Magnesium stress corrosion cracking
来源期刊:中国有色金属学报(英文版)2007年增刊第1期
论文作者:N. Winzer A. Atrens W. Dietzel G. Song K. U. Kainer
文章页码:150 - 155
Key words:stress corrosion cracking; linearly increasing stress test; LIST; CERT; hydrogen
Abstract: The significant positive green environment influence of magnesium alloy usage in transport could be compromised by catastrophic fast fracture caused by stress corrosion cracking (SCC). Transgranular stress corrosion cracking (TGSCC) of AZ91 was evaluated using the linearly increasing stress test (LIST) and the constant extension rate test (CERT). The TGSCC threshold stress was 55-75 MPa in distilled water and in 5 g/L NaCl. The TGSCC velocity was 7×10-10-5×10-9 m/s. A delayed hydride-cracking (DHC) model for TGSCC was implemented using a finite element script in MATLAB and the model predictions were compared with experiment. A key outcome is that, during steady state TGSCC propagation, a high dynamic hydrogen concentration is expected to build up behind the crack tip. A number of recommendations are given for preventing SCC of Mg alloys in service. One of the most important recommendations might be that the total stress in service (i.e. the stress from the service loading + the fabrication stress + the residual stress) should be below a threshold level, which, in the absence of other data, could be (conservatively) estimated to be about 50% of the tensile yield strength.

N. Winzer1, A. Atrens1, 2, W. Dietzel3, G. Song1, K. U. Kainer3
1. Materials Engineering, The University of Queensland, Brisbane, Australia
2. Swiss Federal Laboratories for Materials Science and Technology, EMPA, Dept 136,
?berlandstrasse 129, CH-8600 Dubendorf, Switzerland
3. GKSS-Forschungszentrum Geesthacht GmbH, Germany
Received 15 July 2007; accepted 10 September 2007
Abstract: The significant positive green environment influence of magnesium alloy usage in transport could be compromised by catastrophic fast fracture caused by stress corrosion cracking (SCC). Transgranular stress corrosion cracking (TGSCC) of AZ91 was evaluated using the linearly increasing stress test (LIST) and the constant extension rate test (CERT). The TGSCC threshold stress was 55-75 MPa in distilled water and in 5 g/L NaCl. The TGSCC velocity was 7×10-10-5×10-9 m/s. A delayed hydride-cracking (DHC) model for TGSCC was implemented using a finite element script in MATLAB and the model predictions were compared with experiment. A key outcome is that, during steady state TGSCC propagation, a high dynamic hydrogen concentration is expected to build up behind the crack tip. A number of recommendations are given for preventing SCC of Mg alloys in service. One of the most important recommendations might be that the total stress in service (i.e. the stress from the service loading + the fabrication stress + the residual stress) should be below a threshold level, which, in the absence of other data, could be (conservatively) estimated to be about 50% of the tensile yield strength.
Key words: stress corrosion cracking; linearly increasing stress test; LIST; CERT; hydrogen
1 Introduction
Magnesium offers a high potential as a lightmass structural material for use in transport, and consequently can have significant green environment influence. Consequently, magnesium usage is growing rapidly. The rapid increase is due to the lightmass of magnesium alloys, which provides considerable mass saving potential in automobile and transport industries, although corrosion is an issue[1-17]. Its good processing capability, particularly its ability to be die cast into large thin sections, can also lead to considerable savings. However, there is a significant risk of SCC causing catastrophic fracture of critical Mg components that are exposed to road spray. The total risk increases with the current rapid increase in Mg usage in auto applications. Mg SCC can occur in any application when a stressed Mg component is subject to wet conditions. Catastrophic failure is expected from SCC when the environment (e.g. road splash) decreases the SCC initiation stress to below the operating stress.
