J. Cent. South Univ. Technol. (2009) 16: 0575-0580
DOI: 10.1007/s11771-009-0096-1

Synthesis of modified D401 chelating resin and
its adsorption properties for Pb2+
WANG Fei(王 菲), WANG Lian-jun(王连军), LI Jian-sheng(李健生),
SUN Xiu-yun(孙秀云), ZHANG Liang(张 亮)
(School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China)
Abstract: A novel chelating resin with sulfonic group was synthesized by chemical modification of D401 resin with sulphonation reaction and characterized by FT-IR spectrometry. The adsorption properties of the novel chelating resin for Pb2+ were studied by batch adsorption, and the adsorption process was analyzed from thermodynamics and kinetics aspects. The adsorption mechanism of Pb2+ on the modified D401 chelating resin was discussed by FT-IR spectrometry. Experimental results show that in the Pb2+ concentration range of 200-400 mg/L, the adsorption capacities of the modified D401 chelating resin for Pb2+ increase by 77%-129%, and Langmuir isothermal adsorption model is more suitable for the equilibrium adsorption data. Adsorption is an endothermic process that runs spontaneously. Kinetic analysis shows that the adsorption rate is mainly governed by liquid film diffusion. The best pH value under adsorption condition is 4-5. The saturated resin can be regenerated by 3 mol/L nitric acid, and the adsorption capacity remains stable after five consecutive adsorption-desorption cycles. The maximal static saturated adsorption capacity of the resin is 206 mg/g at 333 K in the Pb2+ concentration range of 200-400 mg/L. The modified D401 chelating resin is an efficient adsorbent for the removal of Pb2+ from its single-metal ion solution.
Key words: modified D401 chelating resin; Pb2+; synthesis; adsorption; desorption
1 Introduction
As industry expands, heavy metals are widely used in many fields, and environmental contamination with heavy metals gains more concern because of their high persistence, nervous system damage, and even cancer, caused by their accumulation at certain levels. The wastewater from mining, painting and printing processes, plumbing, automobile battery and petrochemical industrial contains undesired amounts of Pb2+. In industrial wastewater, Pb2+ concentrations approach 200-500 mg/L [1]. This concentration is very high in relation to water quality standards and is very dangerous to human health. Therefore, many separation methods have been developed for removing Pb2+ from aqueous solution, including precipitation [2-3], biological adsorption [4-6], electrodialysis [7] and resin adsorption [8-9]. Among these technologies, the resin adsorption seems to be the most suitable method for the removal of Pb2+ from aqueous solution because of its low cost, ease of handling and high efficiency. Now, much attention has been focused on chelating resin sorbents due to their stronger coordination for heavy metals [10].
In recent years, to improve the sorption capacity, many new chelating resin sorbents have been synthesized for the removal of heavy metals by using chemical modification method [11-15]. Polymer grafting is the most common synthesis route, and several function groups such as sulfonic group, carboxyl group, amidocyanogen group and aspartate group are grafted on polymers to synthesize the modified chelating resin sorbents for removal and recovery of metal ions. D401 resin is a macroporous styrene chelating resin with diglycolamidic acid as function group, but its adsorption capacity for metal ions is comparatively low. It is therefore necessary to modify D401 resin to increase its adsorption capacity for metal ions and expand its applications.
In the present study, a novel chelating resin with sulfonic group was synthesized by chemical modification of D401 resin with sulphonation reaction, and the influences of experimental parameters such as pH, temperature, initial concentration and contact time on the adsorption of Pb2+ were investigated by using modified D401 chelating resin as adsorbent. The isothermal models and kinetic models used to describe the adsorption data were discussed, and the consecutive adsorption-
desorption procedures were conducted repeatedly to investigate the repeated use capability of the modified D401 chelating resin.
2 Experimental
2.1 Reagents and apparatus
Materials: D401 macroporous chelating resin was provided by the Chemical Plant of Nankai University; lead nitrate, sulfuric acid, and 1, 2-dichloroethane were from Nanjing Chemical Reagent Company; other reagents used in this study were of analytical-reagent grade. The aqueous solutions of lead were prepared by dissolving analytical-reagent grade lead nitrate in deionized water.
Adsorption experiments were carried out in the SHA-B thermostatic vibrator (Changzhou Guohua Appliance Plant, China). The structure of polymer was characterized by MB-154S FTIR spectrometer (Bomem Company, Canada), and solid samples were tested with KBr pressed disc method. The concentrations of Pb2+ were measured by AA-320-CRT atomic absorption spectrometry (Shanghai Analysis Instrument Factory, China). Measurement conditions were as follows: analytical line 228.8 nm, lamp current 5 mA, air flux 0.4 m3/h, ethane flux 0.05 m3/h, bandpass width 0.4 nm.
