Development and characterization of hot dip aluminide coated stainless steel 316L
来源期刊:中南大学学报(英文版)2018年第11期
论文作者:Sehrish MUKHTAR Waqas ASGHAR Zubair BUTT Zaheer ABBAS Mudaser ULLAH Rana ATTA-UR-REHMAN
文章页码:2578 - 2588
Key words:stainless steel; aluminide coating; microstructure; intermetallics; nano-indentation; corrosion testing
Abstract: Stainless steel (SS) grade 316L is used for orthopedic implants due to its biocompatibility; yet the effort should be done to minimize the carcinogenic and inflammatory effects related to SS 316L implants. In this research, aluminide coating of Al–Si alloy on SS 316L is characterized by using optical microscopy, energy dispersive spectroscopy (EDS), nano-indentation and corrosion testing technique. Hot dip aluminizing process is used to coat the SS 316L specimens at 765 °C for 2 min immersion time. Half of the specimens are also diffusion treated in a Muffle furnace at 550 °C for 4 h to produce diffused specimens of SS 316L. Microstructural examination shows the formation of flat coating/substrate interface due to Si addition. EDS analysis confirms the formation of complex intermetallic at the coating/substrate interface which finally results in increasing the hardness and corrosion resistance properties of coating.
Cite this article as: Sehrish MUKHTAR, Waqas ASGHAR, Zubair BUTT, Zaheer ABBAS, Mudaser ULLAH, Rana ATTA-UR-REHMAN. Development and characterization of hot dip aluminide coated stainless steel 316L [J]. Journal of Central South University, 2018, 25(11): 2578–2588. DOI: https://doi.org/10.1007/s11771-018-3937-y.

J. Cent. South Univ. (2018) 25: 2578-2588
DOI: https://doi.org/10.1007/s11771-018-3937-y

Sehrish MUKHTAR1, Waqas ASGHAR2, 3, Zubair BUTT4, Zaheer ABBAS2,Mudaser ULLAH5, 6, Rana ATTA-UR-REHMAN2
1. College of Engineering and Emerging Technologies, University of the Punjab, Lahore, Pakistan;
2. University of Engineering and Technology Taxila, 47050, Taxila, Pakistan;
3. Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy ofSciences (CAS), Ningbo 315201, China;
4. Mechatronics Engineering Department, University of Engineering and Technology Taxila,Chakwal Campus, 48800, Chakwal, Pakistan;
5. MOE Key Lab for Liquid-Solid Structure Evolution and Materials Processing,
Institute of Materials Joining, Shandong University, Ji’nan 250061, China;
6. Department of Mechanical Engineering, CET, University of Sargodha, Pakistan
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: Stainless steel (SS) grade 316L is used for orthopedic implants due to its biocompatibility; yet the effort should be done to minimize the carcinogenic and inflammatory effects related to SS 316L implants. In this research, aluminide coating of Al–Si alloy on SS 316L is characterized by using optical microscopy, energy dispersive spectroscopy (EDS), nano-indentation and corrosion testing technique. Hot dip aluminizing process is used to coat the SS 316L specimens at 765 °C for 2 min immersion time. Half of the specimens are also diffusion treated in a Muffle furnace at 550 °C for 4 h to produce diffused specimens of SS 316L. Microstructural examination shows the formation of flat coating/substrate interface due to Si addition. EDS analysis confirms the formation of complex intermetallic at the coating/substrate interface which finally results in increasing the hardness and corrosion resistance properties of coating.
Key words: stainless steel; aluminide coating; microstructure; intermetallics; nano-indentation; corrosion testing
Cite this article as: Sehrish MUKHTAR, Waqas ASGHAR, Zubair BUTT, Zaheer ABBAS, Mudaser ULLAH, Rana ATTA-UR-REHMAN. Development and characterization of hot dip aluminide coated stainless steel 316L [J]. Journal of Central South University, 2018, 25(11): 2578–2588. DOI: https://doi.org/10.1007/s11771-018-3937-y.
1 Introduction
Stainless steel (SS) grade 316L implants are employed in surgical applications because of their advantages like mechanical properties relevant to those of bone material, ease of fabrication and availibility at low cost [1–3]. SS 316L implants are generally used in support or replacement of broken bones, replacement of joints at hip or knee and fixation of hard tissues in dentistry [4, 5]. SS implants after implantation crode in vivo [6] and cause the release of metal ions (e.g., iron (Fe), Cr, and Ni) [7–9] that penetrate into the biological tissues and extracellular body fluids surrounding the implant [10–12]. Afterwards, the released metal ions spread throughout the human body and partially accumulate in distant organs, such as the liver, kidneys and spleen [13–15]. Degradtion products of corrosion cause inflammatory response locally and systemically [16, 17]. Locally, corrosion products are associated with cessation of bone formation/growth, synovitis and loosening of artificial joint implants [18–20] and systemically, these products may cause neoplasms [16, 18].
It has been proved that failure of more than 90% of the SS 316L implants occurs due to significant localised corrosion attack [21]. The severity of corrosion attack increases with the increase of implantation period [1]. Different ceramic coatings have been applied on SS 316L implants, to cope with the problem of localised corrosion attack and to increase the wear properties of these implants [22–24]. Processes like surface alloying [25–27], ion implantation [28], laser melting [29] and physical and chemical vapor deposition have been used by different reserachers to meet the necessary performance requirements of SS 316L implants [30–38].
