Si purification by solidification of Al-Si melt with super gravity
来源期刊:中国有色金属学报(英文版)2012年第4期
论文作者:李京伟 郭占成 唐惠庆 王志 孙士瞳
文章页码:958 - 963
关键词:超重力;硅;铝硅熔体;凝固;提纯
Key words:super gravity; metallurgical grade Si; Al?Si melt; solidification; purification
摘 要:研究超重力场下铝硅过共晶熔体凝固精炼提纯冶金硅。实验结果表明:超重力作为一种强化分离手段,可以实现铝硅合金中初晶硅颗粒的富集分离。在超重力作用下,铝硅合金中精炼硅颗粒沿超重力方向富集在铝硅合金下部。用王水溶解其中的铝,得到初晶硅颗粒。通过分析初晶硅中杂质含量可知,与冶金硅原样相比,精炼后的硅纯度由99.59%提高到99.92%,硼和磷的质量分数分别由8.33×10-6和33.65×10-6降低到5.25×10-6和13.50×10-6,表明该提纯方法可行。
Abstract:
The investigation on purification of metallurgical grade silicon by solidification of hypereutectic Al-Si melt with super gravity as an intensified separation way was carried out. The results indicate that the refined silicon grains are successfully enriched at the bottom of the Al-Si alloy along the direction of super gravity. Then the refined silicon was collected by aqua regia leaching. The purity of the collected silicon is analyzed as 99.92%, which is obviously improved compared with the purity of the metallurgical grade silicon of 99.59%, proving the feasibility of this purification method. Furthermore, the mass fraction of B is reduced from 8.33×10-6 to 5.25×10-6 and that of P from 33.65×10-6 to13.50×10-6.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 958-963
LI Jing-wei1, GUO Zhan-cheng1, TANG Hui-qing1, WANG Zhi2, SUN Shi-tong1
1. State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing,Beijing 100083, China;
2. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received 1 April 2011; accepted 19 November 2011
Abstract: The investigation on purification of metallurgical grade silicon by solidification of hypereutectic Al-Si melt with super gravity as an intensified separation way was carried out. The results indicate that the refined silicon grains are successfully enriched at the bottom of the Al-Si alloy along the direction of super gravity. Then the refined silicon was collected by aqua regia leaching. The purity of the collected silicon is analyzed as 99.92%, which is obviously improved compared with the purity of the metallurgical grade silicon of 99.59%, proving the feasibility of this purification method. Furthermore, the mass fraction of B is reduced from 8.33×10-6 to 5.25×10-6 and that of P from 33.65×10-6 to13.50×10-6.
Key words: super gravity; metallurgical grade Si; Al-Si melt; solidification; purification
1 Introduction
The rapid growth in the photovoltaic industry has caused a shortage of solar grade silicon (SOG-Si) or Si with the required chemical purity for photovoltaic applications, resulting in increased prices [1]. A large source for SOG-Si has been electronic-grade silicon rejected from the electronic industry. However, because it is difficult to secure a steady supply of this material, a low-cost mass production process for SOG-Si is desired.