2.2 Synthesis of modified D401 chelating resin
The preparation of the modified D401 macroporous chelating resin was carried out as follows. 5 g D401 resin was dipped in 1, 2-dichloroethane solution for over 10 h. 20 g sulfuric acid was added in the mixture, and the process was performed unceasingly at 333 K for over 6 h. At the end of the reaction, the mixture was poured into 100 mL acetone solution containing 1% hydrochloric acid with whisking at 313 K for 1 h, and then the polymer was washed by deionized water.
The polymers were filtered and extracted with ethanol in a Soxhlet apparatus for 8 h, and then washed by deionized water, 3% hydrochloric acid, deionized water, 3% sodium hydroxide, and deionized water 3 times alternately. Finally, the polymers were dried under vacuum before being used.
2.3 Batch adsorption experiments
2.3.1 Adsorption isotherm
At three temperatures (298, 313 and 333 K), 100 mL of Pb2+ solutions in the range of 200-400 mg/L were agitated with the modified D401 chelating resin (0.1 g) at the initial pH until the equilibrium was reached (24 h).
The equilibrium adsorption capacity of the resin Qe (mg/g) was calculated by the following equation:
Qe=V(C0-Ce)/m (1)
where V is the volume of solution, L; C0 and Ce are the initial and equilibrium concentrations of Pb2+, respectively, mg/L; and m is the mass of resin, g.
2.3.2 Kinetics studies
0.1 g modified D401 chelating resin was left in contact with 100 mL of Pb2+ solutions (initial concentration is 300 mg/L) at the initial pH and a constant temperature of 298 K. Kinetics of adsorption was determined by analyzing adsorptive uptake of Pb2+ from aqueous solution at different time intervals.
The instantaneous adsorption capacity of the resin Qt (mg/g) was calculated by the following equation:
Qt=V(C0-Ct)/m (2)
where Ct is the instantaneous concentration of Pb2+ at time t, mg/L.
2.3.3 Effect of pH on adsorption
At 298 K, 100 mL of Pb2+ solutions (initial concentration is 300 mg/L) were agitated with the modified D401 chelating resin (0.1 g) at different pH values until the equilibrium was reached (24 h). The pH value of each solution was adjusted with HNO3, and the adsorption capacity Qe was calculated with Eqn.(1).
3 Results and discussion
3.1 Characterization of modified D401 chelating resin
The modified D401 chelating resin was analyzed by FT-IR spectrum. It is found that the absorption peak of bond S=O is formed at 1 045 cm-1, which indicates that sulfonic group is grafted on D401 resin. Other absorption peaks of function group do not make obvious changes, showing that the modified resin reserves intrinsic function group. Moreover, the mass fraction of element S is changed from 0 to 0.85%, which indirectly indicates that sulfonic group is grafted on D401 resin.
The adsorption capacities of the modified D401 chelating resin for Pb2+ increase by 77%-129% compared with that of the original D401 resin in Pb2+ concentration of 200-400 mg/L. The adsorption capability for Pb2+ is obviously increased after D401 resin is chemically modified with sulfonic group, and the modified D401 chelating resin shows an efficient sorbent for the removal of Pb2+ from its single-metal ion solution, which indicates that the modified D401 chelating resin is more suitable for Pb2+ adsorption.
3.2 Effect of pH on adsorption
Fig.1 shows the relationship between pH of the solution and capacity of the modified D401 chelating resin for Pb2+. As seen from Fig.1, it is observed that the adsorption capacity of Pb2+ increases with the increase of the pH of the solution and then decreases with the further

Fig.1 Effect of pH on adsorption of Pb2+
increase of the pH of the solution. In addition, the optimum adsorption pH of the modified D401 chelating resin for Pb2+ is 4-5.
The adsorption capacity decreases with the decrease of pH under pH<4. This can be explained as follows. With the decrease of the solution pH, the concentration of H+ increases and the dissociation degree of exchange group decreases, the exchange capacity between H+ and Pb2+ decreases. Synchronously, the stability of coordination compound is weakened. So the adsorption capacity decreases. When pH>5, the adsorption capacity of Pb2+ decreases because of hydrolyzation.
3.3 Adsorption isotherm
Adsorption isotherm indicates the relationship between equilibrium adsorption capacity and equilibrium concentration at a certain temperature. The adsorption isotherms of Pb2+ on the modified macroporous chelating resin are shown in Fig.2.
It is seen from Fig.2 that the equilibrium adsorption capacities increase with the increase of temperature.