Surface modification of stainless steel with aluminide layers has gained importance out of all, because of its cost effectiveness and industrial viablity [27, 39–45]. Aluminide coatings improve the erosion–corrosion properties of stainless steels very effeciently [46]. When SS 316L is hot dipped in a molten bath of Al–12.6 wt%Si alloy, it produces Ni-free intermetallic layer on the top of steel [47]. The coating shows good adhesion, besides high hardness and toughness [48], providing a similar corrosion resistance to steel and higher biocompatibility in vitro [49]. Presence of Si in Al–Si melt not only alters the microstructure of the aluminide layer but also transforms the Fe–Al intermetallic compounds into Fe–Al–Si intermetallic compounds [50]. Some researchers have also investigated the formation of permeation barriers by aluminizing [29, 51–53]. Formation of intermetallic aluminide layer not only improves the corrosion resistance of stainless steel implants [46, 54–56], but also reduces the release of metal ions [57].
In this study, biomedical grade SS 316L was coated in Al–Si alloy melts by using hot dip aluminizing process. Depending upon different composition aluminum silicon alloy, three different test specimens were prepared. Finally, different techniques like optical microscopy, energy dispersive spectroscopy (EDS), nano-indentation and corrosion testing were used to characterize the coating.
2 Experimental procedures
The experimental procedure describes the materials used, specimen preparation, coating parameters and characterization techniques.
2.1 Materials
Commercially available SS grade 316L sheet of 2 mm thickness bought from market was used in this research work. Chemical composition of SS grade 316L is shown in Table 1.
Table 1 Chemical composition of SS grade 316L (mass fraction, %) [58]

2.2 Specimen preparation for aluminide coating
Rectangular specimens having dimensions of 15 mm×30 mm were cut from 316L sheet. Schematic diagram of specimen is shown in Figure 1. All the specimens were cleaned by dipping in nitric acid to remove any oil or grease and then washed with tap water followed by cleaning in ethanol.
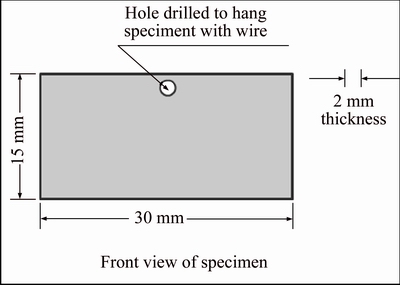
Figure 1 Schematic diagram of SS grade 316L specimen
SS specimens were initially pre-heated at a 700 °C in non-oxidative environment to reduce the effect of strong native oxide, which is present on the surface of SS and inhibits the wetting of coating material. Three different melts of Al–Si alloy with different percentages of silicon (11%, 13% and 15%) were prepared in the laboratory and then temperature of melt was maintained at 750 °C. Al–Si alloy coating was developed on SS 316L specimens by dipping the specimens in molten Al–Si melts for 2 min in reduced atmosphere containing hydrogen gas with the help of nozzle [59].
Afterwards, half of the specimens were diffusion treated in a Muffle furnace at 550 °C for 4 h to produce diffused specimens of SS 316L. Finally, three sets of specimens were produced which are abbreviated as Table 2. Both types of coated specimens (diffused and non-diffused) were finally subjected to different characterization techniques described in the next section.
Table 2 Codes assigned to different specimens prepared

2.3 Specimen preparation for corrosion testing
For corrosion testing, specimens of the size 1 cm2 were cut and then cold mounted in test tubes by using following procedure. A wire attached to corrosion specimen was passed through bent glass tube, as shown in Figure 2. Test tubes were used to hold the corrosion specimens and to encapsulate the attached wire. Position of each specimen was fixed by cold mounting the specimen in mixture of hardener and polyester resin. After cold mounting, specimens were sanded by using sand paper of grade 1000C to remove any remaining impurities (polyester layer) on the surface of test specimens and to establish good electrical connection.
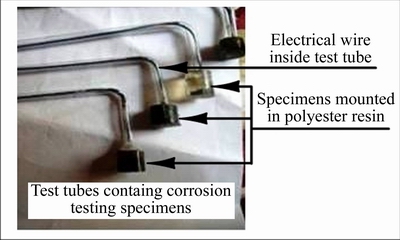
Figure 2 Specimens for corrosion
2.4 Characterization techniques
Following characterization techniques were used to characterize the coating.
2.4.1 Corrosion testing
Polarization (CP) technique using Gamry apparatus was conducted on each specimen to evaluate the corrosion as well as pitting tendency of coated and uncoated SS 316L specimens. Corrosion testing apparatus consists of a reference electrode (calomel), working electrode made of test specimen of SS316L and an auxiliary electrode made of graphite. Ringer solution having pH 7.4 value and a temperature of 37 °C was used as an electrolyte, for the corrosion testing of specimens under simulated body fluid condition. Temperature and pH value of Ringer solution were kept the same as that of human body fluid [60, 61]. Thermostat was used to maintain the temperature of solution. Chemical composition of Ringer solution is shown in Table 3.
Table 3 Chemical composition of Ringer solution (100 mL of water) [62]

Electrochemical cell was developed and clips were attached to auxiliary, working and reference electrodes to provide connection to potentiostat.Before starting the test, all the specimens were allowed to stabilize at open circuit potential for 25 min. After the stabilizing time, all the specimens were cathodically polarized up to 300 mV and polarization curves of coated and uncoated specimens having 15 min and one-week immersion time in Ringer solution were plotted which will be presented in results and discussions section.
2.4.2 Metallography
Initially, cross sections of coated specimens were cut and then sanded by using different grades of sand paper like 180C, 400C, 600C, 800C, 1000C, and 1200C for metallographic analysis. Afterwards, all the specimens were polished by using 1 μm and 6 μm diamond pastes on Teflon cloths. Finally, microstructure of all the specimens was observed under optical microscope of model No. LEICA DFC 500, which will be presented in results and discussions section.
2.4.3 Nano-indentation
Ultra nano-indentation tester provided by CSM instruments, Switzerland was used to perform hardness tests on polished SS 316L specimens by using diamond Berokvich indenter. Ultra nano-indentation tester is shown in Figure 3.

