Article ID: 1003-6326(2005)02-0371-04
Infiltration kinetics of pressureless infiltration in SiCp/Al composites
QIN Zhen-kai(秦振凯), YU Jia-kang(于家康), ZHANG Xiao-yu(张小宇)
( State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi′an 710072, China)
Abstract: The pressureless infiltration kinetics was investigated by plotting the infiltration distance as function of the infiltration time. The effects of key process parameters such as time, temperature, Mg content on the pressureless infiltration of silicon carbide particle compacts were studied and quantified. The preform with high volume fraction SiC was obtained by mixing SiC particles with bimodal size distribution, whose diameters are 5 and 50μm, respectively. The results show that an incubation period exists before infiltration, the influence of temperature on the incubation time exceeds that of Mg content, infiltration rate increases with the increasing temperature and Mg content, infiltration rate decreases as Mg consumes. A model of macroscopical infiltration and microscopical infiltration of liquid alloy in porous SiC preform was proposed.
Key words: SiCp composites; metal matrix composites; pressureless infiltration; kinetics; electronic packaging CLC number: TB333
Document code: A
1 INTRODUCTION
Aluminum infiltrated silicon carbide composite(SiCp/Al) exhibits excellent properties such as high thermal conductivity, low coefficient of thermal expansion(CTE), high modulus and low density, therefore it has extensive potential applications in electronic packaging and thermal management[1]. Electronic packaging materials are required to possess compatible CTEs with the IC, thus the composites with high volume fraction of SiC particles are preferred[2]. Methods to fabricate high volume fraction composites include pressure infiltration[3, 4], liquid-exchange[5] and pressureless infiltration[6, 7]. During pressureless infiltration, porous preform can be infiltrated by liquid metal without the aid of an externally applied force. The method is low-cost and simple alternative, thus it obtains close attention in recent years. Sound composites with high density and excellent properties can be produced by the pressureless infiltration. Recent investigation on pressureless infiltration focus on SiC particle reinforcement with single size[6-9], however, pressureless infiltration with bimodal size particles distribution is little touched upon. It is difficult to infiltrate the SiC preform with bimodal size particles distribution because of the inconstant porosity. To obtain good infiltration, it is very important to understand the infiltration kinetics of pressureless infiltration in SiCp/Al composites. In this paper, incubation period and infiltration rate during pressureless infiltration are investigated.
2 EXPERIMENTAL
Al-Si alloy was used as the matrix material, magnesium was added to help infiltration at the levels of 3%, 6%, 10% and 12%, while SiC particles with bimodal size at the average size of 50μm and 5μm were used as the reinforcement, the particle volume fractions(vp) of preform were 70%. The rectangle preform (60mm×30mm×10mm) was produced by mixing, forming, and sintering. The sintering of SiC particles was carried out in an electric furnace with open air at a pre-set temperature of 1200℃ for 2h. The infiltration experiment is shown in Fig.1. After preheated, preform was pushed into the Al-Mg-Si liquid alloy at 800, 900, 950, 1000 and 1100℃, respectively. The system was run at a very slight overpressure to ambient under N2 atmosphere. Infiltration rates were figured out by infiltration distance (height) as a function of infiltration time.
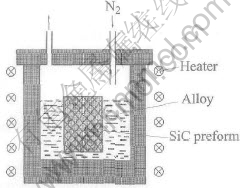
Fig.1 Schematic diagram of infiltration
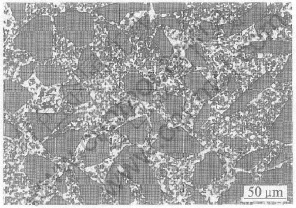
Fig.2 Distribution of SiC particles in composite
3 RESULTS AND DISCUSSION
Fig.2 shows the distribution of coarse and fine SiC particles and the distribution of pores in a composite produced by the infiltration parameters as temperature 1000℃, 70%vp, 10%Mg in alloy and 2h. The value of vp is easily changed from 56% to 75% through varying the proportion of coarse to fine particle.
3.1 Incubation period
When the preform was contacted with the alloy, a period that was called incubation period before infiltration was found. For a given temperature, the incubation time is the minimum time required to initiate infiltration. In the experiment, the incubation time is primarily controlled by infiltration temperature and Mg content.
Figs.3 and 4 show the effects of temperature and Mg content on the incubation time. Fig.3 shows that incubation time decreases from 45min to 6min as temperature increases from 800℃ to 1100℃ at 10%Mg. When the temperature increases to 900℃, the incubation time decreases to 20min, indicating that a sharp decline of incubation time appears at the temperature between 800 and 900℃. When the temperature varies from 1000 to 1100℃, the decrease of incubation time is only from 8 to 6min, which shows that there is not significant effect on incubation time when the temperature is over 1000℃. It is shown in Fig.4 that the incubation time decreases from 18 to 6min at 1000℃, as Mg content increases from 3% to 12%. It is easily found that the effect of temperature on incubation time exceeds that of Mg content.