Several groups have developed promising metallurgical routes such as directional solidification, vacuum melting and oxidation treatment for producing SOG-Si. Directional solidification is an effective way of removing metallic impurities because the segregation coefficients of metals between the solid and liquid silicon are small [2]. Vacuum melting and oxidation treatment are often employed to remove phosphorus [3,4] and boron [5-7]. However, the methods above need very high temperature (>1700 K). So far, a little work has been conducted in alloying metallurgical grade silicon (MG-Si) and then utilizing the fractional crystallization to improve the refining process. Some attempts have been made with copper, aluminum or tin as the alloying element [8-11]. For Cu-Si system, copper has high affinity to a wide range of elements, low activity coefficient and high diffusion coefficient in silicon, which could remove most metallic impurities [8]. But alloying Cu-Si requires high temperature above the Si melting point and it could form complex compound resulting in the difficulty in separating refined silicon from the alloy. For Sn-Si system, the segregation coefficient of boron decreases to 0.038 at 1500 K [10], which is much less than 0.8 at the melting point of silicon. Although the contents of both boron and phosphorus could be reduced to 0.1×10-6, the content of Si in Sn-Si is less than 10% at low temperature, therefore, the recovery of purified silicon would be low for every time alloying treatment. Compared with Cu and Sn, Al-Si alloy is a more effective system for the purification of MG-Si because of its low alloy melting point, high solubility of silicon and single eutectics without complex compound. However, because of the similar densities of primary Si and Al-Si melt, the primary Si grains are distributed in the Al-Si eutectics. It is difficult to separate them under normal gravity. Although acid leaching is a promising method to selectively collect primary Si from the alloy, it would result in considerable loss of aluminum, as well as generation of waste acid. Therefore, it is necessary to make primary Si separated and enriched from the Al-Si alloy for the effective acid leaching process. YOSHIKAWA and MORITA [9,11,12] reported that solidified Si grains were successfully agglomerated in the Al-Si alloy by the use of electromagnetic force under fixed alternating magnetic field. In essence, the reason for such agglomerating effect is temperature gradient together with electromagnetic force. Compared with preceding method, super gravity may control the distribution of different phases, wherein crystals move out from the melt during the solidification process [13-16]. The morphology of crystals would be influenced by super gravity [17]. Based on the above characteristics, the separation efficiency of primary silicon grains could be increased by super gravity to some extent.
In this work, a new method of super gravity plus acid leaching was introduced to the Al-Si solidification refining process for the separation of primary silicon and purification of metallurgical grade silicon. The morphologies of Al-Si alloy and refined silicon before and after super gravity treatment were analyzed.
2 Experimental
2.1 Principle
MG-Si is initially alloyed with aluminum which is used as solvent metal to trap impurities, and later the refined silicon is recovered by super gravity. Al-Si alloy is a typically binary eutectic system. Alloying MG-Si with Al is a process of redistribution of impurities in silicon by taking advantage of the thermodynamic instability of impurity elements in solid silicon at lower temperature, and the segregation ratios of impurity elements between solid Si and Al-Si melt are less than those between solid Si and liquid Si [12]. Therefore, the mass fractions of impurities in primary Si are less than those in MG-Si. In order to obtain primary Si crystal, the proportion of Al-Si should be selected in the hypereutectic region. The primary Si crystal would initially precipitate from liquid alloy when the temperature of Al-Si melt decreases. However, the solidified structure of hypereutectic Al-Si alloy is composed of uniformly distributed needle-like Si crystals and surrounding Al-Si eutectic, which results in the difficult separation of primary silicon and ineffective acid leaching. To intensify the separation, super gravity is introduced into the Al-Si refining process. The primary silicon is separated from the Al-Si melt under super gravity and the refined silicon is obtained after acid leaching.
2.2 Method
The super-gravity field was generated by a centrifugal apparatus in the experiment. Figure 1 shows the sketch of this apparatus. The furnace was fixed into the centrifugal rotor. The dash line represents the furnace in stationary state. The gravity coefficient is calculated as the ratio of super-gravitational acceleration to normal- gravitational acceleration by Eq. (1). The samples were MG-Si and high purity aluminum, and the compositions of which are summarized in Table 1.
 (1)
(1)
where ω is the angular velocity, r/s; N is the rotating speed of the centrifugal, r/min; r is the distance from the centrifugal axis to the sample, m; and g is normal- gravitational acceleration. At N=0, G is equal to 1.