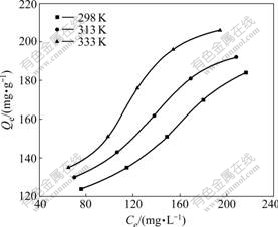
Fig.2 Equilibrium adsorption isotherms of Pb2+ on modified macroporous chelating resin
Higher temperature is favorable for the adsorption process, which indicates that the adsorption is an endothermic process. The equilibrium adsorption capacity increases with the increment of equilibrium concentration in pb2+ concentration range of 200-400 mg/L. The maximal static saturated adsorption capacity of the resin is 206 mg/g at 333 K.
The adsorption isotherms were analyzed by Freundlich-Langmuir isothermal adsorption equations [16-17]. The predigestion isothermal equations are as follows.
Freundlich isothermal adsorption equation:
ln Qe=ln KF+1/n lnCe (3)
Langmuir isothermal adsorption equation:
Ce/Qe=Ce/Qm+1/(KLQm) (4)
where KF and n are the constants of Freundlich isothermal equation, KL and Qm are the constants of Langmuir isothermal equation. The fitting results are listed in Tables 1 and 2, respectively.
Table 1 Correlated results of Freundlich isotherm

Table 2 Correlated results of Langmuir isotherms
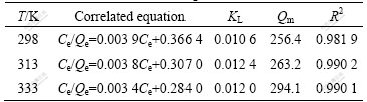
Table 1 shows that all the values of n are larger than 1, indicating that this adsorption is a favorable adsorption process [18-19]. As shown in Table 2, Qm increases with the increment of temperature, and higher temperature is favorable for the increase of the adsorption capability [20]. All the correlation coefficients R2 exceed 0.98, while Langmuir model will be more suitable for the adsorption.
3.4 Adsorption thermodynamic character
At 298, 313 and 333 K, the adsorption thermo- dynamic parameters of Pb2+ on the modified D401 chelating resin are listed in Table 3.
3.4.1 Adsorption enthalpy change
The relationship between adsorption enthalpy ?H and adsorption capacity is closely related, and the isosteric enthalpy can be calculated at a given adsorption capacity with the following equation [21]:
ln Ce=?H/(RT)-ln K0 (5)
Table 3 Thermodynamic parameters of adsorption
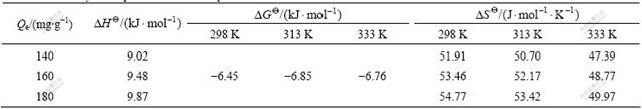
where R is the gas constant, Ce is the equilibrium concentration at a given adsorption capacity, T is the thermodynamic temperature, and K0 is a constant. DH is obtained from the slope of line plotted by ln Ce vs 1/T.
It is seen from Table 3 that the enthalpy changes are positive, which indicates the adsorption is an endothermic process, and higher temperature is favorable to the adsorption process.
3.4.2 Adsorption free energy change
When the adsorption data well fit to Freundlich isothermal equation, DG can be obtained from the following equation [22]:
?G=-nRT (6)
The results show that the adsorption free energy change is negative, which indicates that the process runs spontaneously.
3.4.3 Adsorption entropy change
DS can be obtained from the following equation [22]:
?S=(?H-?G)/T (7)
The entropy change is positive, which shows the adsorption process is impelled by entropy [23].
3.5 Adsorption kinetics character
Using the Boyd liquid film diffusion [24] and the intraparticle diffusion [25] equations to fit the adsorption kinetic data, equations are expressed as follows.
Liquid film diffusion equation:
-ln(1-F)=k1t (8)
Intraparticle diffusion equation:
Qt=k2t0.5 (9)
where F(=Qt/Qe) is the adsorption percentage at time t, k1 and k2 are the rate constants of liquid film diffusion and intraparticle diffusion, respectively, and Qt is the instantaneous adsorption capacity at time t. The fitting results are shown in Figs.3 and 4.
Generally, the diffusion process of adsorption mainly includes the following two steps: (1) diffusion from the liquid film to the particle surface, and (2) diffusion from the surface to the internal site of particle. The adsorption rate is mainly governed by these two diffusion steps.
Fitting results show that the linear relationship
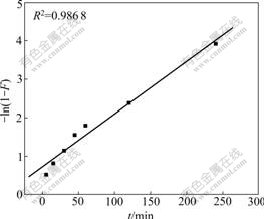
Fig.3 Correlation line of liquid film diffusion
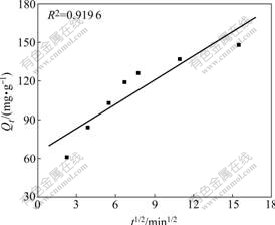
Fig.4 Correlation line of intraparticle diffusion
between -ln(1-F) and t is better (the correlative coefficients are larger than 0.98) than that between Qt and t0.5, which indicates the adsorption rate is mainly governed by liquid film diffusion.
