J. Cent. South Univ. Technol. (2011) 18: 1865-1870
DOI: 10.1007/s11771-011-0915-z
Preparation of basic magnesium carbonate and
its thermal decomposition kinetics in air
LIU Xin-wei(刘欣伟)1, 2, FENG Ya-li(冯雅丽)1, LI Hao-ran(李浩然)2
1. Civil and Environmental Engineering School, University of Science and Technology Beijing, Beijing 100083, China;
2. National Key State Laboratory of Biochemical Engineering, Institute of Process Engineering,Chinese Academy of Sciences, Beijing 100190, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: The thermal decomposition process of basic magnesium carbonate was investigated. Firstly, Basic magnesium carbonate was prepared from magnesite, and the characteristics of the product were detected by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Subsequently, the thermal decomposition process of basic magnesium carbonate in air was studied by thermogravimetry-differential thermogravimetry (TG-DTG). The results of XRD confirm that the chemical composition of basic magnesium carbonate is 4MgCO3·Mg(OH)2·4H2O. And the SEM images show that the sample is in sheet structure, with a diameter of 0.1-1 μm. The TG-DTG results demonstrate that there are two steps in the thermal decomposition process of basic magnesium carbonate. The apparent activation energies (E) were calculated by Flynn-Wall-Ozawa method. It is obtained from Coats-Redfern’s equation and Malek method that the mechanism functions of the two decomposition stages are D3 and A1.5, respectively. And then, the kinetic equations of the two steps were deduced as well.
Key words: basic magnesium carbonate; TG-DTG; thermal decomposition; kinetics; mechanism function
1 Introduction
Basic magnesium carbonate (xMgCO3·Mg(OH)2·yH2O) is considered as one of the most important compounds of the magnesium industry. It has been widely used in various industries, such as toothpaste, painting, cosmetic manufacturing, plastic, rubber, and as precursors for other magnesium-based chemicals [1-2]. Basic magnesium carbonate can also be used as the chemical coolant when the heat is absorbed during the thermal decomposition [3]. In recent years, a series of xMgCO3·Mg(OH)2·yH2O with different morphologies have been obtained via different synthesis method or in different preparation conditions. However, to the best of our knowledge, the report on the thermal decomposition of basic magnesium carbonate is rare. The decomposition reported in previous studies showed that the thermal decomposition of basic magnesium carbonate occurs in at least two stages, dehydration and magnesium carbonate decomposition. KHAN et al [4] studied the influence of heating rate, sample size and atmospheric conditions on the origin of exothermic peak.
The aim of the present work is to investigate the thermal decomposition of basic magnesium carbonate, which was prepared from magnesite, by thermo- gravimetry-differential thermogravimetry (TG-DTG) technology [5-6]. Based on the analysis of the dynamic parameters in thermal decomposition, the basic magnesium carbonate decomposition mechanism was investigated and the key dynamic parameters were also tested.
2 Experimental
2.1 Raw material
The magnesite ore used in the experiment was collected from Haicheng in Liaoning Province, China. Chemical analysis result of the ore is given in Table 1. As can be seen from Table 1, the main component of the ore is MgCO3.
Table 1 Chemical analysis of magnesite (mass fraction, %)

2.2 Experimental procedures
2.2.1 Preparation of basic magnesium carbonate
The light-burned magnesia was prepared from magnesite, which was calcinated at 1 023 K for 1.5 h. Then, basic magnesium carbonate was obtained from the light-burned magnesia. Process flow chart is shown in Fig.1.
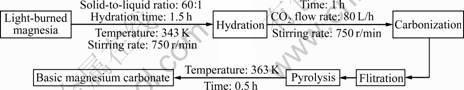
Fig.1 Preparation flow chart of basic magnesium carbonate
2.2.2 Analysis of basic magnesium carbonate
The produced basic magnesium carbonate was analysed by X-ray diffraction (Rigaku D/max-RB type X-ray) with Cu Kα radiation (λ=1.541 8 ? at 40 kV and 70 mA). Scanning electron microscope (SEM) images were taken with SSX-550 field-emission scanning electron microscope.