A total of 9 g MG-Si and 16.7 g high purity Al were imposed in a dense graphite crucible with inner diameter of 22 mm and 13 g covering slag. The covering slag was a mixture of 48% sodium chloride and 52% calcium chloride (mass fraction), and both of them were of analytical grade. The temperature of the furnace was raised to 1473 K and kept for 3 h in an Ar atmosphere,and then the centrifugal apparatus was started and adjusted to the specified angular velocity. The apparatus was kept rotating until the sample was cooled to room temperature at a certain cooling rate. The sample was cut into halves along the direction of the super-gravity, and one of the halves was polished and then investigated on a metallurgical microscope. The left one was cut into two parts along perpendicular direction of super-gravity, and the agglomeration part of primary silicon was dissolved with aqua regia to obtain the refined silicon. Then the concentrations of impurities in refined silicon were examined on an inductively coupled plasma optical emission spectrometer (ICP-OES).

Fig. 1 Schematic diagram of experimental centrifugal apparatus
Table 1 Chemical compositions for MG-Si and high purity Al (×10-6)

3 Results and discussion
3.1 Refined silicon distribution
The comparison of the Al-35%Si alloy solidified structure at a cooling rate of 0.5 K/min with gravity coefficients of 1 and 403 is shown in Fig. 2. At G=1, the solidified structure is composed of uniformly distributed needle-like Si crystals and surrounding Al-Si eutectics (Fig. 2(a)). At G=403, the growth of needle-like primary silicon grains is mainly along the direction of super gravity, the alloy appears obvious stratification. The upper part of the alloy is mainly Al-Si eutectics, and the primary Si distributes in the lower part, including a little Al-Si eutectics. The height of sedimented Si layers accounts for half of the total height, which indicates that the separation of primary Si could be realized under super gravity.
Under normal gravity (G=1), the needle-like primary Si could not be separated from the alloy because of narrow density difference between primary Si and Al-Si melt. However, super gravity as an intensified separation way could affect the structure and property of solidified alloy to some extent. Super gravity may control the distributions of different phases, wherein crystals move out from the melt during the solidification process [14]. Moreover, some research confirmed that there was a significant stratification with respect to density difference [14-17]. For Al-35%Si melt system, the density difference between primary silicon and Al-Si melt is narrow, and increasing super gravity coefficient equivalently improves the gravity acceleration of the silicon in the melt. Therefore, the separation of primary Si from Al-Si melt could be realized under the condition of super gravity.

Fig. 2 Effect of gravity coefficient on solidification structures of Al-35%Si alloy: (a) G=1; (b) G=403
3.2 Refined silicon microstructure
Figure 3 shows the longitudinal-section micro- structures of different parts inside the Al-35%Si alloy at a cooling rate of 0.5 K/min under G=403. As mentioned above, Al-35%Si alloy has a distinct stratification. The results show that the upper part is mainly uniform eutectics of Al-Si alloy accompanying with a little primary silicon, and the length of primary silicon is less than 50 μm, as shown in Fig. 3(a). The center part of the alloy is mainly long coarse needle-like primary silicon surrounding by the eutectics of Al-Si alloy, and the average length of needle-like primary silicon is more than 500 μm, as shown in Fig. 3(b). The primary silicon at the bottom of the alloy is short dendrite-like silicon shown in Fig. 3(d). At the edge of the sample, the average length of needle-like primary silicon is less than 500 μm, as shown in Fig. 3(d).

Fig. 3 Longitudinal-section microstructures of Al-35%Si alloy at cooling rate of 0.5 K/min and G=403: (a) OM image; (b)-(e) Enlarged images of zones A, B, C and D in Fig. 3(a), respectively
During the cooling process, the primary silicon crystal is gradual precipitation. Because of certain temperature gradient existed in the Al-Si system, the primary silicon would preferentially precipitate from the edge of the sample close to the crucible wall. Based on the density difference between silicon and Al-Si melt, the primary silicon would move towards the bottom of the Al-Si alloy along the direction of super gravity. Besides, the primary silicon crystals would be influenced by super gravity companying with the movement process. With the increasing distance from the centrifugal axis to the primary silicon crystals, the effect of super gravity on the primary silicon crystals would be larger. When the super gravity increases to a certain degree, the long coarse primary silicon would be interrupted and change into short dendrite-like particles. Furthermore, the viscosity is of particular importance for considering the relationship between the convection and solidification of the melt. Normally, viscosity increases with decreasing temperature, which could also directly affect the movement of primary silicon in the Al-Si melt obviously. During the cooling process, the dendrite-like silicon is initially sedimented at the bottom along the direction of the super gravity. With the increasing viscosity, the movement of primary silicon is restrained and changed into long needle-like primary silicon in the lower part of the alloy, as shown in Fig. 3(b).