3.6 Desorption and repeated use
The desorption of Pb2+ from the saturated modified D401 chelating resin was carried out using 3 mol/L HNO3 solution at 298 K, initial Pb2+ concentration of 300 mg/L and contact time of 12 h. The consecutive adsorption-desorption procedure was repeated for five cycles to investigate the repeated use capability of the modified D401 chelating resin. The results are listed in Table 4. The adsorption capacities of the resin show a slight decrease and then remain stable. The capacity of Pb2+ after five cycles is 197 mg/g. This shows the modified D401 chelating resin is a good reusable sorbent in removal of Pb2+ from its single-metal ion solution.
Table 4 Adsorption capacities of modified D401 chelating resin

3.7 Adsorption mechanism
The adsorption mechanism of Pb2+ on the modified D401 chelating resin was discussed by FT-IR spectrum. Fig.5 shows the FT-IR spectra of blank and Pb2+ loaded modified D401 chelating resin. It is found that the absorption peak of bond C—N (1 385 cm-1) is strengthened, indicating that atom N combines with Pb2+ by coordination bond. The absorption peak of bond S=O shifts from 1 045 to 1 055 cm-1, showing that H+ ionized by function group —SO3H ionizes exchanges with Pb2+.

Fig.5 FT-IR spectra of blank and Pb2+ loaded modified macro- porous D401 chelating resin
4 Conclusions
(1) A novel chelating resin with sulfonic group is synthesized by chemical modification of D401 resin, and the adsorption of Pb2+ on the modified D401 chelating resin is studied.
(2) The resin shows an efficient adsorbent for the removal of Pb2+ from its single-metal ion solution. The adsorption is influenced by solution pH, initial concentration of Pb2+, temperature and contact time.
(3) The adsorption is an endothermic process that runs spontaneously. Langmuir isothermal adsorption model is more suitable for the equilibrium adsorption data. The adsorption rate is mainly governed by liquid film diffusion, the maximal static saturated adsorption capacity of the resin is 206 mg/g at 333 K in the Pb2+ concentration range of 200-400 mg/L, and the adsorption capacity after five consecutive adsorption-desorption cycles remains stable, showing that the modified D401 chelating resin is a good reusable sorbent for the removal of Pb2+.
References
[1] ?ZACAR M, ?ENGIL ? A, T?RKMENLER H. Equilibrium and kinetic data, and adsorption mechanism for adsorption of lead onto valonia tannin resin [J]. Chemical Engineering Journal, 2008, 143(1/3): 32-42.
[2] ZHENG Rong-guang, ZHANG Li-mei. The research of treatment of wastewater containing lead by dolomite milk [J]. Environment and Exploitation, 2000, 15(4): 35-36. (in Chinese)
[3] GUO Ru-xin. The application of light-burned magnesia and magnesium hydroxide to environmental protection [J]. Environmental Protection of Chemical Industry, 1997, 17(4): 206-210. (in Chinese)
[4] KRATOCHVIL D, VOLESKY B. Advances in the biosorption of heavy metals [J]. Trends Biotechnol, 1998, 16(7): 291-300.
[5] DENG Li-ping, SU Ying-ying, SU Hua, WANG Xin-ting, ZHU Xiao-bin. Sorption and desorption of lead (Ⅱ) from wastewater by green algae cladophora fascicularis [J]. Journal of Hazardous Materials, 2007, 143(1/2): 220-225.
[6] HAN Run-ping, LI Hong-kui, LI Yan-hu, ZHANG Jing-hua, XIAO Hui-jun, SHI Jie. Biosorption of copper and lead ions by waster beer yeast [J]. Journal of Hazardous Materials, 2006, 137(3): 1569-1576.
[7] MAKHLOUFI L, SAIDANI B, HAMMACHE H. Removal of lead ions from acidic aqueous solutions by cementation on iron [J]. Water Research, 2000, 34(9): 2517-2525.
[8] NILCHI A, BABALOU A A, RAFIEE R, KALAL H S. Adsorption properties of amidoxime resins for separation of metal ions from aqueous systems [J]. Reactive and Functional Polymers, 2008, 68(12): 1665-1670.
[9] KOCAOBA S. Comparison of amberlite IR 120 and dolomite’s performances for removal of heavy metals [J]. Journal of Hazardous Materials, 2007, 147(1/2): 488-496.