2.2.3 Thermal decomposition of basic magnesium carbonate
A NETZSCH STA 449C (manufactured by Netzsch in Germany) thermogravimetry differential scanning calorimetry (TG-DSC) system was used to determine the TG and differential TG (DTG) experimental points for basic magnesium carbonate at different heating rates. A given amount of sample was put into an alumina crucible and its mass loss was recorded at heating rate of 15, 20 and 25 K/min in air atmosphere, with a carrier gas flow rate of 50 mL/min. In the TG-DSC system, the masses of samples are 3.271-4.077 mg, and the results obtained are repeatable.
3 Results and discussion
3.1 Detection and analysis of basic magnesium carbonate
The XRD pattern of the product is shown in Fig.2. All the peaks in Fig.1 can be indexed to the crystalline phase of 4MgCO3·Mg(OH)2·4H2O, with unit cell parameters of a=10.11 ?, b=8.94 ?, c=8.38 ? and β= 114.58°, which are in agreement with literature values (JCPDS25-0513).
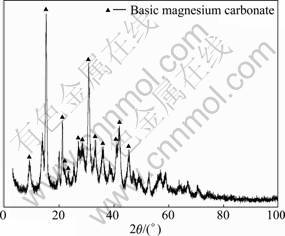
Fig.2 XRD pattern of product synthesized in accordance with Fig.1
Figure 3 shows the SEM images of the basic magnesium carbonate. The products exist as independent flaky units, with a diameter of 0.1-1 μm, which are generally consistent with Ref.[7]. Some flaky units aggregate like roseleaf-shaped basic magnesium carbonate. It is superposed layer by layer, from inside to outside, and the flakes exhibit uniform thickness.

Fig.3 SEM micrographs of 4MgCO3·Mg(OH)2·4H2O
3.2 Thermal decomposition of basic magnesium carbonate
Figure 4 shows TG-DTG curves of the thermal decomposition of 4MgCO3·Mg(OH)2·4H2O.
The TG curves indicate that there are two stages in the thermal decomposition process of basic magnesium carbonate. The first decomposition stage is in the range of 423-573 K attributed to the water loss of crystallization, and this decomposition is accompanied by an endothermic peak at 508.4 K. The mass loss is measured to be about 14.98 % in this stage. The second decomposition stage is in the range of 573-823 K, and this decomposition is accompanied by an endothermic peak at 729 K. The mass loss is measured to be about 56.88%, which is slightly smaller than the theoretical value (57.08%). Basic magnesium carbonate has been completely decomposed into magnesium oxide, carbon dioxide and water in the second stage. The thermal decomposition of basic magnesium carbonate can be expressed as follows.
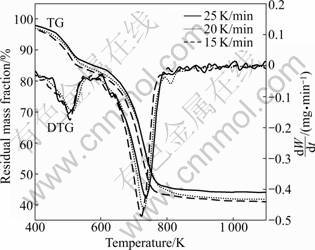
Fig.4 TG-DTG curves of 4MgCO3·Mg(OH)2·4H2O thermal decomposition at different heating rates
In first stage:
4MgCO3·Mg(OH)2·4H2O→4MgCO3·Mg(OH)2+4H2O
In second stage:
4MgCO3·Mg(OH)2→5MgO+H2O+4CO2
3.3 Calculation of dynamic parameters
According to non-isothermal kinetic theory, the kinetic equation of solid-state thermal transformation under linear temperature increasing condition could be generally described as [8-9]
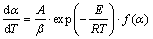 (1)
(1)
where α is the extent of conversion, dα/dT is the reaction rate and f(α) is the reaction model; A is the pre-exponential term; β is the heating rate; E is the energy of activation; R is the universal gas constants and T is the absolute temperature.
Methods for solving kinetic parameters can be attributed to the approximate treatment of Eq.(1). In this work, Flynn-Wall-Ozawa (FWO) method [10] was used. FWO equation can be written as:
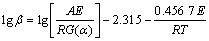 (2)
(2)
From Eq.(2), it can be seen that, when the reaction mechanism function is unchanged, by plotting ln β vs 1/T, straight lines can be produced, and the slopes can be used to calculate the activation energy E, which was obtained without the mechanism functions [11].
