fund of Ministry of Education of China.
Corresponding author: HU Lian-xi; Tel: +86-451-86418613; E-mail: hulx@hit.edu.cn