
Crystallization behavior of electroless Co-Ni-B alloy plated in magnetic field in presence of cerium
XUAN Tian-peng(宣天鹏), ZHANG Lei(章 磊), HUANG Qin-hua(黄芹华)
Institute of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China
Received 8 July 2005; accepted 7 November 2005
Abstract: The electrochemical property, chemical composition and crystal structure of electroless Co-Ni-B-Ce alloy plated in general state as well as in magnetic field were studied using potentiometer, plasma emission spectrometer, X-ray diffractometer, transmission electron microscope. The results show that the static potential and polarizability of electroless Co-Ni-B alloy are remarkably improved as the plating is carried out in magnetic field in the presence of a little amount of cerium in plating bath. Because of the action of magnetic field and rare earth element cerium, the boron content in alloy decreases, while cobalt and nickel contents increase. As a result, the amorphous Co-Ni-B alloy transforms to the microcrystalline Co-Ni-B-Ce alloy when the plating is in general state, and the Co-Ni-B alloy makes a crystalline transformation because of the action of magnetic field and rare earth element cerium.
Key words: Co-Ni-B alloy; crystallization behavior; crystalline transformation; crystal structure; cerium; magnetic field
1 Introduction
Electroless Co-Ni-B soft magnetic thin film has the property of high microhardness, wearability and excellent soft magnetism[1, 2], and possesses many advantages such as simple preparation and low cost. It can be used as a new type of soft magnetic film in the electronic industry and computers. However, the bath stability, depositing speed and behaviour reproduction of electroless Co-Ni-B alloy coating are not very ideal. This influences the research and application of the coating directly. In order to solve these problems, rare earth element cerium is added in the electroless Co-Ni-B alloy plating bath to improve the processing property and service performance of the alloy coating[3-5]. On the basis of this experiment, the effect of magnetic field on preparing process and crystal structure of electroless Co-Ni-B-Ce alloy coating is studied. Although magnetic field has obvious effect on the crystallization, the figuration of the alloy as well as the mechanical, physical, electrochemical properties of the material[6-12], no report has been found on the electroless Co-Ni-B alloy plated in magnetic field by far. The electrochemical property, chemical composition and structure of eletroless Co-Ni-B coating under different conditions are presented in this paper. The mechanism of magnetic field and rare earth element cerium effect is also studied.
2 Experimental
The sample material was red copper sheet with the size of 20 mm×5 mm×1 mm. The starting formulation of plating bath was: CoCl2·6H2O 7 g/L; NiCl2·6H2O 3 g/L; Na2C4H4O6·2H2O 60 g/L; Na2B4O4·10H2O 4 g/L; NaBH4 1.0 g/L; Ce 0.8 g/L, temperature 20-25℃; pH value 10; loadage 0.4 dm2/L. Rare earth element cerium was added into plating bath in the form of chloride.
Electroless Co-Ni-B-Ce plating bath was placed in open magnetic field with a copper sample appended in the middle of two magnetic poles. The line of magnetization was cut vertically by the copper sheet. The magnetic field induction was (400±10) ×10-4 T. The cathode polarization curve of Co-Ni-B-Ce alloy was measured by the electrochemical online system of the potentiometer (type TD3690). The working electrode was pure copper sheet with an area of 1 cm2 and insulated with single side. The material of auxiliary electrode was platinum sheet. The reference electrode was HgO electrode. The scanning speed of the potentiometer was 3.6 mV/s. The chemical composition was determined by ICP/AES (Inductive Coupling Plasma/ Appearance Emission Spectrometre) with the type of Plasma-Spec. The structure was examined by X-ray diffractometry with Dmax/rb revolving anode and H-800 transmission electron microscope.
3 Results and discussion
3.1 Cathode polarization curves of Co-Ni-B-Ce alloys
The cathode polarization curves of Co-Ni-B-Ce alloys are shown in Fig.1. In general state, the static potential of electroless Co-Ni-B alloy is -0.512 V and the polarization curve is very steep with great polarizability (curve a). When the rare earth element cerium is added in plating bath, the static potential of electroless Co-Ni-B-Ce alloy increases to -0.496 V in general state. Moreover, the polarization curve becomes smooth and the polarizability decreases(curve b). In the magnetic field, the static potential of electroless Co-Ni-B-Ce alloy increases even more with the value reaching -0.457 V, then the polarization curve becomes more smooth with the least polarizability(curve c). In the electrode reaction of electrochemical process, the lower the static potential is, the more difficultly the alloy deposits. With the polarizability increasing, the resistance to the reduction and deposition of alloy becomes greater, and the electrode reaction goes on more difficultly[13].