The plot of ln β vs 1/T was established based on the same conversion at different heating rates and activation energy was calculated from the slope of the regression line. In this work, the experiments were conducted at three heating rates of 15, 20 and 25 K/min. And nine conversion values were chosen from 10% to 90% with an increment of 10% at each heating rate. The regression lines of ln β vs 1/T are plotted in Fig.5. From Fig.5, it can be observed that when basic magnesium carbonate conversion rates are 10%, 20% and 30%, the three regression lines are parallel to each other. However, when the conversion rates are between 40% and 90%, this part of regression lines are parallel to each other. This also shows that there are two steps in the thermal decomposition process of basic magnesium carbonate.
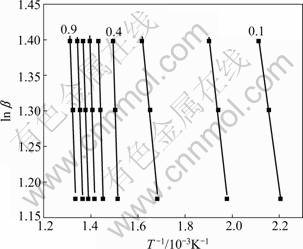
Fig.5 ln β-1/T curves at different decomposition rates
The plot of basic magnesium carbonate decomposition activation energy versus conversion rate is constructed, as shown in Fig.6. From Fig.6, it can be seen that the decomposition apparent activation energy is divided into two parts. The first part of the activation energy is about 51.84 kJ/mol, and the second part is about 191.97 kJ/mol, which further explains that the
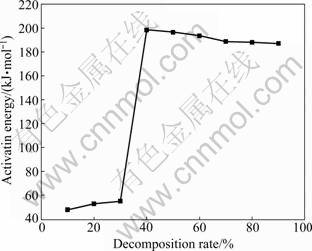
Fig.6 Thermal decomposition activation energy variation curve of 4MgCO3·Mg(OH)2·4H2O
thermal decomposition of basic magnesium carbonate is divided to two-step reactions.
3.4 Mechanism functions determination
In order to study the non-isothermal reaction kinetics of basic magnesium carbonate thermal decomposition from single TG curve. Coats-Redfern equation [12-13] was used. The equation can be written as
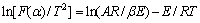 (3)
(3)
where 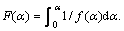
Linear-regression was used to analyze the kinetic characteristics and reaction mechanism of the entire basic magnesium carbonate decomposition. According to Eq.(3), a plot of ln[F(α)/T2] vs 1/T was constructed. Eight different values of F(α) from documents [14-18] were tested. The form of F(α) that gave the best straight line with high correlation coefficient (R-squared value) of linear regression analysis was selected and the mechanism corresponding to this value of F(α) was assigned as the mechanism for the reaction. The slope of this particular plot yielded the energy of activation while the y-intercept provided the pre-exponential factor (lgA-value) for the reaction. The values obtained are listed in Table 2.
From the regression results, it can be deduced that in the first stage of basic magnesium carbonate decomposition process, D3, D4 and 3D mechanism functions have higher correlation coefficient and are close to each other. In the second stage, 3D and A1.5 mechanism functions have higher correlation coefficient and are close to each other. In the case of correlation coefficients close to one another, Malek method is an effective method to further determine the most probable mechanism function [19]. The standard curve equation of mechanism function can be written as
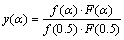 (4)
(4)
Experimental curve equation can be written as
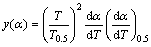 (5)
(5)
where F(α) and f(α) are the reaction models, and dα/dt is obtained from the TG curve. Plotting y(α) vs α curves, if the experimental curve and the standard curve overlap or all experimental data points fall on a standard curve, the corresponding standard curve, F(α) or f(α), is the most probable mechanism function.
Taking 20 K/min as an example, the two stages y(α) vs α curves are given in Fig.7 and Fig.8. Experimental curve is expressed by the dashed line and labeled by “p”.