An overview of Mg SCC is emerging from our critical literature review[18] and current research[19-25] for existing Mg alloys and alloys being developed. Our recent critical review of SCC[18] indicated that many common Mg alloys show SCC in common environments such as distilled water and dilute chloride solutions and that the threshold stress for SCC is commonly about half the yield stress. There can be, nevertheless, significant SCC differences between alloys[18,21-22]. Intergranular stress corrosion cracking (IGSCC) is not the subject of our research because Mg IGSCC is well understood. IGSCC in Mg alloys is typically caused[23] by corrosion associated with a continuous second phase along the grain boundaries, a microstructure typical of cast creep-resistant Mg alloys.
Transgranular stress corrosion cracking (TGSCC) occurs through the Mg matrix. TGSCC is the intrinsic form of SCC and TGSCC can occur in alloys resistant to IGSCC. TGSCC is the focus of our research. The main features of Mg TGSCC have been established[18]. PUGH and co-workers[26-30] provided convincing evidence for a brittle cleavage mechanism involving hydrogen. Particularly noteworthy were the stepped and facetted interlocking fracture surfaces. The slow strain rate tests[1-2] indicated a mechanism involving strain induced breaking of a crack tip film leading to corrosion and hydrogen production, with crack advance due to hydrogen. MAKAR et al[1] confirmed the fractography, the importance of strain rate and hydrogen, but they proposed a brittle hydride model. Thus, there is agreement that hydrogen is a key part of Mg TGSCC crack propagation but considerable disagreement concerning the details. Concerning the environment, the mechanistic hypothesis[18] is that TGSCC occurs for conditions leading to the local breakdown of a partially protective surface film. Film breakdown can be caused by pitting by chloride ions or by the applied stress. The surface film also inhibits hydrogen ingress into the metallic Mg alloy, the hydrogen being produced as part of the Mg corrosion reaction.
In summary, mechanistic understanding indicates that hydrogen plays a key role[18] in Mg TGSCC. It is most likely[18] that TGSCC involves repeated cycles of (1) hydrogen diffusion to the stressed region ahead of the crack tip and (2) stress corrosion crack advance through a hydrogen influenced process zone. Research needs[18-19, 21-22] are for a fundamental understanding to overcome Mg TGSCC, which is the inherent SCC response.
2 Characterization of TGSCC
SCC tests[19-21] were performed using high purity AZ91 (>90.11%Mg, 8.99%Al, 0.78%Zn, 0.21%Mn, mass fraction) using the linearly increasing stress test (LIST) or the constant extension rate test (CERT). In the LIST apparatus[34-42] (Fig.1) the specimen is attached to one end of a lever arm. To the opposite end of the arm a known mass is attached such that the tensile load applied to the specimen increases linearly as the distance between the fulcrum and the mass is increased by means of a screw thread and synchronous motor. The CERT apparatus (Fig.2) maintains a constant extension rate by means of an open-loop control system; the average specimen elongation is measured by two high-resolution LVDTs in parallel with the specimen whilst a geared synchronous motor increases the elongation. SCC susceptibility was characterised according to the threshold stress, which was determined using the DC potential drop (DCPD) method[43-47]. The stress corrosion crack velocity, vc, was calculated for both LIST and CERT according to vc = a/l, where a is the length of the stress corrosion crack at the end of the test as estimated from the fracture surface and t is the time for stress corrosion crack growth from crack initiation (identified using the DCPD method) to the end of the test.
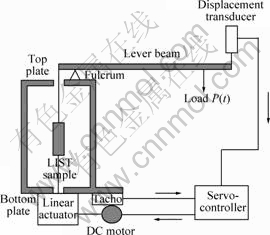
Fig.1 Schematic illustration of LIST apparatus
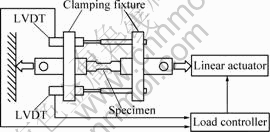
Fig.2 Schematic illustration of CERT apparatus
Figs.3 and 4[19-21] show typical stress—strain behaviour and DCPD measurements for CERT. There was a considerable reduction in the UTS relative to air for all of the samples exposed to distilled water. Moreover, the UTS decreased with decreasing strain rate. The threshold stress corresponded to the point where the DCPD curve became non-linear (Fig.4). The SCC threshold stress was 55-75 MPa in distilled water and 5 g/L NaCl. The measured velocity was in the range of 7×10-10-5×10-9 m/s. These values are compared with literature values in Table 1.