Figure 3 Ultra nano-indentation tester
The most common approach for evaluation of hardness has been developed by OLIVER et al [63]. According to this approach, hardness of coating is given by Eq. (1) where Ac can be found by using Eq. (2). In this study, load applied by ultra nano-indentation tester was ranging from 0 to 300 mN.
 (1)
(1)
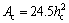 (2)
(2)
Value of plastic depth, hc, in Eq. (2) was calculated by using Eq. (3) [63]. In this research, value of total penetration depth used was h=200 μm. Elastic depth, he, was calculated by using Eq. (4) [63].
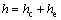 (3)
(3)
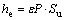 (4)
(4)
In Eq. (4), ε is a constant quantity, which depends on indenter geometry (ε=0.75 for Berkovich indenters). Slope of the unloading load– displacement curve, Su, was calculated by using modified form of Sneddon’s flat punch [64]:
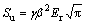 (5)
(5)
The constant β was introduced to correct the non-symmetric effect of Berkovich indenters (β=1.034 used in this study). The constant γ in above mentioned Sneddon’s flat punch equation was introduced by JOSLIN et al [65] to account for deviations from the ideal Sneddon behaviour predicted by finite element investigations. HAY et al [66] proposed the value of constant γ=1.067 for the mechanical testing of those specimens which exhibits Poisson ratio of 0.3 by using Berkovich indenter. Reduced modulus, Er, was calculated by using Eq. (6) [67].
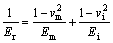 (6)
(6)
where Ei=1141 GPa and vi=0.07 [68]. Em=193 GPa and vm=0.3 are the elastic modulus and Poison ratio of the test material (SS 316L).
2.4.4 EDS analysis
EDS analysis was carried out to find out the chemical composition corresponding to nano- indents. EDS analysis provided information about the composition of intermetallics formed and their spread over the coated specimen.
3 Results and discussion
In this research work, aluminide formed on SS 316L has been cahrecterized by using techniques like optical microscopy, energy dispersive spectroscopy (EDS), nano-indentation and corrosion testing. It has been found that brittle coating having excellent adhesion with substrate and exhibiting flat coating/substrate interface was formed due to formation of complex intermetallic. An intermetallic compound formed due to coating inhibits the localized corrosion, which finally limits the corrosion kinetics.
3.1 Optical microscopy
When SS 316L specimens were dipped in three melts of Al–Si alloy, coatings were developed as shown in Figures 4–6. Coating thickness was measured by using optical microscopy.
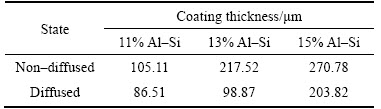
Cross sectional examination of coated specimens confirms the formation of different zones: top layer Al–Si alloy, intermetallics and steel substrate. Flat substrate/coating interface was formed in both diffused and non-diffused coated specimens as observed by other researchers [69]. It is clear from the figures that coating thickness increases as the percentage of the silicon increases. Variation in coating thickness with respect to silicon percentage is shown in Table 4. Diffusion treated specimens give different trends of coating thickness as compared to non-diffused specimens.
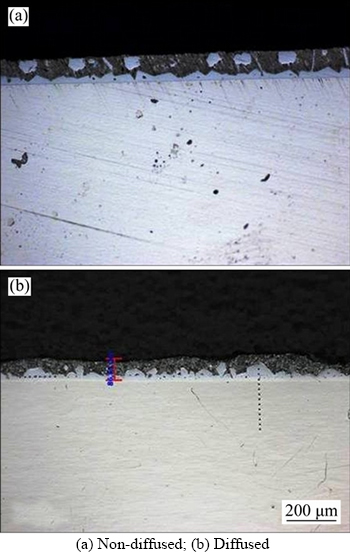
Figure 4 Microstructure of 11% Al–Si specimens:
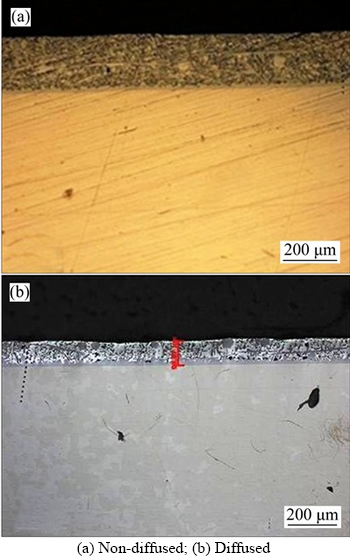
Figure 5 Microstructure of 13% Al–Si specimens:
CHENG et al [50] reported that Al–Si alloy containing 10 wt% silicon is the most common composition used for hot dip aluminizing of steel. Thickness of intermetallic layer developed as a result of aluminizing shows decrease up to 10 wt%, but afterwards, this trend changes and increase in Si content results in thicker intermetallic layer, which resists the localized corrosion attack more efficiently. Table 4 confirms that coating thickness increases in both diffused and non-diffused specimens, with the increase of Si percentage. Non-diffused specimens have shown superior behaviour in terms of coating thickness as compared to diffused specimen. The maximum coating thickness is achieved in 15% Al–Si specimens (both diffused and non-diffused), that’s why only these specimens are further characterized in this research.
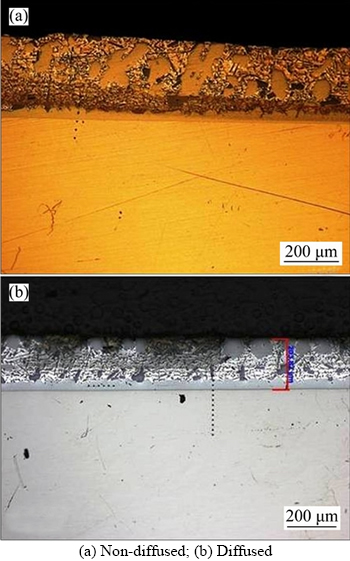
Figure 6 Microstructure of 15% Al–Si specimens:
Table 4 Coating thickness with respect to silicon percent
3.2 EDS analysis
EDS analysis of non-diffused specimen was carried out to find the variation of chemical composition with coating thickness. Figure 7 shows all the points where the EDS was carried out, while details of intermetallic formed at specified locations are presented in Table 5.

