J. Cent. South Univ. (2012) 19: 17-21
DOI: 10.1007/s11771-012-0966-9
Effect of heat treatment of Mn-Cu precursors on morphology of dealloyed nanoporous copper
TAN Xiu-lan(谭秀兰)1, LI Kai(李恺)1, NIU Gao(牛高)1, YI Zao(易早)1, 2, LUO Jiang-shan(罗江山)1,
LIU Ying(刘颖)3, HAN Shang-jun(韩尚君)1, WU Wei-dong(吴卫东)1, TANG Yong-jian(唐永建)1
1. Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China;
2. College of Physical Science and Technology, Central South University, Changsha 410083, China;
3. Department of Materials Science and Engineering, Sichuan University, Chengdu 610065, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Nanoporous copper with nano-scale pore size was synthesized by dealloying Mn-Cu precursor alloy using a free corrosion method. The effects of heat treatment of Mn-Cu precursors on alloy phase, morphology and composition of the resultant nanoporous copper were investigated. It is revealed that the compositions distribute homogeneously in the bulk Mn-Cu precursors, which consequently results in a more fully dealloying for forming nanoporous copper. The alloy phase changes from Cu0.49Mn0.51 and Cu0.21Mn0.79 of non-thermally treated precursor to Cu0.33Mn0.67 of heat treated alloy. The residual Mn content in nanoporous copper is decreased from 12.97% to 2.04% (molar fraction) made from the precursor without and with 95 h heat treatment. The typical pore shape of nanoporous copper prepared by dealloying the precursor without the heat treatment is divided into two different zones: the uniform bi-continuous structure zone and the blurry or no pore structure zone. Nanoporous copper is of a uniform sponge-like morphology made from the heat-treated precursor, and the average ligament diameter is 40 nm, far smaller than that from the non-thermally treated precursor, in which the average ligament diameter is estimated to be about 70 nm.
Key words: nanoporous copper; preparation; dealloying; heat treatment; morphology
1 Introduction
Nanoporous metals often display novel physical, chemical, and mechanical properties due to their high surface-to-volume ratio [1-6]. In recent years, extensive studies have been carried out on the dealloying technique, specially in the nanoporous gold made by dealloying the Ag-Au alloy [7-13]. Furthermore, the dealloying technique was applied to preparing other nanoporous metals, like nanoporous platinum [14-15], nanoporous palladium [16], nanoporous titanium [17] and nanoporous copper (Cu) [18]. Some researches indicate that nanoporous Cu prepared by dealloying the bulk exhibits a high yield strength despite the presence of inevitable cracks [18-19]. However, the effect of heat treatment of precursors on the morphology of nanoporous Cu in dealloying was less researched. Research on corrosion dealloying of cold-rolled samples (43%-51% Mn, 3%-5% Al and balance Cu, molar fraction) shows that the initial structure of the bulk precursor plays an important role in the structure of final pores [20]. SEKER et al’s [21] study illustrates that annealing after release, yet prior to dealloying, prevents failure during the selective dissolution step, and nanoporous gold with low residual stress is obtained [21]. The heat treatment of the initial precursor before dealloying may have an influence on the resultant nanoporous structure. In this work, nanoporous Cu with nano-scale pore size was synthesized by dealloying the bulk Mn-Cu precursor using free corrosion method in acid solution. The effects of the heat treatment of the precursors on the structure of bulk Mn-Cu alloy, the morphology of nanoporous Cu and the residual Mn content after dealloying are explored.
2 Experimental
The commercially analytical reagent (AR) Cu (≥99.9%) and AR Mn (≥99.9%) powders were firstly mixed. The mixture was compacted into a d10 mm cylinder. This cylinder was then smelted at 1 573 K and quenched to form a bulk Mn-Cu precursor in a high-frequency induction heating equipment. The smelting process was conducted under the argon-flow protection. The bulk Mn-Cu precursor without or with the heat treatment was mechanically cut into about 200 μm-thick disks. These disks were polished and freely corroded in HCl solution. In the heat treatment, the Mn-Cu bulk was heated for 95 h at 1 253 K in a vacuum quartz tube and quenched in water to room temperature. Before corrosion, HCl was continuously deoxygenated for 30 min with high-purity argon gas. The bulk Mn-Cu disks were corroded in 0.1 mol/L deoxygenated HCl solution for 1-12 d. Afterwards, the disks were diluted in de-ionized water for 1 h to remove the HCl solution. The morphology and the composition of the resultant disks were examined by the field-emission scanning electron microscope (FE-SEM, S-4800 and S440) equipped with an energy dispersive X-ray (EDX) analyzer. The phase structures of the resultant disks were analyzed by X-ray diffractometer (DX2000) with a Cu Kα radiation (λ= 1.540 5 ?).