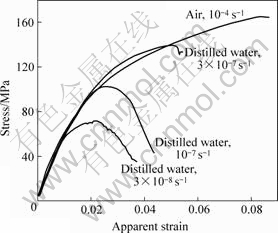
Fig.3 Stress—apparent strain curves for AZ91 in distilled water and air[19-21]
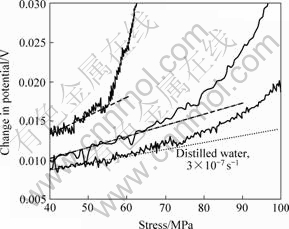
Fig.4 DCPD results for AZ91 in distilled water[19-21]
3 Delayed hydride cracking model
A DHC model[24] was proposed based on transient hydrogen diffusion towards and, when the H solvus concentration is exceeded, hydride precipitates in the region ahead of the crack tip. The model evaluates vc based on the time-to-reach the critical hydride dimension during stage-2 TGSCC crack growth. A finite element script was developed in MATLAB to solve for the transient H distribution and hydride precipitation. A typical result for the DHC model is presented in Fig.5. It shows contours of equal values of φ. The region bounded by φ=1 is comprised completely of hydride, whereas at the next contour, φ=0.95, there is 95 % hydride and 5% Mg metal. In this case, DHC was simulated for K1=K1SCC?6 MPa?m1/2, neglecting the influence of the plastic zone, for a period of time corresponding to φ=1 at a distance of about 0.8 m ahead of the crack tip.
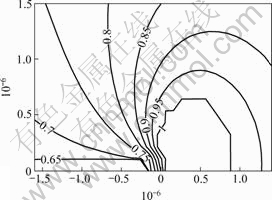
Fig.5 Distribution of hydride volume fraction φ for K1= 6 MPa?m1/2 and t = 6.2 s [24]
For most of the simulation (about 4.5 s) φ<0 for the entire domain; the greatest period of time (t about 4.5 s) is that which is required for the hydrogen concentration to reach the solvus concentration at the point of maximum stress, then hydride first forms and thereafter the hydride grows rapidly. Fig.6 shows the hydride distribution for various times between (1) complete hydride throughout the first element (at the point of maximum stress) and (2) when φ=1 front is 0.8 μm ahead of the crack tip (assuming K1 = 6 MPa/m1/2).
Table 1 Measured stress corrosion crack velocity values
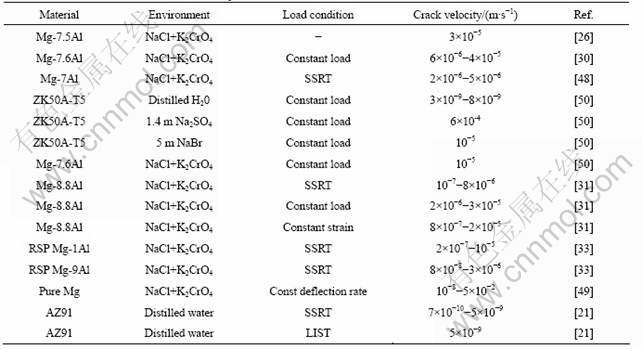
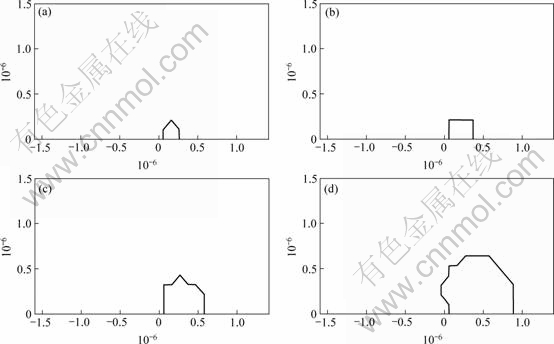
Fig.6 φ=1 zone after 6.19, 6.2, 6.21 and 6.22 s for K1 = 6 MPa?m1/2
Assuming a critical hydride size of 0.8 μm for all values of K1, the values predicted by the present DHC model for vc range from 4.6×10-8 m/s to 3.3×10-7 m/s. These DHC model predictions are consistent with the lower measured crack velocities reported by previous workers and thus could explain the stress corrosion crack velocity for Mg alloys in distilled water. However, the DHC model as presently formulated does not predict stress corrosion crack velocity values of 10-5 m/s as measured e.g. by SPEIDEL et al[50] for the stress corrosion cracking of ZK50 in dilute NaBr solution or by PUGH and co-workers[26-30] for Mg-Al alloys in NaCl + K2CrO4.