[10] WANG Shuai, ZHONG Hong, LIU Guang-yi, ZHANG Qian, LI Ting. Synthesis and adsorption properties for Au(Ⅲ) of alkoxycarbonyl thiourea resin [J]. Journal of Central South University of Technology, 2008, 15(4): 463-468.
[11] ATIA A A, DONIA A M, YOUSIF A M. Removal of some hazardous heavy metals from aqueous solution using magnetic chelating resin with iminodiacetate functionality [J]. Separation and Purification Technology, 2008, 61(3): 348-357.
[12] CHEN C Y, LIN M S, HSU K R. Recovery of Cu(Ⅱ) and Cd(Ⅱ) by a chelating resin containing aspartate groups [J]. Journal of Hazardous Materials, 2008, 152(3): 986-993.
[13] DINU M V, DRAGAN E S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters [J]. Reactive and Functional Polymers, 2008, 68(9): 1346-1354.
[14] ZHANG Q, GAO Y, ZHAI Y A, LIU F Q, GAO G. Synthesis of sesbania gum supported dithiocarbamate chelating resin and studies on its adsorption performance for metal ions [J]. Carbohydrate Polymers, 2008, 73(2): 359-363.
[15] WONGKAEW M, IMYIM A, EAMCHAN P. Extraction of heavy metal ions from leachate of cement-based stabilized waste using purpurin functionalized resin [J]. Journal of Hazardous Materials, 2008, 154(1/3): 739-747.
[16] WANG Yu-hua, LAN Ye, HUANG Chuan-bing. Adsorption behavior of Pb2+ and Cd2+ ions on bauxite flotation tailings [J]. Journal of Central South University of Technology, 2008, 15(2): 183-187.
[17] POWELL T, BRION G M, JAGTOYEN M. Investigating the effect of carbon shape on virus adsorption [J]. Environ Sci Technol, 2000, 34(13): 2779-2783.
[18] SALEM I A, EI-MAAZAWI M S. Kinetics and mechanism of color removal of methylene blue with hydrogen peroxide catalyzed by some supported alumina surfaces [J]. Chemosphere, 2000, 41(8): 1173-1180.
[19] CARMO A M, HUNDAL L S, THOMPSON M L. Sorption of hydrophobic organic compounds by soil materials: Application of unit equivalent Freundlich coefficients [J]. Environ Sci Technol, 2000, 34(20): 4363-4369.
[20] WEI Rui-xia, CHEN Lian-long, CHEN Jin-long. Studies of the adsorption thermodynamics and kinetics of lipoic acid on three types of resin [J]. Journal of Functional Polymers, 2004, 17(2): 193-199. (in Chinese)
[21] GARCLA-DELGADO R A, COTOUELO-MINGUEZ L M, RODFIGUEZ J J. Equilibrium study of single-solute adsorption of anionic surfactants with polymeric XAD resins [J]. Sep Sci Technol, 1992, 27(7): 975-987.
[22] LI Ai-min, ZHANG Quan-xing, LIU Fu-qiang, FEI Zheng-hao, WANG Xue-jiang, CHEN Jin-long. Thermodynamic study of adsorption of phenolic compounds on a phenol hydroxyl modified polystyrene [J]. Ion Exchange and Adsorption, 2001, 17(6): 515-525. (in Chinese)
[23] WANG Mu-jun, SUN Yue, ZHOU Wei, FEI Zheng-hao, ZHANG Quan-xing, REN Huai-xing. Study on thermodynamic properties for adsorption of o-phthalic acid from aqueous solution by macroreticular resin [J]. Ion Exchange and Adsorption, 2004, 20(6): 533-540. (in Chinese)
[24] MA Hong-mei, ZHU Zhi-liang, ZHANG Rong-hua, LIN Jian-wei, ZHAO Jian-fu. Kinetics of adsorption of copper from water by weak base epoxy anion-exchange resin [J]. Ion Exchange and Adsorption, 2006, 22(6): 519-526. (in Chinese)
[25] WANG Xue-jiang, ZHANG Quan-xing, LI Ai-min, CHEN Jin-long. Adsorption of salicylic acid from aqueous solution by NDA-100 macroreticular resin [J]. Acta Scientiae Circumstantiae, 2002, 22(5): 658-660. (in Chinese)
(Edited by CHEN Wei-ping)
Foundation item: Project(708049) supported by the Important Item Cultivation Foundation of Scientific Innovation Project of Colleges and Universities of China
Received date: 2008-10-20; Accepted date: 2009-01-09
Corresponding author: WANG Lian-jun, Professor; Tel: +86-25-84315518; E-mail: wanglj@mail.njust.edu.cn