3 Results and discussion
3.1 Precursor alloy without and with heat treatment
The prototypical metallic alloy system of Mn-Cu for preparing nanoporous Cu using dealloying is employed, because the FCC γ-phase of the Cu-Mn binary alloys at elevated temperatures (700-871 °C) has a continuous solid solution between copper and γ form of manganese. The oxidation potentials of the elemental Mn and Cu (φ0(Cu2+/Cu)=0.34 V, φ0(Mn2+/Mn)=-1.19 V) are different enough to allow the selective removal of Mn constituent. Figure 1 shows the backscattered-electron (BSE) images of the bulk Mn-Cu disk without and with heat treatment. Some inclusions are present on the precursor surface, which are determined to consist mainly of Mn and CuO precipitates as indicated by EDX and XRD. The bulk Mn-Cu precursor without the heat treatment is divided into two different zones, the light-grey zone, x(Cu):x(Mn)=49:51, in Fig. 1(a), and the dark-grey zone, x(Cu):x(Mn)=21:79, in Fig. 1(a), by EDX. The non-thermally treated precursor is composed mainly of the FCC crystal structure of Cu0.49Mn0.51 and Cu0.21Mn0.79 phases as indicated by EDX and XRD. The Cu and Mn components tend to distribute homogeneously so that the color in the BSE image keeps homogeneous in 95 h heat-treated precursor (see Fig. 1(b1)). The heat-treated precursor shows a smoother surface than the non-thermally treated one. Comparison of the XRD in Fig. 1(b2) with Fig. 1(a2) indicates that the heat-treated sample is composed of single Cu0.33Mn0.67 phase. There is some different result from the volatilization of Mn during smelting and heat treatment process.
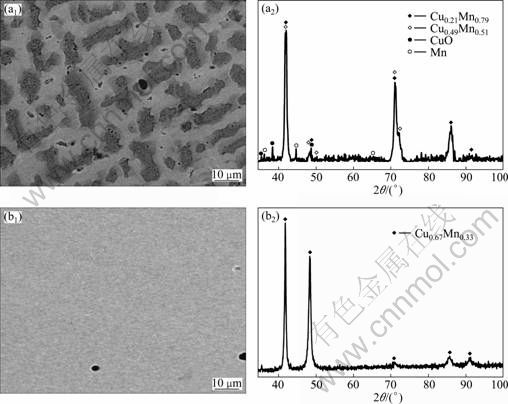
Fig. 1 BSE images (a1, b1) and corresponding XRD patterns (a2, b2) of bulk Mn-Cu precursor without heat treatment (a1, a2) and with heat treatment (b1, b2)
3.2 Nanoporous Cu prepared by dealloying bulk disks without heat treatment
Figure 2 shows the morphologies of nanoporous Cu prepared by dealloying the bulk disks without the heat treatment. The pore shape grows from honeycombs (Fig. 2(a)) to the bi-continuous structure of interconnecting ligaments (Fig. 2(b)) with increasing the corrosion time. The shape formed after 5 d corrosion exhibits uniform ligament morphology with an average ligament diameter of 70 nm. The nanoporous copper has been obtained by dealloying the bulk disks without the heat treatment although this sample is not fully dealloyed. The sample after dealloying the Mn-Cu bulk without the heat treatment is divided into two zones: uniform bi-continuous structure zone (light-grey color in Fig. 2(b)), and the blurry or no pore structure zone (dark-grey color in Fig. 2(b)). The ligament is gradually coarsened with increasing the corrosion time, and there are some granules in the ligament as seen in Fig. 2(c), which may be resulted from the aggregation of the copper atoms.
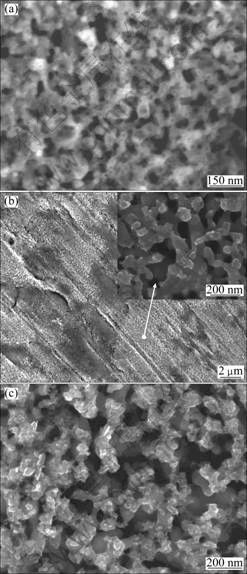
Fig. 2 FESEM images of nanoporous Cu made by corroding non-heat-treated bulk precursor for 2 d (a), 5 d (b) and 12 d (c)
The ligament coarsening is also found in the Ag-Au system [22]. The previous research on the Ag-Au system showed that the compositional threshold (or parting limit) is a necessary condition to induce dealloying [7-8]. For the Au-Ag film with the Au content greater than 44% (molar fraction), the dissolution of Ag is limited. Au atoms aggregate at the partially-dealloyed zones with individual pores randomly distributing on the surface [9-10]. It is the case that the composition distribution in the bulk Mn-Cu precursor is inhomogeneous. The Cu content may be more than the threshold in some zones especially with 49% (molar fraction) Cu atoms (see Fig. 1). Therefore, it might be expected that the selective corrosion is present only at the surface monolayers of these zones to dissolve Mn atoms, resulting in the apparent copper-rich layers at their surfaces. The copper-rich layers prevent Mn atoms from further dissolution. As a result, this sample is not fully dealloyed and more Mn atoms are residual in these nanopores than in those made from the dealloyed Mn-Cu with the heat treatment (Table 1). Table 1 gives the EDX results of nanoporous Cu prepared by corroding the bulk precursor without and with the heat treatment. The residual Mn contents in nanoporous Cu are 12.97% and 2.04% (molar fraction) made from the precursor without and with 95 h heat treatment, respectively. Therefore, it is reasonable that the heat treatment of the precursors can drive the compositions to distribute homogeneously in the bulk Mn-Cu precursor and consequently result in a more sufficient dealloying for forming nanoporous Cu.