Figure 7 SEM micrograph of non-diffused 15% Al–Si specimen
Table 5 Intermetallics formed at specified locations

Table 5 confirms that iron and chromium present in steel, migrating more outward towards the coating as compared to inward diffusion of aluminum towards the steel substrate as shown in Figure 7. Si acts as a diffusion limiting agent for Fe and Cr. High electron affinity of Si towards Fe is responsible for the diffusion limiting behaviour of Si as reported by EGGELER et al [70]. Step by step moving to equilibrium composition close to coating/substrate interface confirms the reduction of coating growth rate. It is also evident that increase in the silicon percentage of coating enhances the coating thickness but at the same time Si hinders the diffusion of the aluminum inside the lattice. Same behaviour has been reported by AWAN et al [69]. Detailed study of the nano-mechanical properties of coating and their correlation with microstructure is explained in the following section.
3.3 Nano-indentation
Nano-indentation was performed on coated specimens with a Berkovich intender. A nano scale close view of indentation of diffused and non- diffused 15% Al–Si specimen is shown in Figures 8 and 9. After indentation, crack was developed only in the case of non-diffused specimen and it is absent in diffused specimen.

Figure 8 Nano-indentation close view of non-diffused 15% Al–Si specimen

Figure 9 Nano-indentation close view of diffused 15% Al–Si specimen
It is evident from the comparison of both figures that relatively soft intermetallic was developed in the case of diffused specimen. Crack developed in non-diffused specimen runs along the parallel direction in this phase only and does not penetrate into the metal or in the Al–Si coating. Development of crack in non-diffused specimen confirms the formation of more brittle coating in non-diffused specimen which will finally increase the hardness of coating.Silicon addition plays an important role in this increase of hardness as reported by FRUTOS et al [47] and this increases the capability of orthopedic implants to bear the maximum stresses especially at knee and hip joints.
3.4 Hardness testing
Figure 10 shows the hardness profile of diffused and non-diffused 15% Al–Si alloy specimens. Hardness of coatings of both specimens initially increases to a maximum value and then decreases and attains almost a constant trend. When indenter touches the specimen, first of all, it touches the top surface of coating layer. Coating layer of Al–Si alloy exhibits low hardness as compared to intermetallic zone, that’s why initial hardness value is very small. When indenter moves onward, it comes in contact with intermetallic zone at coating/ steel interface, due to which hardness increases and finally it decreases and attends almost a constant trend when indenter comes in contact with base metal. It is evident from Figure 10 that the maximum hardness of 11.4 GPa is observed in the intermetallic zone, which has been also reported by another researchers [71–74].
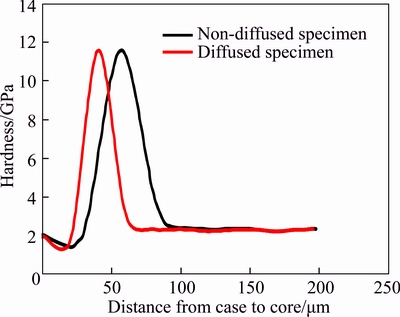
Figure 10 Hardness profile of diffused and non-diffused 15% Al–Si specimen
As discussed earlier, coating thickness of the non-diffused sample is more as compared to the diffused sample thickness, that’s why indenter covers more distance to reach intermetallic zone in the case of non-diffused specimens. Due to this reason, hardness profile of non-diffused specimen is shifted toward right along x-axis and its hardness reaches peak value after covering relatively more case to core distance as compared to diffused samples.
3.5 Corrosion testing
Polarization curves of coated and uncoated specimens of SS 316L after dipping for 15 min in Ringer solution are shown in Figure 11.
Potentiodynamic polarization curves (potential vs current density) of both specimens show passivity in anodic region. This is attributed to the passive behaviour under test conditions of intermetallic steel compound, i.e., Al13Si9Fe2Cr formed at the steel and coating interface.Corrosion response of the material under passive behaviour becomes difficult to evaluate, that’s why it is evaluated by using current density.

Figure 11 Polarization curves of coated and uncoated specimens of SS 316L (after 15 min immersion time in Ringer solution)
Both coated and uncoated specimens show the similar values of current density, i.e., 1.039×10–7 A/cm2 but propensity to localized attack can also be evaluated by the length of passive branch or area enclosed by the loop. Current density technique reveals that uncoated specimen has shown nobler behaviour than that of coated one,i.e., –216 and –425 mV (vs SCE) respectively. The pitting potential (φpit) for the coated specimen is about –155 mV (vs SCE), which is much lower than the uncoated specimen whose value is 693 mV (vs SCE). Therefore, the length of passivity for coated specimen is shorter than the uncoated one, i.e., 580 vs 909 mV, respectively. Therefore, it is confirmed that after a short immersion time of 15 min, coated specimens show higher susceptibility to localized corrosion than uncoated specimen. However, both have the same passivation current density and similar corrosion rate. Polarization curves of both the coated and uncoated specimens after one-week immersion time showed active behaviour, followed by passivation behaviour as shown in Figure 12.
Polarization curves of both specimens plotted after one week show an increase in corrosion potential up to 600 mV. Afterwards, a steady state is attained in both the specimens, which continues until pitting potential is reached. This shape of the curve shows the growth of an oxide layer in active phase.

