
Structural characteristics of BaTiO3 films prepared by microarc oxidation
LI Wen-fang(李文芳), HAN Bing(韩 冰), DU Jun(杜 军), PENG Ji-hua(彭继华), GAO Yin-hui(高引慧)
College of Mechanical Engineering, South China University of Technology, Guangzhou 510640, China
Received 20 October 2005; accepted 2 April 2006
Abstract: BaTiO3 ferroelectric films were prepared on titanium substrate by microarc oxidation(MAO) technology. The effects of current density and electrolytic concentration on chemical composition, crystal phase, and surface morphologies of the films were characterized by XRD, SEM and EDS. The results show that the films made by MAO technology have a two layer structure with a good combinability between them and with the substrate. The inner layer is composed of Ti oxide without Ba, and the outer layer is mainly composed of BaTiO3 and Ti oxide. The compactability of the films decreases and the surface roughness of the films increases with the increase of current density and electrolytic concentration. Films with a high content of primitive tetragonal and end-centered orthorhombic BaTiO3 are obtained in 0.2 mol/L Ba(OH)2 solution with current density of 5 A/cm2 after 18 min.
Key words: microarc oxidation; ferroelectric film; BaTiO3; structural characteristics
1 Introduction
BaTiO3, with a relatively large dielectric constant and unusual electro-optical coefficient, has been widely used in the electronics industry. BaTiO3 ferroelectric films have been proposed for applications such as thin film capacitors, pyroelectric detectors, light modulators, optical switches and dynamic random access memories[1, 2].
Microarc oxidation(MAO) is a new technology to produce ceramic films. Films obtained by MAO treat- ment on valve metals such as Al, Ti and Mg have super hardness, wear resistance and favorable combinability with the substrate in comparison with films prepared by other methods such as classical anodization and plating. MAO technology has been widely used in depositing wear resistance and corrosion resistance coatings on Al, Mg and their alloy in recent years[3-6]. Now the use of MAO technology to produce functional films has been interested[7,8]. As for producing BaTiO3 films, a relative large current density was used only by GNEDENKOV et al[8]. Compared with the other technologies such as sol-gel[9], metal organic chemical vapor deposition[2], pulsed laser deposition[10] and radio-frequency sputtering[11], depositing BaTiO3 films in aqueous solutions by electrochemical deposition[12] and MAO needs a lower deposition temperature (<100 ℃). Tetragonal BaTiO3 can be got directly without annealing after MAO treatment. Furthermore, MAO technology has a relatively higher film deposition rate compared with electrochemical deposition.
In the present study, 2-4 μm thick films are prepared on Ti substrate by MAO treatment. Their microstructure, surface morphologies and chemical composition are analyzed by scanning electron microscopy(SEM) with energy dispersive spectroscope (EDS). Their phase composition is analyzed by X-ray diffraction(XRD). The influence of current density and electrolytic concentration on chemical composition, crystal phase, and surface morphologies of the films is studied.
2 Experimental
The substrates were industrial pure Ti (99.5%) plates. After abraded by 900# emery paper, they were etched and polished in a mixed acid composed of 25% HF and 75% HNO3 (volume fraction), and then washed in distilled water. The power supply was a homemade 42 kW alternating current microarc oxidation equipment whose current is continuously adjustable in the range of 3-60 A, and frequency can be set at 50, 100, 150, 200 and 250 Hz, respectively. The substrate and the wall of a stainless steel container were used as two electrodes, respectively. Three different concentrative Ba(OH)2 aqueous solutions namely 0.1, 0.15 and 0.2 mol/L were used as the electrolyte whose temperatures were controlled in the range of 25-40 ℃ using a chiller. The current density was 1, 2, 3, 4 and 5 A/cm2, respectively, at each concentrative electrolyte. The frequency was 250 Hz. The oxidation time was about 18 min.
XRD analysis was performed using Philips X’ pert MPD diffractometer (Cu Kα1 radiation, λ=1.540 56 ?, monochromator used, 40 kV, 40 mA, scanning velocity 0.04 (?)/min) to identify the phases of the films. The microstructure, surface morphologies and chemical composition of the films were analyzed by SEM (LEO 1530VP) with EDS (OXFORD INCA 300).