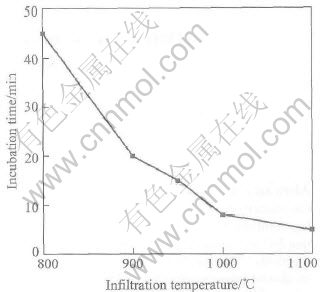
Fig.3 Effect of infiltration temperature on incubation time
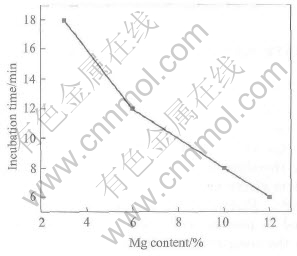
Fig.4 Effect of Mg content on incubation time
Eqn.(1) shows the chemical reaction on the surface of preoxied SiC particles during incubation period, the reaction promotes the wetting ability between SiC and aluminum alloy[10, 11] and thus induce the infiltration:
SiO2+Mg +Al→MgAl2O4+Si(1)
Many researchers[8] indicate that Eqns.(2) and (3) also plays an important role on wetting,
Mg+N2→Mg3N2(2)
Al+Mg3N2→AlN+Mg(3)
The increase of Mg content promotes Eqn.(1) along positive direction reaction, and the increase of temperatures decreases the Gibbs free energy. Furthermore, the increases of both Mg content and temperatures decrease the alloy viscosity and liquid-vapor surface tension[6, 12], and consequently decrease the incubation time.
3.2 Infiltration rate
The infiltration in the porous preform is not regular and stable. Fig.5 gives a model of the typical infiltration process. The gray region, dark gray region and white region represent the infiltrated area, the congregation of fine particles and the porous preforms, respectively. The process is also shown in Fig.6. At first, the large pores among coarse particles in the preform are infiltrated, and this process is called macroscopical infiltration. And then the small pores among fine particles are gradually infiltrated, and this process is called microscopical infiltration.
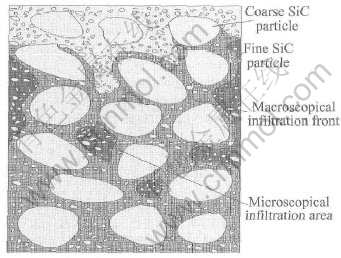
Fig.5 Infiltration model
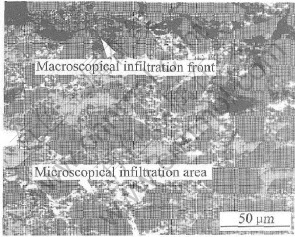
Fig.6 Optical micrograph of infiltration front
The above infiltration processes can be explained according to the equation proposed by Semlak and Rhines[13], as shown in Eqn.(4):
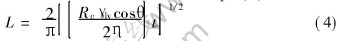
where Rc is the radius of the pore that will be infiltrated by the liquid phase, γlv is the liquid-vapor surface tension, θ is the contact angle, and η is the viscosity of liquid alloy. The value of Rc among coarse particles is 10 times than that among fine particle, thus infiltration rate of liquid alloy in large pores is about three times than that in small pores. Besides, the gas among coarse particles can be gradually expelled by liquid alloy, but the gas among fine particles must be consumed by surrounding liquid alloys that have enveloped the fine particles.
The effects of Mg content and temperature on the macroscopical infiltration are shown in Figs.7 and 8. The infiltration rate increases as Mg content and temperature increase. When infiltration temperature is 1000℃ and Mg content is over 6% for 2h, the preform can be infiltrated completely. When Mg content is over 10%, the infiltration rate is quickened. It is known that the completion of macroscopical infiltration does not mean the infiltration period is over, because it requires longer time to carry through microscopical infiltration. When Mg content is over 10% and temperature is over 1000℃ for 2h, the infiltration is accomplished.