Figure 12 Polarization curves of coated and uncoated specimens of SS 316L (after one-week immersion time in Ringer solution)
Grown oxide layer is attributed to the passive behaviour in oxidizing environment. It is further noted that the corrosion potential of coated specimen shifts more towards cathodic values with time, i.e., –754 mV (vs SCE) but φcorr of uncoated specimen increases to nobler values with time, i.e., –152 mV (vs SCE) as compared to its previous initial φcorr value. Jcorr values estimated from Tafel slopes, i.e., 7.49×10–9 and 3.29×10–9 A/cm2, for coated and uncoated specimens, are lower by two orders of magnitude as compared to initial Jcorr values for both coated and uncoated specimens. This confirms that with the passage of time, the protective oxide layer gets coarsened and thereby reduces the electrochemical activity. Also, shift of pitting potential (about 1 V) towards nobler values for the coated specimen after 1 week is comparable to the bare steel, i.e., 876 mV (vs SCE). This behaviour depicts a remarkable improvement in propensity of localized corrosion.
Localized corrosion was inhibited due to the formation of passive film formation on both coated and uncoated specimens. Coated SS 316L specimens were immersed in a Ringer solution for one week, and then their intermetallic (Al13Si9Fe2Cr) shifted more towards the nobler values as compared to the intermetallic of specimens having only 15 min immersion time in Ringer solution. Due to this reason, coated specimens having more immersion time showed a superior behaviour and restricted the corrosion kinetics very efficiently. The same behaviour was reported by ARENAS et al [49].
4 Conclusions
In this research work, SS 316L was coated by hot sinking in Al–Si alloy and the developed coating was characterized by using different techniques like optical microscopy, energy dispersive spectroscopy (EDS), nano-indentation and corrosion testing. Following conclusions are drawn from research work.
1) The growth mode of the coating changes with the incorporation of Si and develops the flat substrate/coating interface instead of tongue like structure.
2) High hardness, i.e., 11.4 GPa, is observed in the intermetallic zone due to diffusion of Al–Si alloy towards the trace elements of stainless steel which finally results in the formation of intermetallics.
3) Formation of complex intermetallic compound (Al13Si9Fe2Cr) at coating/substrate interface inhibits the localized corrosion.
4) Decrement of Jcorr values, i.e., 1.039×10–7 to 3.29×10–9 A/cm2, with the passage of time, confirms that intermetallic compound is efficiently limiting the corrosion kinetics.
Acknowledgements
This research was sponsored and funded by Metallurgy and Materials Engineering Department, College of Engineering and Emerging Technologies, University of the Punjab (grant no. PU/ASR&TD/ RG-348 dated 26-1-2012). Alloy was developed in in Foundry Laboratory and specimen preparation was carried out in Advanced Metallography Laboratory of the said department. Mechanical Testing Laboratory was used for mechanical experimentation and corrosion analysis was carried out in Corrosion Laboratory of the said department which is gratefully acknowledged.
Nomenclature
Acronyms
SS
Stainless steel
SEM
Scanning electron microscopy
EDS
Energy dispersive spectroscopy
EIS
Electrochemical impedance spectroscopy
Symbols
φcorr
Corrosion potential
Jcorr
Corrosion current
φpit
Pitting potential
P
Indenter load applied by machine
H
Hardness
Ac
Contact area of Berkovich indenter
h
Total penetration depth
hc
Plastic depth or contact depth
he
Elastic depth
Su
Slope of the unloading load-displacement curve at maximum load
Er
Reduced modulus
Em
Elastic modulus of test material
Ei
Elastic modulus of indenter
vi
Poisson ratio of indenter
vm
Poisson ratio of test material
β
Constant
γ
Correction factor for Sneddon equation
References
[1] CHEW K K, ZEIN S H S, AHMAD A L. The corrosion scenario in human body: Stainless steel 316L orthopaedic implants [J]. Natural Science, 2012, 4(3): 184–188.
[2] MORAIS S, PEREIRA M. Application of stripping voltammetry and microelectrodes in vitro biocompatibility and in vivo toxicity tests of AISI 316L corrosion products [J]. Journal of Trace Elements in Medicine and Biology, 2000, 14(1): 48–54.
[3] SCALES J T, WINTER G, SHIRLEY H. Corrosion of orthopaedic implants [J]. Bone & Joint Journal, 1959, 41(4): 810–820.
[4] CIGADA A, RONDELLI G, VICENTINI B, BRUNELLA F, DALLASPEIIA G. Corrosion behaviour of high nitrogen stainless steels for biomedical applications [C]// Proceedings of the Symposium on Compatability of Biomedical Implants. Sam Francisco, USA: The Electrochemical Society, 1994: 185–195.
[5] SHIRLEY H. Corrosion of orthopaedic implants: screws, plates and femoral nail-plates [J]. J Bone Joint Surg B, 1959, 41: 810–820.
[6] SUTOW E, POLLACK S. Biocompatibility of clinical implant materials I [M]. Boca Raton: CRC Press, 1981: 45–48.
[7] SRIDHAR T, MUDALI U K, SUBBAIYAN M. Preparation and characterisation of electrophoretically deposited hydroxyapatite coatings on type 316L stainless steel [J]. Corrosion Science, 2003, 45(2): 237–252.
[8] GURAPPA I. Development of appropriate thickness ceramic coatings on 316 L stainless steel for biomedical applications [J]. Surface and Coatings Technology, 2002, 161(1): 70–78.
[9] TUKEN T. Polypyrrole films on stainless steel [J]. Surface and Coatings Technology, 2006, 200(16, 17): 4713–4719.
[10] COHEN J. Metal implants—Historical background and biological response to implantation [J]. Biomater Reconstruct Surg, 1979: 46–61.
[11] NAJABAT ALI M, ANSARI U, SAMI J, QAYYUM F, MIR M. To develop a biocompatible and biodegradable polymer- metal composite with good mechanical and drug release properties [J]. J Mater Sci Eng, 2016, 5(5): 274.