3 Results and discussion
3.1 Phase composition of films
Figs.1 and 2 show the phase composition of the films with the change of current density in two different kinds of electrolytic concentrations. The relative intensity of diffraction peaks of BaTiO3 increases and the ones of Ti decrease with the increase of current density, when the electrolytic concentration is small (Fig.1). It is likely that the relative areas of microarc discharge holes, where X-ray can penetrate more easily, decrease with the increase of current density (Figs.3 and 4), and consequently, the relative intensity of diffraction peaks of Ti decreases. The increase of relative intensity of diffraction peaks due to BaTiO3 manifests that the formation ability of BaTiO3 increases with the increase of current density. This can also be seen in Fig.2. There is considerable intensity of diffraction peaks of TiO2 (Tetragonal, JCPDS 83-2243) in the XRD patterns when the electrolytic concentration is small. The presence of TiO2 in the film makes the capabilities of the film bad. No distinct diffraction peaks of TiO2 are observed when the electrolytic concentration is high. Only the diffraction peaks of BaTiO3 and Ti substrate are observed and BaTiO3 is the major phase in the film when the current density and electrolytic concentration are 5 A/cm2 and 0.2 mol/L, respectively. It is reasonable to confirm those data by other methods. When the electrolytic concentration increases, there is more Ba2+ taking part in the reaction and the products tend to be substances with a higher content of Ba. The height of the diffraction base line is high at low diffraction angles, and there are weak intensity diffraction peaks in the XRD patterns, manifesting that there are amorphous phase and crystallite in the films. It is likely that the melted substances, produced in the process of microarc discharge, are cooled rapidly by the aqueous solutions. Some of them do not crystallize in the cooling process. The structure of Ti substrate is primitive hexagonal (JCPDS 05-0682). And the BaTiO3 in the films are composed of several phases, they are mainly primitive tetragonal (JCPDS 74-1965) and end-centered orthor- hombic (JCPDS 81-2199), which is also manifested by the separation of the diffraction peaks of BaTiO3 in XRD patterns[13]. It is likely that the electrolyte temperature increases with the increase of oxidation time, resultantly, the cooling rate decreases. Moreover, the molten substance produced in the microarc discharge has a higher cooling rate at the surface layer contacted with the electrolyte than in the under layer. The difference of cooling rate leads to different phase formed in the films[3,14]. The relative intensities of the BaTiO3 peaks with respect to those of the Ti substrate increase with the increase of current density when it changes in the range of 2-5 A/cm2.
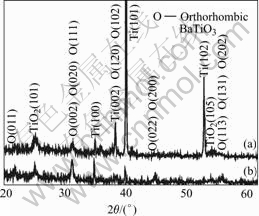
Fig.1 XRD patterns of BaTiO3 film prepared in 0.1 mol/L Ba(OH)2 solution: (a) 2 A/cm2; (b) 5 A/cm2
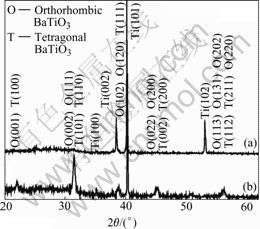
Fig.2 XRD patterns of BaTiO3 film prepared in 0.2 mol/L Ba(OH)2 solution: (a) 2 A/cm2; (b) 5 A/cm2
3.2 Microstructure of BaTiO3 films
Figs.3 and 4 show SEM micrographs of surface morphologies of the films. The films are composed of solidified molten substances, and those substances combine into a compact whole without distinct boundaries when the current density is small. While they are loose when the current density is large. The surface roughness of the film increases with the increase of electrolytic concentration. This manifests that the combinability between those solidified molten substances gets worse with the increase of current density and electrolytic concentration. There are holes from hundreds nanometer to several microns in the film. They are the channels of microarc discharge. Most of the holes are filled partially, and their size and uniformities decrease with the increase of current density. It may be due to that the increased current density in the process of discharge makes the splash in microarc molten region increase and those destroyed region can not be healed completely[3-5]. The defaults such as holes and sparseness form in the film. The amount of those defaults increases with the increase of current density and electrolytic concentration, resultantly, the surface roughness of the film increases and the density of the ceramic layer decreases. The defaults may be affected by the ones on the surface of the substrate. There are small granules stuck on the film surface that can be removed easily. The amount of those small granules increases with the increase of current density and the decrease of electrolytic concentration, and they connect with each other when the current density and electrolytic density are 5 A/cm2 and 0.1 mol/L, respectively. The composition of those small granules is 2.3% Ba, 36%-39%Ti and 58-62%O (mole fraction) analyzed by EDS. Additionally, there are extended cracks in the films caused by thermal stress, while the molten substance is cooled in the electrolyte. Most of the cracks are across the center of the discharge hole. The amount of the cracks decreases with the increase of the current density, while their width increases with the increase of the current density. The result is similar to the one of MAO treated alumina alloy in Ref.[15].