Based on the above analysis, it can be determined that the first stage of basic magnesium carbonate decomposition is D3 mechanism, and mechanism function can be represented as
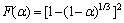
or
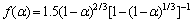
Table 2 Kinetics parameters and regression calculation results
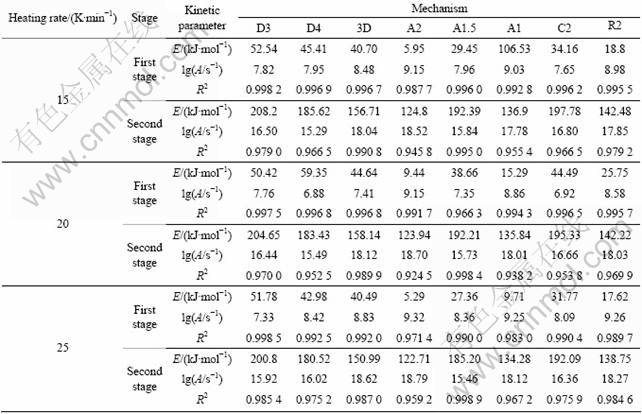
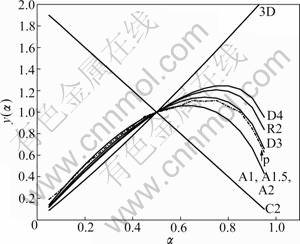
Fig.7 y(α)-α curves of first phase
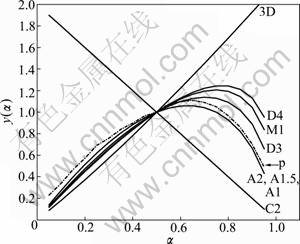
Fig.8 y(α)-α curves of second phase
The second stage is A1.5 mechanism, and mechanism function can be represented as
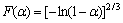
or
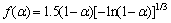
The average values of two stage pre-exponential factor lg A are 7.64 and 15.68, respectively. Therefore, the corresponding kinetic equations can be represented as follows.
In the first stage:
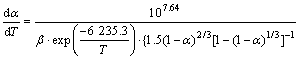 (6)
(6)
In the second stage:
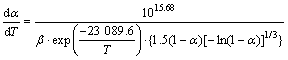 (7)
(7)
4 Conclusions
1) Basic magnesium carbonate is prepared from magnisite, and the product is in sheet structure, with diameters of 0.1-1 μm, and the chemical formula is 4MgCO3·Mg(OH)2·4H2O.
2) The thermal decomposition process of basic magnesium carbonate in air is studied by TG-DTG. The results demonstrate that there are two steps in the thermal decomposition process of basic magnesium carbonate, and decomposition products are mainly magnesium oxide, carbon dioxide and water. Dehydration reaction is in the first reaction, and complete decomposition reaction is in the second stage.
3) It is obtained from Coats-Redfern equation and Malek method that the mechanism functions of the two decomposition stages are D3 and A1.5, respectively. The apparent activation energy (E) are 51.84 kJ/mol and 191.97 kJ/mol, respectively, and pre-exponential factor (lgA) are 7.64 s-1 and 15.68 s-1, respectively.
References
[1] TU Jie, XU Wang-sheng. New technology of producing basic magnesium carbonate from dolomite by pressurized carbonation [J]. Non-Metallic Mines, 2010, 33(1): 45-48. (in Chinese)
[2] HAO Zhi-hai, DU Fang-lin. Synthesis of basic magnesium carbonate microrods with a “house of cards” surface structure using rod-like particle template [J]. Journal of Physics and Chemistry of Solids, 2009, 70(2): 401-404.
[3] LAOUTID F, GAUDON P, TAULEMESSE J M, LOPEZ CUESTA J M, VELASCO J I, PIECHACZYK A. Study of hydromagnesite and magnesium hydroxide based fire retardant systems for ethylene–vinyl acetate containing organo-modified montmorillonite [J]. Polymer Degradation and Stability, 2006, 91(12): 3074-3082.
[4] KHAN N, DOLLINORE D, ALEXANDER K, WILBURN F W. The origin of the exothermic peak in the thermal decomposition of basic magnesium carbonate [J]. Thermochimica Acia, 2001, 367/368(3): 321-333.