A key outcome of the modeling [24] is that the standard initial condition for DHC models is unlikely to be correct. It was assumed that the region near the crack tip was free of H and that H diffuses from the crack surface only. This assumption, although made in previous DHC models, is valid only for the initial crack propagation step in a “virgin” H-free material and is not valid for steady state TGSCC propagation. In the case of the initial crack propagation in a “virgin” H-free material it is appropriate to choose a low, uniform H concentration as an initial condition. For steady state TGSCC propagation the appropriate hydrogen concentration around the crack tip can be deduced from Fig.5 by moving the point of origin to the right by a distance corresponding to the prior crack advance (about 0.8 μm), such that the new crack tip is in a region with a hydride volume fracture φ=0.95 rather than a hydrogen concentration of about 0. Fig.6 shows that in a “virgin” material the growth of the region corresponding to φ=1 grew to about 0.8 μm in 0.02 s. Given an initial condition of φ=0.95 at the crack tip, the predicted stress corrosion crack velocity is then
vc = 0.8 μm/0.02 s = 4×10-5 m/s
This estimation shows that the DHC model may be developed to predict a TGSCC crack velocity sufficiently high to explain the stress corrosion crack velocities measured by SPEIDEL et al [50], PUGH et al [26-30], EBTEHAJ et al [31] and MAKAR et al [33].
4 Recommendations to avoid SCC
The general principle for SCC prevention is to avoid loading a susceptible alloy above a critical stress during exposure to a SCC producing environment. SCC prevention is a serious task for Mg alloys, since SCC can be produced in distilled water for applied stresses above 50% YS [18]. There is inadequate data, partly because the use of Mg alloys as a structural material is relatively recent and reported service failures are not numerous. Fig.7 schematically illustrates how threshold values can be included in an assessment of structural integrity. In an inert environment, the mechanical limits can be defined by the yield stress, σy, and the fracture toughness, K1C. In a SCC environment, the mechanical limits are reduced to the threshold stress intensity factor, K1SCC, and the threshold stress, σSCC, determined on smooth tensile specimens of the same material and in the same environment. These two parameters define an “acceptable region” with respect to immunity against SCC. Depending on the susceptibility of the material/environment combination under investigation, this region can be significantly smaller than the original “acceptable region” derived from tests in air.
There is a general trend in SCC prevention to start with a design that avoids concentration of stresses at the start, or during use, or through increasing susceptibility to different forms of degradation in service, especially localised corrosion, galvanic corrosion and (corrosion) fatigue. SCC is expected to not be an issue for dry atmospheres provided the relative humidity is less than 95% and provided that there are no crevices. For crevices, capillary condensation can cause the formation of a liquid in the crevices at lower values of relative humidity[51]. It would be prudent to fill crevices with a corrosion-inhibited putty. Furthermore, crevices are part of the microstructure of diecast alloys, which might be part of the reason that MILLER[52] measured threshold valued of 40%-50% of YS for AZ91, AM60 and AS41 in distilled water. The total stress in service (stress from the service loading + the fabrication stress + the residual stress) should be below the threshold level which, in the absence of other data, could be estimated to be about 40%-50% of the tensile yield strength.