Table 1 Composition distributions from EDX of nanoporous Cu synthesized by corroding bulk precursor for 2 d

3.3 Nanoporous Cu prepared by dealloying bulk disks with heat-treatment
Figure 3 shows the FESEM images of nanoporous Cu made from the bulk Mn-Cu precursor with 95 h heat treatment as a function of corrosion time. Comparison of Fig. 3 with Fig. 2 indicates a significant difference in pore morphology made with and without the heat treatment under the same corrosion process. There is no significant morphology evolution process of nanoporous made from the heat-treated precursor with increasing the corrosion time, and there are fewer dark-grey color zones on the sample surface than the one from the non-heat treated precursor. The sponge-like shape of nanoporous phase with an average ligament diameter of 30 nm made from 95 h heat-treated disk is formed by the regular channels and the interconnected ligament after selective corrosion for 1 d (Fig. 3(a)). Free corrosion for 2 d produces nanoporous phases with a uniform sponge-like morphology (Fig. 3(b)). The average diameter of ligaments increases from 30 nm after 1 d, 40 nm after 2 d, to 60 nm after 3 d corrosion (Fig. 3(c)).
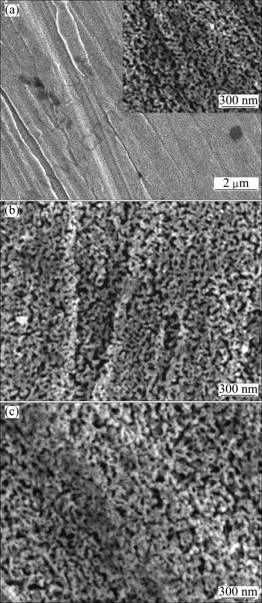
Fig. 3 FESEM images of nanoporous Cu made by corroding heat-treated precursor for different time: (a) 1 d; (b) 2 d; (c) 3 d
4 Conclusions
1) The heat treatment can drive the composition to homogeneously distribute in the Mn-Cu precursor. The non-thermally treated precursor is composed mainly of Cu0.49Mn0.51 and Cu0.21Mn0.79 phases, and the heat treated sample is composed of single Cu0.33Mn0.67 phase. The heat treatment of the precursors could result in a more sufficient dealloying for forming nanoporous Cu. The residual Mn content in nanoporous Cu is decreased from 12.97% to 2.04% (molar fraction) made from the precursor without and with 95 h heat treatment.
2) The heat treatment of the precursors has significant effect on the morphology of nanoporous Cu. The pore shape of nanoporous Cu prepared by dealloying the precursor without the heat treatment is divided into two different zones: the uniform bi-continuous structure zone and the blurry or no pore structure zone. And the pore is gradually coarsened with increasing the corrosion time. There is no striking morphology evolution in the nanoporous copper made from the heat-treated precursor, in which the pores are formed by the regular channels through interconnected ligaments. Correspondingly, the nanoporous Cu is of a uniform sponge-like morphology with an average ligament diameter of 40 nm, far smaller than those in nanoporous Cu from the non-thermally treated precursor, in which the average ligament diameter is estimated to be about 70 nm.
References
[1] DING Y, KIM Y J, ERLEBACHER J. Nanoporous gold leaf: “Ancient technology” [J]. Advanced Materials, 2004, 16(21): 1897-1900.
[2] VOLKERT C A, LILLEODDEN E T, KRAMER D, WEISSM?LLER J. Approaching the theoretical strength in nanoporous Au [J]. Applied Physics Letters, 2006, 89: 061920.
[3] ERTENBERG R W, ANDRAKA B, TAKANO Y. Prospects of porous gold as a low-temperature heat exchanger for liquid and solid helium [J]. Physica B, 2000, 2022: 284-288.