[12] BLACK J, MAITIV E C, GELMAN H, MORRIS D M. Serum concentrations of chromium, cobalt and nickel after total hip replacement: A six month study [J]. Biomaterials, 1983, 4(3): 160–164.
[13] PANIGUTTI M, PANIGUTTI M A, MERRITT K, BRUNER R J, KRAAY M J, BROWN S A. Correlation of allergy, metal levels, implant alloy, and implant damage in patients undergoing revision joint arthroplasties [J]. Trans Soc Biomaterials, 1992, 15: 7.
[14] PEREIRA M D C D S. Products in blood filtration organs: An experimental study in mice [D]. Porto, Portugal: Faculty of Engineering, University of Porto, 1998.
[15] MORAIS S, SOUSA J P, FERNANDES M H, CARVALHO G S, de BRUIJN J D, van BLITTERSWIJK C A. Effects of AISI 316L corrosion products in in vitro bone formation [J]. Biomaterials, 1998, 19(11): 999–1007.
[16] PATTERSON S P, DAFFNER R H, GALLO R A. Electrochemical corrosion of metal implants [J]. American Journal of Roentgenology, 2005, 184(4): 1219–1222.
[17] SILVER F, DOILLON C. Biocompatibility [M]// Interactions of Biological and Implantable Materials. New York: VCH Publ Inc, 1989.
[18] JACOBS J J, GILBERT J L, URBAN R M. Corrosion of metal orthopaedic implants [J]. JBJS, 1998, 80(2): 268–282.
[19] KONG H, WILKINSON J L, COE J Y, GU X, URNESS M, KIM T H, BASS J L. Corrosive behaviour of Amplatzer devices in experimental and biological environments [J]. Cardiology in the Young, 2002, 12(3): 260–265.
devices in experimental and biological environments [J]. Cardiology in the Young, 2002, 12(3): 260–265.
[20] FERNANDES M H. Effect of stainless steel corrosion products on in vitro biomineralization [J]. Journal of Biomaterials Applications, 1999, 14(2): 113–168.
[21] SIVAKUMAR M, RAJESWARI S. Investigation of failures in stainless steel orthopaedic implant devices: Pit-induced stress corrosion cracking [J]. Journal of Materials Science Letters, 1992, 11(15): 1039–1042.
[22] GURRAPPA I. Corrosion and its importance in selection of materials for biomedical applications [J]. Corrosion Prevention & Control, 2001, 48(1): 23–37.
[23] FENG B, CHEN J Y, ZHANG X D. Calcium phosphate coating on titanium induced by phosphating in key engineering materials [J]. Trans Tech Publ, 2001, 192: 167–170.
[24] GAO W, LIU Z, LI Z. Nano-and microcrystal coatings and their high-temperature applications [J]. Advanced Materials, 2001, 13(12, 13): 1001–1004.
[25] UHLIG H H, REVIE R W. Corrosion and corrosion control [M]. New York: John Wiley & Sons, 1971: 92–95.
[26] ASGHAR W, NASIR M A, QAYYUM F, SHAH M, AZEEM M, NAUMAN S, KHUSHNOOD S. Investigation of fatigue crack growth rate in CARALL, ARALL and GLARE [J]. Fatigue & Fracture of Engineering Materials & Structures, 2017, 40(7): 1086–1100.
[27] BHUVANESWARAN N, MUDALI U K, SHANKAR P. Characterization of aluminide coatings formed by diffusion alloying on nitrogen-containing type 316L stainless steels [J]. Scripta Materialia, 2003, 49(11): 1133–1138.
[28] AGARWAL P, NATH P, DOERR H J, BUNSHAH R F, KUHLMAN G, KOURY A J. Coatings for the prevention of localized corrosion of M-50 bearing steel [J]. Thin Solid Films, 1981, 83(1): 37–46.
[29] MUDALI U K, BHUVANESWARAN N, SHANKAR P, BALDEV RAJ N. Corrosion behaviour of intermetallic aluminide coatings on nitrogen-containing austenitic stainless steels [J]. Corrosion Science, 2004, 46(12): 2867–2892.
[30] TIWARI S K, MISHRA T, GUNJAN M K, BHATTACHARYYA A S, SINGH T B, SINGH R. Development and characterization of sol–gel silica–alumina composite coatings on AISI 316L for implant applications [J]. Surface and Coatings Technology, 2007, 201(16): 7582– 7588.
[31] QAYYUM F, SHAH M, SHAKEEL O, MUKHTAR F, SALEM M, REZAI-ARIA F. Numerical simulation of thermal fatigue behavior in a cracked disc of AISI H-11 tool steel [J]. Engineering Failure Analysis, 2016, 62: 242–253.
[32] WANG R, MERZ M. Corrosion resistance of amorphous FeNiCrW alloys [J]. Corrosion, 1984, 40(6): 272–280.
[33] MAHAN J E. Physical vapor deposition of thin films [M]. Weinheim: Wiley-VCH, 2000: 336.
[34] WESTWOOD W. Physical vapor deposition in microelectronic materials and processes [M]. Berlin: Springer, 1989: 133–201.
[35] BUTT Z, ANJUM Z, SULTAN A, QAYYUM F, KHURRAM ALI H M, MEHMOOD S. Investigation of electrical properties & mechanical quality factor of piezoelectric material (PZT-4A) [J]. Journal of Electrical Engineering and Technology, 2017, 12(2): 846–851.
[36] HAMPDEN-SMITH M J, KODAS T T. Chemical vapor deposition of metals: Part 1. An overview of CVD processes [J]. Chemical Vapor Deposition, 1995, 1(1): 8–23.