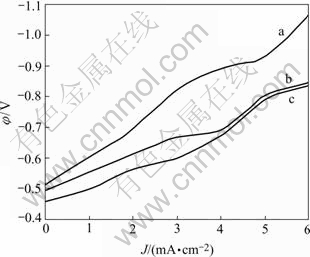
Fig.1 Cathode polarization curves of electroless Co-Ni-B-Ce alloys: (a) Co-Ni-B (in general state); (b) Co-Ni-B-Ce (in general state); (c) Co-Ni-B-Ce(in magnetic field)
The electronic structure of rare earth element cerium atom is [Xe]4f15d16s2. Its 4f electron encloses atomic nuclear imprecisely, so the shielding factor is less than that of other inner electrons with the same principal quantum number. Accordingly cerium atom possesses larger valid nuclear charge number and represents powerful adsorptive capacity to the electrons around it. After being added in electroless Co-Ni-B plating bath, rare earth element cerium with lively character will superiorly be adsorbed on the surface of matrix where crystal defects exist (such as end of dislocation, grain boundary, vacant position). In this way, rare earth element can reduce the surface energy of matrix, accelerate the adsorption of metallic complex ions and reductant ions 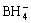 to the surface of metal. At the same time, rare earth element can also accelerate the exchange of electrons on the interface, improve the static potential and decrease the polarizability. Furthermore, the reaction speed of electrode is increased and the reduction ability of electroless Co-Ni-B alloy is also reinforced, so the depositing speed of alloy is promoted remarkably[4].
to the surface of metal. At the same time, rare earth element can also accelerate the exchange of electrons on the interface, improve the static potential and decrease the polarizability. Furthermore, the reaction speed of electrode is increased and the reduction ability of electroless Co-Ni-B alloy is also reinforced, so the depositing speed of alloy is promoted remarkably[4].
With the magnetic field being applied to electroless Co-Ni-B-Ce plating, the solution on the boundary surface moves to the catalyzing surface of the sample. Such phenomenon is called magnetohydrodynamic (MIID). Under the influence of magnetic field, the moving of charged ions to a certain direction accelerates the convection of electroless plating bath, and slight stir action of plating bath occurs. And the thickness of diffusion layer and the concentration polarization are decreased, so the ability of mass transmission in plating bath is improved, and the structure of electrical double layer is changed[6]. Consequently the static potential of alloy increases and the polarizability ameliorates, the discharging, reducing and depositing of metallic ions are facilitated on the surface of matrix.
3.2 Chemical composition of electroless Co-Ni-B-Ce alloy coatings
The chemical composition of the electroless Co-Ni-B-Ce alloy coatings is shown in Table 1. It shows that the boron content decreases obviously, and the cobalt and nickel contents of coatings increase with the increasing of cerium in the coating.
Table 1 Chemical composition of electroless Co-Ni-B-Ce alloy coatings (mass fraction, %)
It is generally believed that rare earth element can not be deposited easily from aqueous solution directly because of the lower electronegativity. But under the inducement effects of the appropriate complexing agent and transition metal, the electrode potential of rare earth element increases in the positive direction while the electrode potential of transition metal increases in the negative direction. Then the reduction and co-deposition of rare earth metal and transition metal can be achieved. Moreover, the rare earth metal ion is one of the few cations which can be characteristically adsorbed on the surface of electrode[14]. After being adsorbed on the surface of matrix, the rare earth metal ion facilitates the adsorption, discharge, reducing and deposition of the metal ions on the surface of the sample. Thus electroless Co-Ni-B alloy coating containing rare earth element cerium is formed.
Rare earth elements have low electronegativity and great activity. In alkaline plating bath, rare earth element cerium easily turns into cation to react as reductant, which can accelerate the reduction of metallic ions and reduce the consumption of reductant NaBH4[4], then the quantity of reduced boron atoms decreases accordingly. When coating contains a little amount of cerium, the boron content decreases, while the cobalt and nickel contents of coating increase.
Under the effect of Lorentz force which is generated by the magnetic field, metal ions move in the direction of line of magnetization[8]. This is useful for the reduction and deposition of the ions of transition metal and rare earth metal. Therefore, the quantity of cerium atoms in Co-Ni-B-Ce coating plated in magnetic field is more than that of coating plated in general state. Accordingly the boron content decreases, while the cobalt and nickel contents of coating plated in magnetic field increase further.