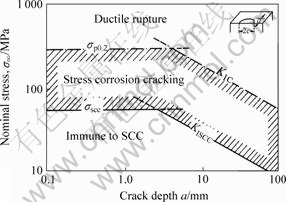
Fig.7 Mechanical limits of typical system involving an inert and SCC environment[53-54]
5 Summary
1) The TGSCC threshold stress is 55-75 MPa for AZ91 in distilled water and 5 g/L NaCl. The measured TGSCC velocity is in the range of 7×10-10-5×10-9 m/s.
2) Stress corrosion crack velocities about 10-4 m/s, which are typical for Mg alloys in aqueous solutions, cannot be predicted by the DHC model based on the time to reach a critical hydride size for material with a low initial H concentration throughout. Such TGSCC velocities might be predicted by a DHC model based on the time to reach the critical hydride size in steady state, when a significant hydrogen concentration would have built up at the crack tip.
3) During steady state stress corrosion crack propagation of Mg in aqueous solutions, a high dynamic hydrogen concentration would be expected to build up just behind the crack tip. This may be a feature of all cases of SCC where the crack propagation mechanism is Hydrogen Environment Assisted Cracking (HEAC).
4) The total stress in service (stress from the service loading + the fabrication stress + the residual stress) should be below the threshold level which, in the absence of other data could be estimated to be about 40%-50% of the tensile yield strength.
Acknowledgements
This research was supported by an ARC Linkage Grant in collaboration with the GM Technical Centre at Warren Mi. N. Winzer and A. Atrens also wish to thank GKSS-Forschunszentrum GmbH for their support.
References
[1] SONG G, ATRENS A. Corrosion mechanisms of magnesium alloys[J]. Advanced Engineering Materials, 1999, 1: 11-33.
[2] SONG G L, ATRENS A. Understanding magnesium corrosion mechanism: A framework for improved alloy performance [J]. Advanced Engineering Materials, 2003, 5: 837.
[3] SONG G, ATRENS A. Recent insights into the mechanism of magnesium corrosion and research suggestions[J]. Advanced Engineering Materials, 2007, 9: 177-183.
[4] ATRENS A, DIETZEL W. The negative difference effect and unipositive Mg+ [J]. Advanced Engineering Materials, 2007, 9: 292-297.
[5] JIA J X, SONG G L, ATRENS A. Influence of geometry on galvanic corrosion of AZ91D coupled to steel [J]. Corrosion Science, 2006, 48: 2133-2153.
[6] JIA J X, SONG G, ATRENS A. Experimental measurement and computer simulation of galvanic corrosion of magnesium coupled to steel[J]. Advanced Engineering Materials, 2007, 9: 65-74.
[7] SONG G L, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of diecast AZ91D[J]. Corrosion Science, 1999, 41: 249-273.
[8] SONG G L, ATRENS A, WU X, ZHANG B. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride[J]. Corrosion Science, 1998, 40: 1769-1791.
[9] SONG G L, ATRENS A, STJOHN D H, NAIRN J, LANG Y. Electrochemical corrosion of pure magnesium in 1N NaCl[J]. Corrosion Science, 1997, 39: 855-875.
[10] SONG G L, ATRENS A, STJOHN D, WU X, NAIRN J. The anodic dissolution of magnesium in chloride and sulphate solutions[J]. Corrosion Science, 1997, 39: 1981-2004.
[11] SHI Z, SONG G, ATRENS A. The corrosion performance of anodised magnesium alloys[J]. Corrosion Science, 2006, 48: 3531-3546.
[12] SHI Z, SONG G L, ATRENS A. Influence of anodising current on the corrosion resistance of anodised AZ91D magnesium alloy[J]. Corrosion Science, 2006, 48: 1939-1959.
[13] SHI Z, SONG G L, ATRENS A. Corrosion resistance of anodised single-phase Mg alloys[J]. Surface and Coatings Technology, 2006, 201: 492-50.
[14] JIA J X, SONG G, ATRENS A. Boundary element predictions of the influence of the electrolyte on the galvanic corrosion of AZ91D coupled to steel[J]. Materials and Corrosion, 2005, 56: 259-270.