3.3 Refined silicon composition
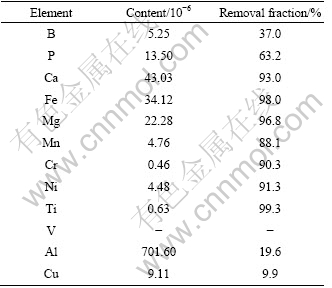
The concentrations of different impurities in the refined silicon together with removal fractions are summarized in Table 2, where the removal fraction is defined from the ratio of the impurity content of refined Si to that of MG-Si. As can be seen, the purity of refined Si at the lower part increases from 99.59% to 99.92%. For refined Si at the lower part, the removal fractions of most metallic impurities, such as Ca, Fe, Mg, Cr, Ni, Ti and V, are higher than 90%, furthermore, the mass fraction of B is reduced from 8.33×10-6 to 5.25×10-6 and that of P from 33.65×10-6 to 13.50×10-6. During the solidification of Al-Si melt with super gravity, needle-like primary silicon is gradually sedimented towards the bottom of the sample, while the eutectic Al-Si phase remains in the above part. The results of refined silicon confirm that the refining of Al-Si alloy with the use of super gravity would improve the purity of MG-Si. Although problems concerning the solidification process to obtain ideal purification remain, the solidification of silicon from the Al-Si melt is effective for the purification with super gravity, especially for most metallic impurities, such as B and P. To achieve further effective purification of metallurgical silicon, much more research is needed.
Table 2 Impurity contents of collected Si and removal fraction after refining test
4 Conclusions
1) For the separation and agglomeration of refined silicon grains solidified from Al-Si melt during the solidification refining, the use of super gravity is found to be effective to obtain agglomerated refined Si grains in the solidified Al-Si alloy.
2) Laboratory scale refining test of the solidification refining of silicon with Al-Si melt is demonstrated using super gravity, and the high purification ability of this refining is confirmed. For the refined primary Si in the lower part, the purity increases from 99.59% to 99.92%, the mass fraction of B reduces from 8.33×10-6 to 5.25×10-6 and that of P from 33.65×10-6 to13.50×10-6.
References
[1] PIZZINI S. Towards solar grade silicon challenges and benefits for low cost photovoltaics [J]. Solar Energy Materials & Solar Cells, 2010, 94: 1528-1533.
[2] YUGE N, HANAZAWA K, KATO Y. Removal of metal impurities in molten silicon by directional solidification with electron beam heating [J]. Mater Trans, 2004, 45: 850-857.
[3] ZHENG Song-sheng, SAFARIAN J, SEOK S, KIM S, MERETE T, LUO Xue-tao. Elimination of phosphorus vaporizing from molten silicon at finite reduced pressure [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 697-702.
[4] PIRES J C S, BRAGA A F B, MEI P R. Profile of impurities in polycrystalline silicon samples purified in an electron beam melting furnace [J]. Solar Energy Materials & Solar Cells, 2003, 79(3): 347-355.
[5] ALEMANY C, TRASSY C, PATEYRON B, LI K I, EDLANNOY Y. Refining of metallurgical-grade silicon by inductive plasma [J]. Solar Energy Materials & Solar Cells, 2002, 72(1-4): 41-48.
[6] NAKAMURA N, BABA H, SAKAGUCHIET Y, KATO Y. Born removal in molten silicon by a steam-added plasma melting method [J]. Materials Transactions, 2004, 45(3): 858-864.