3.3 Crystal structure of electroless Co-Ni-B-Ce alloy coatings
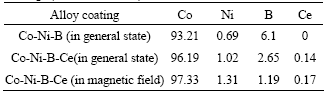
The X-ray diffraction(XRD) patterns of electroless Co-Ni-B-Ce alloy coatings in different conditions are shown in Fig.2. The XRD pattern of electroless Co-Ni-B alloy coating comes forth a diffraction peak with the shape of steamed bread at 2θ=45?, which is the character of amorphous diffraction. In general state, the pattern of electroless Co-Ni-B-Ce coating shows a resemblance with that of electroless Co-Ni-B coating, but the half-width height of its diffraction peak decreases by about 1?. So it presents certain trend to crystallization, which possesses the character of microcrystalline structure. When magnetic field is applied to the plating, electroless Co-Ni-B-Ce coating has come into the structure of crystallization with the presentation of diffraction peaks of Co(100), Co(002), Co(101),Co(110) and so on.
The TEM electron diffraction patterns of electroless Co-Ni-B-Ce alloy coatings under different conditions are shown in Fig.3. From Fig.3(a), it can be seen that the diffraction pattern of electroless Co-Ni-B alloy coating is a broadening diffraction halo, which indicates that such film is amorphous structure. The diffraction pattern of electroless Co-Ni-B-Ce alloy coating in general state (Fig.3(b)) shows that the inner halos are clear and distinct, and the exterior slender rings do not take on the character of broadening. This can be considered micro- crystalline structure. Fig.3(c) shows the diffraction pattern of electroless Co-Ni-B-Ce alloy plated in magnetic field, which is made up of many clear scattered halos. It indicates that the coating transforms to crystalline with the presentation of diffraction halos of Co(100), Co(002), Co(101),Co(110) and so on. The TEM morphologies of electroless Co-Ni-B-Ce alloy coatings in different conditions are shown in Fig.4. From Fig.4(a), it is known that the electroless Co-Ni-B coating shows black and white regions with indistinct borders, taking on the amorphous morphology character. The morphology of electroless Co-Ni-B-Ce alloy coating plated in general state is changed (Fig.4(b)), the borders between strong and weak regions are clear and fine crystal grains can be examined faintly. The clear massive crystalline grains of electroless Co-Ni-B-Ce alloy coating plated in magnetic field are observed in Fig.4(c).
Fig.2 XRD patterns of electroless Co-Ni-B-Ce alloy coatings: (a) Co-Ni-B(in general state); (b) Co-Ni-B-Ce(in general state); (c) Co-Ni-B-Ce(in magnetic field)
The difference between the negativity of cobalt or nickel and that of boron is so great that the interaction between them is intensive. Thus their alloy will form into amorphous state easily. What’s more, the higher the content of the metalloid is, the greater the probability and the stability of forming amorphous state are. At the same time the co-deposition of Ni, Co and B increases the degree that the atoms accumulate randomly, which also enhances the trend of forming amorphous state[15].
After being added in electroless Co-Ni-B plating bath, rare earth element cerium can adsorb the other depositing atoms to the surface. And metallic atoms deposit along the lattice orientation of matrix. Then the three-dimensional epitaxial growth occurs. The regular distribution of atoms and the decrease of boron content for the existence of cerium will make the trend of forming amorphous descend, and it shows some trend of crystallization[16]. When magnetic field is applied to electroless Co-Ni-B-Ce plating process, the contents of Ce, Co and Ni increase further and the B content of electroless Co-Ni-B-Ce coating decreases. In this case the adsorption, reduction and deposition of atoms are significantly affected. This restrains the trend of forming amorphous in alloy coating, so the coating transforms to crystalline from microcrystalline.
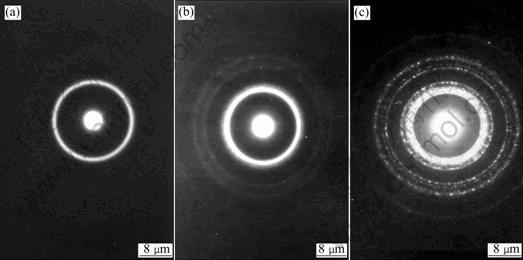
Fig.3 TEM electron diffraction patterns of electroless Co-Ni-B-Ce coatings: (a) Co-Ni-B(in general state); (b) Co-Ni-B-Ce(in general state); (c) Co-Ni-B-Ce(in magnetic field)
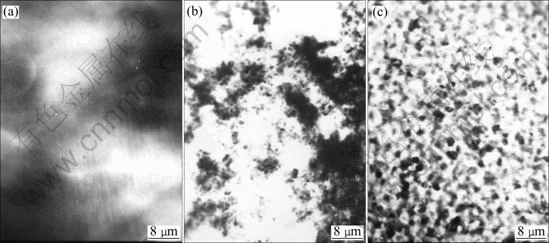
Fig.4 Bright field TEM images of electroless Co-Ni-B-Ce coatings: (a) Co-Ni-B(in general state); (b) Co-Ni-B-Ce(in general state); (c)Co-Ni-B-Ce(in magnetic field)
4 Conclusions
1) When rare earth element cerium is added to plating bath and plating is carried out in magnetic field, the static potential increases evidently and the polarizability of electroless Co-Ni-B alloys decreases, the reduction and deposition of Co-Ni-B alloy is accelerated.