[15] JIA J X, ATRENS A, SONG G, MUSTER T. Simulation of galvanic corrosion of magnesium coupled to a steel fastener in NaCl solution[J]. Materials and Corrosion, 2005, 56: 468-474.
[16] SHI Z, SONG G, ATRENS A. Influence of the b phase on the corrosion performance of anodised coatings on magnesium- aluminium alloys[J]. Corrosion Sci, 2005, 47: 2760-2777.
[17] ATRENS A. Suggestions for research directions in magnesium corrosion arising from the Wolfsburg Conference[J]. Advanced Engineering Materials, 2004, 6: 83-84.
[18] WINZER N, ATRENS A, SONG G, GHALI E, DIETZEL W, KAINER K U, HORT N, BLAWERT C. A critical review of the stress corrosion cracking (SCC) of magnesium alloys[J]. Advanced Engineering Materials, 2005, 7: 659-693.
[19] WINZER N, ATRENS A, DIETZEL W, SONG G, KAINER K U. Comparison of the linearly increasing stress test and the constant extension rate test in the evaluation of transgranular stress corrosion cracking of magnesium[J]. Mater Sci Eng A, 2007, accepted.
[20] WINZER N, SONG G, ATRENS A, DIETZEL W, BLAWERT C, KAINER K U. Evaluation of Mg SCC using LIST and SSRT[C]//7th International Conference on Magnesium Alloys and Their Applications. Deutsche Gesellschaft fuer Materialkunde, 2006.
[21] WINZER N, SONG G, ATRENS A, DIETZEL W, KAINER K U. Stress corrosion cracking of Mg[C]//Invited Keynote Paper, 3rd International Conference on Environmental Degradation of Engineering Materials. Gdansk Poland, 2007.
[22] SONG R G, BLAWERT C, DIETZEL W, ATRENS A. A study of the stress corrosion cracking and hydrogen embrittlement of AZ31 magnesium alloy[J]. Mater Sci Eng A, 2005, 399: 308-317.
[23] KANNAN M B, ATRENS A, DIETZEL W, LYON P, BLAWERT C. SCC evaluation of Mg alloys AZ80, ZE41, QE22 and EV21[J]. Mater Sci Eng A, 2007, accepted.
[24] WINZER N, SONG G, ATRENS A, DIETZEL W, KAINER K U. Evaluation of the delayed hydride cracking mechanism for transgranular stress corrosion cracking of magnesium alloys[J]. Mater Sci Eng A, 2007, 466: 18-31.
[25] ATRENS A, WINZER N, SONG G L, DIETZEL W, BLAWERT C. Stress corrosion cracking and hydrogen diffusion in magnesium[J]. Advanced Engineering Materials, 2006, 8: 749-751.
[26] BURSLE A J, PUGH E N. Mechanisms of environment sensitive cracking of materials[C]//Materials Society (London), 1977: 471.
[27] CHAKRAPANI D G, PUGH E N. Metallurgical Transactions, 1975, 6A: 1155.
[28] CHAKRAPANI D G, PUGH E N. Corrosion, 1975, 31: 247.
[29] CHAKRAPANI D G, PUGH E N. Metallurgical Transactions, 1976, 7A: 173.
[30] PUGH E H, GREEN J A S, SLATTERY P W. Fracture 1969: The proceedings of the second international conference on fracture[C]// Pratt P L. Chapman and Hall Ltd, London 1969, 387.
[31] EBTEHAJ K, HARDIE D, PARKINS R N. Corrosion Science, 1993, 28: 811.
[32] STAMPELLA R S, PROCTER R P M, ASHWORTH V. Corrosion Science, 1984, 24: 325.
[33] MAKAR G L, KRUGER J, SIERADZKI K. Corrosion Science, 1993, 34: 1311.
[34] ATRENS A, BROSNAN C C, RAMAMURTHY S, OEHLERT A, SMITH I O. Linearly increasing stress test (LIST) for SCC research[J]. Meas Sci Technol, 1993, 4: 1281-1292.