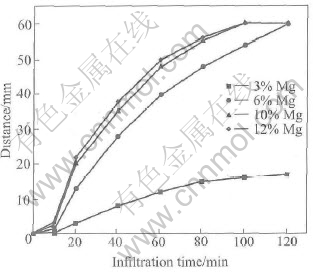
Fig.7 Effect of Mg content on infiltration rate
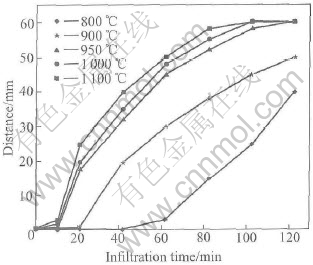
Fig.8 Effect of temperature on infiltration rate
According to Eqns.(1) and (4), the increase of temperature can increase the chemical reactions at interface between the liquid alloy and SiC preform, and thus promotes the infiltration rate. Mg is a powerful surfactant and scavenges any oxygen that may be present at the SiC/Al interface[13], therefore decreases the fine pores among the fine particles. The infiltration rate is influenced by Mg content due to its change of chemical composition during infiltration. At 1000℃, Mg content is decreased from 10% to 5% after infiltration by 2h, this decrease is due to its low vapor pressure. Part Mg vapor is condensed on the furnace wall as Mg, MgO or Mg2N3 and part is taken out by the flowing nitrogen[14]. The infiltration rate decreases after 60min resulted from Mg loss after long time infiltration.
4 CONCLUSIONS
1) There exists an incubation period which is determined by the chemical reactions between molten alloys and silicon oxide at SiC particle surfaces. When the chemical composition of silicon is fixed, the period will decrease with the increase of infiltration temperature and the chemical composition of magnesium.
2) The infiltration rate of molten alloys into porous SiC is related to the loss of magnesium and the temperature. When the atmosphere is inert, the rate will decrease severely by the loss of magnesium and lower temperature(800-1100℃).
3) A model of macroscopical infiltration and microscopical infiltration of liquid alloy in porous SiC preform was proposed.
REFERENCES
[1]Zweben C. Metal matrix composites for electronic packaging [J]. JOM, 1992, 44(7): 15-23.
[2]Zweben C. Advances in composite materials for thermal management in electronic packaging [J]. JOM, 1998, 50(6): 47-51.
[3]Molia J M, Saravanan R A, Arpon R, et al. Pressure infiltration of liquid aluminium into packed SiC particulate with a bimodal size distribution [J]. Acta Mater, 2002, 50: 247-257.
[4]ZHANG Qiang, CHEN Guo-qin, WU Gao-hui. Fabrication and property of SiCp/Al composites with high content of SiCp [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(5): 1180-1183.(in Chinese)
[5]Hozer L, Chiang Y M, Inanova S, et al. Liquid-exchange processing and properties of SiC-Al composites [J]. Mater Res, 1997, 12(7): 1785-1789.
[6]Zulfia A, Hand R J. Role of Mg and Mg plus Si as external dopants in production of pure Al-SiC metal matrix composites by pressureless infiltration [J]. Mater Sci Technol, 2000, 16 (7-8): 867-872 .
[7]Aghajanian M K, Rocazella M A, Burke J T, et al. The fabrication of metal matrix composites by a pressureless infiltration technique [J]. J Mater Sci, 1991, 26(5): 447-454.
[8]Pech-Canul M I, Katz R N, Makhlouf M M. Optimum conditions for preessureless infiltration of SiC preforms by aluminium alloy [J]. J Mater Pro Technol, 2000, 108(1): 68-77.
[9]Aguilar-Martinez J A, Pech-Canul M I, Rodriguez-Reyes M, et al. Effect of processing parameters on the degree of infiltration of SiCp preforms by Al-Si-Mg alloy [J]. Mater Lett, 2003, 57(26-27): 4332-4335.
[10]Shi Z L, Yang J M, Lee J C, et al. The interfacial characterization of oxidized SiCp/2014 Al composites [J]. Mater Sci Eng A, 2001, A303: 46-53.
[11]ZHANG Quan-ping, XU Bo-fan, WU Xin-jie, et al. Effect of Mg on microstructure and properties of SiCp/Al composite fabricated by pressuerless infiltration [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(1): 147-150. (in Chinese)
[12]Hashim J, Looney L, Hashim M S J. The wettability of SiC particles by molten aluminum alloy [J]. J Mater Pro Technol, 2001, 119: 324-328.
[13]Muscat D, Drew R A L. The effect of pore size on the infiltration kinetics of aluminum in titanium carbide preforms [J]. Acta Metall Mater, 1994, 42(12): 4155-4163.
[14]Rodriguez-Reyes M, Pech-Canul M I, Parras-Medecigo E E, et al. Effect of Mg loss on the kinetics of pressureless infiltration in the processing of Al-Si-Mg/SiCp composites [J]. Mater Lett, 2003, 57(13-14): 2081-2089.
Foundation item: Project(69976022) supported by the National Natural Science Foundation of China
Received date: 2004-11-24; Accepted date: 2005-01-18
Correspondence: QIN Zhen-kai; Tel: +86-29-13759923938; E-mail: qinzhenkai@163.com
(Edited by LONG Huai-zhong)