[37] KHAN U A, HUSSAIN A, SHAH M, SHUAIB M, QAYYUM F. Investigation of mechanical properties based on grain growth and microstructure evolution of alumina ceramics during two step sintering process [C]// IOP Conference Series: Materials Science and Engineering. 2016, IOP Publishing.
[38] MEINERT K, UERPMANN C, MATSCHULLAT J, WOLF G K. Corrosion and leaching of silver doped ceramic IBAD coatings on SS 316L under simulated physiological conditions [J]. Surface and Coatings Technology, 1998. 103: 58–65.
[39] XIA Y, PENG D. Review on new technique by ultrasonic hot dip aluminized coating [J]. Transactions of Materials and Heat Treatment (China), 2001, 22(4): 25–30.
[40] ATTA-UR-RAHMAN R, NASIR M A, ULLAH M, PASHA R A, ANJUM N A, MEHMOOD S, MUDDASSAR M, FAROOQI I, ASGHAR W, IMRAN M. Demarcation of fatigue crack cumulative damage (initiation+ stage I) of aluminum alloy under combined loading [J]. Life Science Journal, 2013, 10(12s): 678–683.
[41] BASKES M, BAUER W, WILSON K. Tritium permeation in fusion reactor first walls [J]. Journal of Nuclear Materials, 1982, 111: 663–666.
[42] QAYYUM F, SHAH M, MUQEET A, AFZAL J. The effect of anisotropy on the intermediate and final form in deep drawing of SS304L, with high draw ratios: Experimentation and numerical simulation [C]// IOP Conference Series: Materials Science and Engineering. IOP Publishing, 2016: 012031
[43] ANJUM N, KHAN M, SHAH M, KHALIL M S, PASHA R A, QAYYUM F, ANWAR W. Shear strain model for equal channel angular pressing in high elastic extruded plastics [J]. Nucleus, 2015, 52(4): 169–175.
[44] KHAN F, QAYYUM F, ASGHAR W, AZEEM M, ANJUM Z, NASIR A, SHAH M. Effect of various surface preparation techniques on the delamination properties of vacuum infused Carbon fiber reinforced aluminum laminates (CARALL): Experimentation and numerical simulation [J]. Journal of Mechanical Science and Technology, 2017, 31(11): 5265–5272.
[45] ROBERTS R M, ELLEMAN T S, VERGHESE K. Hydrogen permeability of sintered aluminum oxide [J]. Journal of the American Ceramic Society, 1979, 62(9, 10): 495–499.
[46] MANIVASAGAM G, DHINASEKARAN D, RAJAMANICKAM A. Biomedical implants: corrosion and its prevention—A review [J]. Recent Patents on Corrosion Science, 2010, 2: 40–54.
[47] FRUTOS E, GONZ LEZ–CARRASCO J L, CAPDEVILA C, JIM
LEZ–CARRASCO J L, CAPDEVILA C, JIM NEZ J A, HOUBAERT Y. Development of hard intermetallic coatings on austenitic stainless steel by hot dipping in an Al–Si alloy [J]. Surface and Coatings Technology, 2009, 203(19): 2916–2920.
NEZ J A, HOUBAERT Y. Development of hard intermetallic coatings on austenitic stainless steel by hot dipping in an Al–Si alloy [J]. Surface and Coatings Technology, 2009, 203(19): 2916–2920.
[48] FRUTOS E, GONZ LEZ-CARRASCO J. A method to assess the fracture toughness of intermetallic coatings by ultramicroindentation techniques: Applicability to coated medical stainless steel [J]. Acta Materialia, 2013, 61(6): 1886–1894.
LEZ-CARRASCO J. A method to assess the fracture toughness of intermetallic coatings by ultramicroindentation techniques: Applicability to coated medical stainless steel [J]. Acta Materialia, 2013, 61(6): 1886–1894.
[49] ARENAS M A, FRUTOS E, SALDA A L, CONDE A, LABAJOS-BRONCANO L, GONZ
A L, CONDE A, LABAJOS-BRONCANO L, GONZ LEZ-MART
LEZ-MART N M L, GONZ
N M L, GONZ LEZ-CARRASCO J L, VILABOA N. Corrosion behaviour and biocompatibility of a novel Ni-free intermetallic coating growth on austenitic steel by hot dipping in an Al–12.6% Si alloy [J]. Journal of Materials Science: Materials in Medicine, 2011, 22(4): 1005–1014.
LEZ-CARRASCO J L, VILABOA N. Corrosion behaviour and biocompatibility of a novel Ni-free intermetallic coating growth on austenitic steel by hot dipping in an Al–12.6% Si alloy [J]. Journal of Materials Science: Materials in Medicine, 2011, 22(4): 1005–1014.
[50] CHENG W J, WANG C J. Observation of high-temperature phase transformation in the Si-modified aluminide coating on mild steel using EBSD [J]. Materials Characterization, 2010, 61(4): 467–473.
[51] WITTENBERG L. Proceedings: Tritium technology in fission, fusion, and isotopic applications [M]. La Grange Park, IL: American Nuclear Society, 1980.
[52] MUDASERULLAH R P, RAHMAN R, ASGHAR W. Fatigue life estimation of different welding zones of oxy acetylene welded aluminum alloy (AA 5052-H32) [J]. Nucleus, 2013, 50(3): 261–265.
[53] ELAHI H, BUTT Z, EUGNEI M, GAUDENZI P, ISRAR A. Effects of variable resistance on smart structures of cubic reconnaissance satellites in various thermal and frequency shocking conditions [J]. Journal of Mechanical Science and Technology, 2017, 31(9): 4151–4157.
[54] BURROW G, DOWN M, BAGNALL C. Corrosion inhibition experiments in liquid lithium [J]. Journal of Nuclear Materials, 1981, 103: 657–662.