[4] ZIELASEK V, J?RGENS B, SCHULZ C, BIENER J, MONIKA M B, HAMZA A V, B?UMER M. Gold catalyst: Nanoporous gold foams [J]. Angew Chem Int Ed, 2006, 45: 8241-8244.
[5] XU Cai-xia, XU Xiao-hong, SU Ji-xin, DING Y. Research on unsupported nanoporous gold catalyst for CO oxidation [J]. Journal of Catalysis, 2007, 252: 243-248.
[6] ZEIS R, MATHUR A, FRITZ G, LEE J, ERLEBACHER J. Platinum-plated nanoporous gold: An efficient, low Pt loading electrocatalyst for PEM fuel cells [J]. Journal of Power Sources, 2007, 165: 65-72.
[7] ERLEBACHER J, AZIZ M J, KARMA A, DIMITROV N, SIERADZKI K. Evolution of nanoporosity in dealloying [J]. Nature, 2001, 410: 450-453.
[8] ERLEBACHER J, SIERADZKI K. Pattern formation during dealloying [J]. Scripta Materialia, 2003, 49: 991-996.
[9] LU X, BALK T J, SPOLENAKA R, ARZT E. Dealloying of Au-Ag thin films with a composition gradient: Influence on morphology of nanoporous Au [J]. Thin Solid Films, 2007, 515: 7122-7126.
[10] LU X, BISCHOFF E, SPOLENAKA R, BALK T J. Investigation of dealloying in Au-Ag thin films by quantitative electron probe microanalysis [J]. Scripta Materialia, 2007, 56: 557-560.
[11] HAKAMADA M, MABUCHI M. Microstructural evolution in nanoporous gold by thermal and acid treatments [J]. Materials Letters, 2008, 62: 483-486.
[12] SENIOR N A, NEWMAN R C. Synthesis of tough nanoporous metals by controlled electrolytic dealloying [J]. Nanotechnology, 2006, 17, 2311-2316.
[13] PARIDA S, KRAMER S, VOLKERT C A, R?SNER H, ERLEBACHER J, WEISSM?LLER J. Volume change during the formation of nanoporous gold by dealloying [J]. Physics Review Letters, 2006, 97: 035504.
[14] THORP J C, SIERADZKI K, TANG Lei. Formation of nanoporous noble metal thin films by electrochemical dealloying of PtxSi1–x [J]. Applied Physics Letters, 2006, 88: 033110.
[15] PUGH D V, DURSUN A, CORCORAN S G. Formation of nanoporous platinum by selective dissolution of Cu from Cu0.75Pt 0.25 [J]. Journal of Materials Research, 2003, 18(1): 216-221.
[16] MEYERHEIM H L, SOYKA E, KIRSCHNER J. Alloying and dealloying in pulsed laser deposited Pd films on Cu(100) [J]. Physical Review B, 2006, 74: 085405.
[17] BAYOUMI F M, ATEYA B G. Formation of self-organized titania nano-tubes by dealloying and anodic oxidation [J]. Electrochemistry Communications, 2006, 8(1): 38-44.
[18] HAYES J R, HODGE A M, BIENER J, HAMZA A V, SIERADZKI K. Monolithic nanoporous copper by dealloying Mn-Cu [J]. Journal of Materials Research, 2006, 21(10): 2611-2616.
[19] LU H, LI Y, WANG F. Synthesis of porous copper from nanocrystalline two-phase Cu-Zr film by dealloying [J]. Scripta Materialia, 2007, 56: 165-168.
[20] MIN U S, LI J C M. The microstructure and dealloying kinetics of a Cu-Mn alloy [J]. Journal of Materials Research, 1994, 9: 2878-2883.
[21] SEKER E, GASKINS J T, BART-SMITH H, ZHU J, REED M L, ZANGARI G, KELLY R, BEGLEY M R. The effects of annealing prior to dealloying on the mechanical properties of nanoporous gold microbeams [J]. Acta Materialia, 2008, 56: 324-332.
[22] KUCHEYEV S O, HAYES J R, BIENER J, HUSER T, TALLEY E, HAMZA A V. Surface-enhanced Raman scattering on nanoporous Au [J]. Applied Physics Letters, 2006, 89: 053102.
(Edited by YANG Bing)
Foundation item: Project(10804101) supported by the National Natural Science Foundation of China; Project(9140C6805021008) supported by the State Key Development Program for Basic Research of China; Project(2007B08007) supported by the Science and Technology Development Foundation of Chinese Academy of Engineering Physics
Received date: 2010-10-21; Accepted date: 2011-03-17
Corresponding author: TANG Yong-jian, Professor; Tel: +86-816-2484233, E-mail: txl725@tom.com