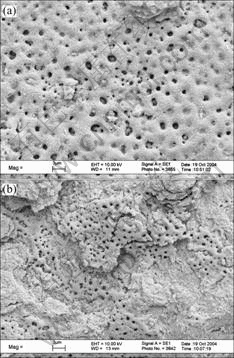
Fig.3 SEM micrographs of surface morphologies of films prepared in 0.1 mol/L Ba(OH)2 solution: (a) 3 A/cm2; (b) 5 A/cm2
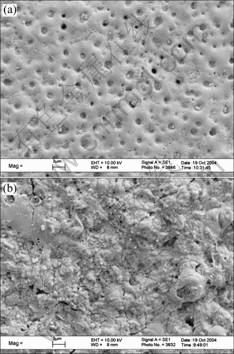
Fig.4 SEM micrographs of surface morphologies of films prepared in 0.2 mol/L Ba(OH)2 solution: (a) 3 A/cm2; (b) 5 A/cm2
Fig.5 shows a BES micrograph of cross-section of the film formed in the conditions of 0.2 mol/L Ba(OH)2 and 2 A/cm2. From the graph we can see that the film contains two layers, a uniform inside layer of hundreds nanometer thickness and a compact outer layer of 2-4 μm. Most of discharge holes in the outside layer are blind whose depths are shallow. The change of thickness of the films has no obvious relationship with the change of technical parameters. There are no obvious boundary between the substance and the inner layer, and the inner layer and the outer layer, which manifests that they combine well with each other. MAO treated films can achieve metallurgic connection with the substance as a whole.

Fig.5 BSE micrograph of cross-section morphology of film: I Resin; II Outer layer; III Inner layer; IV Ti substrate
The outer layer film is composed of 15.93%Ba, 32.76%Ti and 51.31%O (mole fraction) analyzed by EDS element mapping when the current density and electrolytic concentration are 5 A/cm2 and 0.2 mol/L, respectively. Besides BaTiO3, there are small amounts of Ti oxides in the outer layer, consulting with the result of XRD analysis. The inner layer film is composed of 43.29%Ti and 56.71%O analyzed by EDS point analysis when the current density and electrolytic concentration are 5 A/cm2 and 0.2 mol/L, respectively. There is no Ba in the inner layer. This manifests that the Ti oxide in the inner layer is both a precursor for the formation of BaTiO3 and a transition layer, which decreases the internal stress between the substrate and the outer layer[16].
4 Conclusions
BaTiO3 films were prepared on titanium substrates by microarc oxidation technology. The current density and electrolytic concentration are important parameters affecting the films’ properties such as chemical composition, phase, microstructure, and surface morphologies. The films are mainly composed of BaTiO3, Ti and its oxide. BaTiO3 mainly presents in the forms of primitive tetragonal and end-centered orthorhombic phases. When the current density and electrolytic concentration are 5 A/cm2 and 0.2 mol/L, respectively, the film have a high content of BaTiO3. Films made by MAO technology have a two layer structure with good combinability between them and with the substrate. The inner layer is composed of Ti oxide without Ba, while the outer layer is composed of BaTiO3 and Ti oxide. The compactness of the film decreases and the surface roughness of the films increases with the increase of current density and electrolytic concentration.
References
[1] MCCORMICK M A, SLAMOVICH E B. Microstructure development and dielectric properties of hydrothermal BaTiO3 thin films [J]. Journal of the European Ceramic Society, 2003, 23: 2143-2152.