[55] TORTORELLI P F, DE VAN J H, SELLE J E, UPTON H D. Corrosion inhibition in lithium/Ni-bearing alloy systems [C]// Proc. IEEE 8th Symposium on Engineering Problems of Fusion Research. San Francisco, CA. 1979.
[56] QAYYUM, F, ATIF K, ASGHAR A, SHAH M. 3D numerical simulation of thermal fatigue damage in wedge specimen of AISI H13 tool steel [J]. Engineering Fracture Mechanics, 2017, 180: 240–253.
[57] NIELSEN K. Corrosion of metallic implants [J]. British Corrosion Journal, 1987, 22(4): 272–278.
[58] COMMITTEE A I H. ASM handbook: Materials selection and Design: Vol. 20 [M]. Boca Raton: CRC Press. 1997.
[59] BUTT Z, RAHMAN S U, PASHA R A, MEHMOOD S, ABBAS S, ELAHI H. Characterizing barium titanate piezoelectric material using the finite element method [J]. Transactions on Electrical and Electronic materials, 2017, 18(3): 163–168.
[60] AZAR V, HASHEMI B, YAZDI M R. The effect of shot peening on fatigue and corrosion behavior of 316L stainless steel in Ringer’s solution [J]. Surface and Coatings Technology, 2010, 204(21, 22): 3546–3551.
[61] YANG H, YANG K, ZHANG B. Pitting corrosion resistance of La added 316L stainless steel in simulated body fluids [J]. Materials Letters, 2007, 61(4, 5): 1154–1157.
[62] SONGUR M,  ELIKKAN H, G
ELIKKAN H, G KME
KME E F,
E F,  IM
IM EK S A, ALTUN N
EK S A, ALTUN N  , AKSU M L. Electrochemical corrosion properties of metal alloys used in orthopaedic implants [J]. Journal of Applied Electrochemistry, 2009, 39(8): 1259– 1265.
, AKSU M L. Electrochemical corrosion properties of metal alloys used in orthopaedic implants [J]. Journal of Applied Electrochemistry, 2009, 39(8): 1259– 1265.
[63] OLIVER W C, PHARR G M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments [J]. Journal of Materials Research, 1992, 7(6): 1564–1583.
[64] SNEDDON I N. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile [J]. International Journal of Engineering Science, 1965, 3(1): 47–57.
[65] JOSLIN D, OLIVER W. A new method for analyzing data from continuous depth-sensing microindentation tests [J]. Journal of Materials Research, 1990, 5(1): 123–126.
[66] HAY J C, BOLSHAKOV A, PHARR G. A critical examination of the fundamental relations used in the analysis of nanoindentation data [J]. Journal of Materials Research, 1999, 14(6): 2296–2305.
[67] KING R. Elastic analysis of some punch problems for a layered medium [J]. International Journal of Solids and Structures, 1987, 23(12): 1657–1664.
[68] QASMI M, DELOBELLE P. Influence of the average roughness R ms on the precision of the Young’s modulus and hardness determination using nanoindentation technique with a Berkovich indenter [J]. Surface and Coatings Technology, 2006, 201(3): 1191–1199.
[69] AWAN G H, UL HASAN F. The morphology of coating/substrate interface in hot-dip-aluminized steels [J]. Materials Science and Engineering A, 2008, 472(1): 157–165.
[70] EGGELER G, AUER W, KAESCHE H. On the influence of silicon on the growth of the alloy layer during hot dip aluminizing [J]. Journal of Materials Science, 1986, 21(9): 3348–3350.
[71] CHEN C L, RICHTER A, THOMSON R. Mechanical properties of intermetallic phases in multi-component Al–Si alloys using nanoindentation [J]. Intermetallics, 2009, 17(8): 634–641.
[72] BUTT Z, PASHA RA, QAYYUM F, ANJUM Z, AHMAD N, ELAHI H. Generation of electrical energy using lead zirconate titanate (PZT-5A) piezoelectric material: Analytical, numerical and experimental verifications [J].Journal of Mechanical Science and Technology, 2016,30(8): 3553–3558.
[73] JIN T, WU M X, TANG K, CHEN L X. Hydrogenation reaction characteristics and properties of its hydrides for magnetic regenerative material HoCu2 [J]. Journal of Central South University, 2016, 23(7): 1564–1568. DOI: 10.1007/ s11771-016-3209-7.
[74] LIU X L, ZHANG N, LI D, LI Z C, YOU W X, ZHANG Q, XIA L B, YANG B. Fuel combustion synthesis and upconversion properties of Yb3+ and Er3+ dual-doped ZrO2 nanocrystals [J]. Journal of Central South University, 2017, 24(10): 2209–2214. DOI: https://doi.org/10.1007/s11771- 017-3629-z.
(Edited by YANG Hua)
中文导读
316 L热浸镀铝不锈钢的研制与表征
摘要:不锈钢316 L因其生物相容性而被应用于骨科种植体,但应尽量减少SS316L种植体可能引起的相关致癌性和炎症效应。本研究采用光学显微镜、能量色散谱(EDS)、纳米压痕和腐蚀测试技术,对SS 316 L上Al–Si合金涂层进行表征。采用热浸镀铝工艺,在765 °C热浸2 min对SS 316 L试样进行热浸镀。一半试样在马弗炉中于550 ℃扩散4 h,得到SS 316 L扩散试样。显微组织观察表明,由于Si的加入,形成了平坦的涂层/基体界面。EDS分析证实涂层/基体界面形成了复杂的金属间化合物,最终提高了涂层的硬度和耐蚀性。
关键词:不锈钢;铝化物涂层;显微组织;金属间化合物;纳米压痕;腐蚀试验
Received date: 2017-08-16; Accepted date: 2018-03-26
Corresponding author: Zubair BUTT, Mechanical MS, Lecturer; E-mail: zubair.butt@uettaxila.edu.pk; ORCID: 0000-0002-9750-5819