[35] RAMAMURTHY S, ATRENS A. The stress corrosion cracking of As-quenched 4340 and 3.5NiCrMoV steels under stress rate control in distilled water at 90C[J]. Corrosion Science, 1993, 34: 1385-1402.
[36] WANG Z F, ATRENS A. Initiation of stress corrosion cracking for pipeline steels in a carbonate-bicarbonate solution[J]. Metallurgical and Materials Transactions, 1996, 27A: 2686-2691.
[37] SALMOND J, ATRENS A. SCC of copper using the linearly increasing stress test[J]. Scripta Metallurgica et Materialia, 1992, 26: 1447-1450.
[38] ATRENS A, OEHLERT A. Linearly increasing stress test (LIST) of carbon steel in 4N NaNO3 and in Bayer liquor[J]. J Materials Science, 1998, 33: 783-788.
[39] WANG J, ATRENS A. SCC initiation for X65 pipeline steel in “high” pH carbonate/bicarbonate solution[J]. Corrosion Science, 2003, 45: 2199-2217.
[40] WANG J Q, ATRENS A. Analysis of service stress corrosion cracking in a natural gas transmission pipeline, active or dormant[J]. Engineering Failure Analysis, 2004, 11: 3-18.
[41] GAMBOA E, ATRENS A. Material influence on the stress corrosion cracking of rock bolts[J]. Engineering Failure Analysis, 2005, 12: 201-225.
[42] GAMBOA E, ATRENS A. Environmental influence on the stress corrosion cracking of rock bolts[J]. Engineering Failure Analysis, 2003, 10: 521-558.
[43] OEHLERT A, ATRENS A. Stress corrosion crack propagation in AerMet 100[J]. J Mater Sci, 1998, 33: 775-781.
[44] OEHLERT A, ATRENS A. Environmental assisted fracture for 4340 steel in water and air of various humidities[J]. J Mater Sci, 1997, 32: 6519-6523.
[45] OEHLERT A, ATRENS A. The initiation and propagation of stress corrosion cracking in AISI 4340 and 3.5 Ni-Cr-Mo-V rotor steel in constant load tests[J]. Corros Sci, 1996, 38: 1159-1170.
[46] OEHLERT A, ATRENS A. Room temperature creep of high strength steels[J]. Acta Metall Mater, 1994, 42: 1493-1508.
[47] DIETZEL W, SCHWALBE K H. Monitoring stable crack growth using a combined A.C./D.C. potential drop technique[J]. Z Materialprüfung, 1986, 28: 368-372.
[48] WEARMOUTH W R, DEAN G P, PARKINS R N. Role of stress in the stress corrosion cracking of a Mg-Al alloy[J]. Corrosion, 1979, 29: 251-258.
[49] LYNCH S P, TREVENA P. Stress corrosion cracking and liquid metal embrittlement in pure magnesium[J]. Corrosion, 1988, 44: 113-124.
[50] SPEIDEL M O, BLACKBURN M J, BECK T R, FEENEY J A. Corrosion fatigue and stress corrosion crack growth in high strength aluminium alloys[C]//Magnesium Alloys and Titanium Alloys Exposed to Aqueous Solutions. Corrosion Fatigue: Chemistry, Mechanics and Microstructure, NACE-2, 1972: 324-345.
[51] RIECK R M, ATRENS A, SMITH I O. The role of crack tip strain rate in the stress corrosion cracking of high strength steels in water[J]. Met Trans A, 1989, 20A: 889-895.
[52] MILLER W K. Mat Res Soc Symp Proc, 1988, 125: 253.
[53] ATRENS A, WANG Z F. Materials Forum, 1995, 19: 9.
[54] DIETZEL W. Encyclopedia of Materials: Science and Technology. BUSCHOW K H J, CAHN R W, FLEMINGS M C, ILSCHNER B, KRAMER E J, MAHAJAN S, Eds. Elsevier Science Ltd., Amsterdam, 2001, 8883.
Corresponding author: A. Atrens: E-mail: a.atrens@minmet.uq.edu.au
(Edited by CHEN Ai-hua)