[5] JIN Hui-jie, LI Yan-hong, REN Bao-zeng, KONG Hai-ping, LUO Ting-liang, LIU Guo-ji. Thermal decomposition of SnSO4 in catalyst preparation [J]. Journal of Chemical Industry and Engineering, 2008, 59(4): 917-919. (in Chinese)
[6] QU Hong-qiang, WU Wei-hong, JIAO Yun-hong, XU Jian-zhong. ZnO and metal hydroxides as flame-retardants and smoke suppressants for flexible poly (vinyl chloride) [J]. Journal of Chemical Industry and Engineering, 2006, 57(5): 1259-1263. (in Chinese)
[7] HAO Zhi-hua, PAN Jie, DU Fang-lin. Synthesis of basic magnesium carbonate microrods with a surface of “house of cards” structure [J]. Materials Letters, 2009, 63(12): 985-988.
[8] NIU Sheng-li, HAN Kui-hua, LU Chun-mei, SUN Rong-yue. Thermogravimetric analysis of the relationship among calcium magnesium acetate, calcium acetate and magnesium acetate [J]. Applied Energy, 2010, 87(7): 2237-2242.
[9] AL-OTHMAN ASMA A, AL-FARHAN KHALID, MAHFOUZ REFAAT M. Kinetics analysis of nonisothermal decomposition of (Mg5(CO3)4(OH)2·4H2O/5Cr2O3) crystalline mixture [J]. Journal of King Saud University (Science), 2009, 21: 133-143.
[10] CONG Chang-jie, LUO Shi-ting, TAO You-tian, ZHANG Li-ke. Kinetics of thermal decomposition of ZnAc2·H2O in air atmosphere [J]. Chemical Journal of Chinese University, 2005, 26(12): 2327-2330. (in Chinese)
[11] ZHENG Hong-xia, LIAO Xin-sheng, WANG Qi, LI Jing. TG kinetics of decomposition of magnesite power and its pellet [J]. Journal of University of Science and Technology Liaoning, 2008, 31(1): 29-31. (in Chinese)
[12] DEMIR F, DONMEZ B, OKER H, SEVIM F. Calcination kinetic of magnesite from the thermogravimetric data [J]. Institution of Chemical Engineers, 2003, 81(3): 618-622.
[13] LU Chang-bo, SONG Wen-li, LIN Wei-gang. Kinetics of biomass catalytic pyrolysis [J]. Biotechnology Advances, 2009, 27(5): 583-587.
[14] SAMTAIN M, DOLLIMORE D, ALEXANDER K S. Comparison of dolomite decomposition kinetics with related carbonates and the effect of procedural variables on its kinetics parameters [J]. Thermochimica Acta, 2002, 392/393(15): 135-145.
[15] NING Zhi-qiang, ZHAI Yu-chun, SUN Li-qin. Study on the thermal decomposition kinetics of magnesium hydroxide [J]. Journal of Molecular Science, 2009, 25(1): 27-30. (in Chinese)
[16] ZHENG Ying, CHEN Xiao-hua, ZHOU Ying-biao, ZHENG Chu-guang. The decomposition mechanism of CaCO3 and its kinetics parameters [J]. Journal of Huazhong University of Science and Technology: Nature Science Edition, 2002, 32(12): 86-88. (in Chinese)
[17] ZHANG Bao-sheng, LIU Jian-zhong, ZHOU Jun-hu, FENG Zhan-guan, QIN Ke-fa. Experimental study on the impaction of particle size to limestone decomposition kinetics by thermogravimetry [J]. Proceedings of the CSEE, 2010, 30(2): 51-55. (in Chinese)
[18] WANG Shi-jie, LU Ji-dong, ZHOU Hu, HU Zhi-juan, ZHANG Bu-ting. Kinetics model study on thermal decomposition of limestone particles [J]. Journal of Engineering Thermophysics, 2003, 24(4): 699-702. (in Chinese)
[19] HU Rong-zu, SHI Qi-zhen. Thermal analysis kinetics [M]. Beijing: Science Press, 2001: 125. (in Chinese)
(Edited by HE Yun-bin)
Foundation item: Project(20876160) supported by the National Natural Science Foundation of China
Received date: 2010-12-02; Accepted date: 2011-03-11
Corresponding author: FENG Ya-li, Professor, PhD; Tel: +86-10-82627064; E-mail: ylfeng126@126.com