2) In electroless Co-Ni-B-Ce alloys which are plated in general state and in magnetic field, the boron content decreases, and the cobalt and nickel contents of alloy coatings increase with the increasing of cerium content of the alloys.
3)The operating condition and chemical composition of electroless Co-Ni-B-Ce alloy affect the crystalline structure of alloy remarkably. The crystal structure of electroless Co-Ni-B alloy coating in general state is amorphous. The alloy containing rare earth element cerium transforms to microcrystalline state. When magnetic field is applied to the electroless plating process, the electroless Co-Ni-B-Ce alloy transforms to crystalline structure.
References
[1] Osaka T. Co-B based soft magnetic films produced by electroless deposition[J]. J Electrochem Soc, 1992, 139(5): 985-989.
[2] XUAN Tian-peng, ZHANG Lei, HUANG Qin-hua. Effect of rare earth metal on structure and properties of electroless Co-B alloy coating[J]. Journal of Rare Earths, 2002, 20(5): 512-516.
[3] XUAN Tian-peng, HUANG Qin-hua, ZHANG Lei. Effect of rare earth metal on plating technology and structure of electroless Co-B coating[J]. Journal of Rare Earths, 2002, 20(suppl.): 129-132. (in Chinese)
[4] XUAN Tian-peng, ZHANG Lei, HUANG Qin-hua. Modifying behaviors of ultrasonic irradiation and rare earth metal cerium on electroless Co-Ni-B alloy coating[J]. Journal of Rare Earths, 2003, 21(suppl.): 185-189.
[5] ZHANG Lei, XUAN Tian-peng, HUAN Qin-hua. Effect of rare earth elements on the electrochemical behaviour of electroless Co-Ni-B alloy[J]. Electroplating and Finishing, 2002, 21(3): 21-23.(in Chinese)
[6] Matsuda H. Development of an elevtroless CoNiZnP solution for high coercivity films[J]. J Appl Phys, 1993, 23: 1083-090.
[7] Farr J P G, Noshani A A. Some properties of electroless Ni-P, Co-P, and Ni-Co-P deposits[J]. Transactions of the Institute of Metal Finishing, 1996, 74(6): 221-225.
[8] Judgem j s, Morrisonm k r. The effect of the concentration of hypophosphiteion on the magnetic properties of chemically depositing CoP films[J]. Journal of Electrochemical Society, 1999, 109(11): 1040-1044.
[9] Saiyo t, Sato t, Matsuoka m. Effect of heat-treatment on magnetic properties of electroless Ni-B films[J]. Plating & Surface Finishing, 1999, 86(2): 53-56.
[10] Yamasaki t, Tomohira r, Ogino y. Formation of ductile amorphous and nanocrystalline Ni-W alloys by electrodeposition[J]. Plating & Surface Finishing, 2000, 87(5): 148-152.
[11] Matsuda h, Takano o. On a role of zinc in electroless deposited CoNiZnP film with high coercivity[J]. Trans IMF, 1995, 73(4): 147-150.
[12] Chiba a, Gotou t, Kobayashi k. Influence of sonic wave on nickel plating in a nickel sulphate bath[J]. Journal of Applied Electrochemistry, 2000,10(5): 24-28.
[13] LI Di. Theory of Electrochemistry[M]. Beijing: Beijing University of Aeronautics and Astronautics Press, 1999. 193-195.(in Chinese)
[14] XU Guo-bao, YAO Shi-bing, ZOU Shao-ming. Development of study on electrodeposition of rare earth alloy[J]. Materials Protection, 1995, 28(11): 4-6.(in Chinese)
[15] GUO Yi-cheng, WANG Zheng-xi. Physics of Noncrystalline State[M]. Beijing: Science Press, 1984. 84-90.(in Chinese)
[16] XUAN Tian-peng, ZHANG Lei, HUANG Qin-hua. Effect of rare earth lanthanum on crystal structure of electroless Co-Fe-B alloy coating[J]. Transactions of Materials and Heat Treatment, 2003, 24(4): 66-69.(in Chinese)
Foundation item: Project(50371023) supported by the National Natural Science Foundation of China
Corresponding author: XUAN Tian-peng; Tel: +86-551-2903124; E-mail: xtpxm@mail.hf.ah.cn
(Edited by YUAN Sai-qian)