[7] WU Ji-jun, MA Wen-hui, DAI Yong-nian, MORITA K. Boron removal from metallurgical grade silicon by oxidizing refining [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 463-467.
[8] ALEKSANDAR M, Mitra?inovi? T A, Utigard. Refining silicon for solar cell application by copper alloying [J]. Silicon, 2009, 1(4): 239-248.
[9] YOSHIKAWA T, MORITA K. Refining of silicon by the solidification of Si-Al melt with electromagnetic force [J]. ISIJ International, 2005, 45(7): 967-971.
[10] ZHAO Li-xin, WANG Zhi, GUO Zhuan-cheng, LI Cheng-yi. Low-temperature purification process of metallurgical silicon [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1185-1192.
[11] MORITA K, YOSHIKAWA T. Thermodynamic evaluation of new metallurgical refining processes for SOG-silicon production [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 685-690.
[12] YOSHIKAWA T, MORITA K. Refining of silicon during its solidification from a Si-Al melt [J]. Journal of Crystal Growth, 2009, 311(3): 776-779.
[13] VOLKOV M P, GURIN V N, NIKANOROV S P, BURENKOV Y A, DERKACHENKO L I, KARDASHEV B K, REGEL L L, WILCOX W R. Structure and mechanical properties of Al-Si (Ge) alloys upon melt centrifugation and quenching [J]. Physics of the Solid State, 2005, 47(5): 913-919.
[14] XIE Yong, LIU Chang-ming, ZHAI Yan-bo, WANG Kai, LING Xue-dong. Centrifugal casting processes of manufacturing in situ functionally gradient composite materials of Al-19Si-5Mg alloy [J]. Rare Metals, 2009, 28(4): 405-411.
[15] L?FFLER J F, JOHNSON W L. Crystallization of Mg-Al and Al-based metallic liquids under ultra-high gravity [J]. Intermetallics, 2002, 10(11-12): 1167-1175.
[16] GURIN V N, NIKANOROV S P, VOLKOV M P, DERKACHENKO L I, POPOVA T B, KORKIN I V, WILCOX B R, REGEL L L. Crystallization in the Al-Si, Al-Ge, and Al-Si-Ge systems at centrifugation [J]. Technical Physics, 2005, 50(3): 341-346.
[17] LI Ke, WANG Jun, ZHOU Ming, ZHANG Xue-ping, SUN Bao-de, ZHOU Yao-he. Microstructure and properties of in situ hypereutectic Al-Si functional graded materials [J]. The Chinese of Journal Nonferrous Metals, 2002, 12(3): 521-524. (in Chinese)
李京伟1,郭占成1,唐惠庆1,王 志2,孙士瞳1
1. 北京科技大学 钢铁冶金新技术国家重点实验室,北京 100083;
2. 中国科学院 过程工程研究所 湿法冶金清洁生产技术国家工程实验室,北京 100190
摘 要:研究超重力场下铝硅过共晶熔体凝固精炼提纯冶金硅。实验结果表明:超重力作为一种强化分离手段,可以实现铝硅合金中初晶硅颗粒的富集分离。在超重力作用下,铝硅合金中精炼硅颗粒沿超重力方向富集在铝硅合金下部。用王水溶解其中的铝,得到初晶硅颗粒。通过分析初晶硅中杂质含量可知,与冶金硅原样相比,精炼后的硅纯度由99.59%提高到99.92%,硼和磷的质量分数分别由8.33×10-6和33.65×10-6降低到5.25×10-6和13.50×10-6,表明该提纯方法可行。
关键词:超重力;硅;铝硅熔体;凝固;提纯
(Edited by FANG Jing-hua)
Foundation item: Project(51174187) supported by the National Natural Science Foundation of China; Project(2011BAE03B01) supported by the National Technology R & D Program of China
Corresponding author: GUO Zhan-cheng; Tel: +86-10-82375041; E-mail: zcguo@metall.ustb.edu.cn
DOI: 10.1016/S1003-6326(11)61270-3