[2] ZENG Jian-ming, WANG Hong, WANG Ming, SHANG Su-xia, WANG Zuo, LIN Cheng-lu. Preparation and ferroelectric properties of BaTiO3 thin films by atmospheric-pressure metalorganic chemical vapor deposition [J]. Thin Solid Films, 1998, 322: 104-107.
[3] XUE Wen-bin, DENG Zhi-wei, CHEN Ru-yi, ZHANG Tong-he, MA Hui. Microstructure and properties of ceramic coatings produced on 2024 aluminum alloy by microarc oxidation [J]. Journal of Materials Science, 2001, 36: 2615-2619.
[4] APELFELD A V, BESPALOVA O V, BORISOV A M, DUNKIN O N, GORYAGA N G, KULIKAUSKAS V S, ROMANOVSKY E A, SEMENOV S V, SOUMINOV I V. Application of the particle backscattering methods for the study of new oxide protective coatings at the surface of Al and Mg alloys [J]. Nuclear Instruments and Methods in Physics Research B, 2000, 161-163: 553-557.
[5] XUE Wen-bin, WANG chao, CHEN Ru-yi, DENG Zhi-wei. Structure and properties characterization of ceramic coatings produced on Ti-6Al-4V alloy by microarc oxidation in aluminate solution [J]. Materials Letters, 2002, 52: 435-441.
[6] TENG Min, HE Xiao-dong, LI Yao. Effects of plasma microarc oxidation on mechanical properties of aluminum alloy [J]. Trans Nonferrous Met Soc China, 2005, 15(3): 407-410.
[7] HAN Yong, XU Ke-wei. Structural characterization of micro-arc oxidation formed titanium dioxide films containing Ca and P [J]. Journal of Inorganic Materials, 2001, 16(5): 951-956.
[8] GNEDENKOV S V, GORDIENKO P S, KHRISANFOVA O A, SCOROBOGATOVA T M, SINEBRUKHOV S L. Formation of BaTiO3 coatings on titanium by microarc oxidation method [J]. Journal of Materials Science, 2002, 37: 2263-2265.
[9] MANSO-SILVAN M, FUENTES-COBAS L, MARTIN-PALMA R J, HERNANDEZ-VELEZ M, MARTINEZ-DUART J M. BaTiO3 thin films obtained by sol-gel spin coating [J]. Surface and Coatings Technology, 2002, 151/152: 118-121.
[10] KREUTZ E W, GOTTMANN J. PLD of perovskite coatings for optoelectronics, microelectronics, and microtechnology [J]. Journal of the European Ceramic Society, 2004, 24: 979-984.
[11] LILIANA P, LAURENT C, BERNARD D, BANDET J, IANCULESCU ADELINA. Structural character- ristics of RF-sputtered BaTiO3 thin films [J]. Thin Solid Films, 2001, 389: 43-50.
[12] LU Fu-hsing, WU Chu-tsun, HUANG Chueh-ya. Barium titanate films synthesized by an anodic oxidation-based electrochemical method [J]. Surface and Coatings Technology, 2002, 153: 276-283.
[13] YANG Jue-ming, GUO Zhen-qi. Quantitative analysis of hexagonal to monoclinic in barium feldspar by X-ray diffraction [J]. Journal of Xi’an Institute of Technology, 1998, 18(4): 300-305.
[14] SUNDARARAJAN G, RAMA KRISHNA L. Mechanisms underlying the formation of thick alumina coatings through the MAO coating technology [J]. Surface and Coatings Technology, 2003, 167: 269-277.
[15] LU Li-hong, SHEN De-jiu, WANG Yu-lin. Influence of technical parameters on the properties of micro-arc oxidation film on casted aluminum-silicon alloy [J]. Plating and Finishing, 2001, 23(1): 32-35.
[16] SHANG F, KITAMURA T, HIRAKATA H, KANNO I, KOTERA H, TERADA K. Experimental and theoretical investigations of delamination at free edge of interface between piezoelectric thin films on a substrate [J]. International Journal of Solids and Structures, 2005, 42: 1729-1741.
Foundation item: Project(51412020203JW1609) supported by the Advanced Research Foundation of Weapon Equipment, China
Corresponding author: LI Wen-fang; Tel: +86-20-87110201; Fax: +86-20-87113802; E-mail: mewfli@scut.edu.cn
(Edited by YUAN Sai-qian)