
Corrosion protection of composite coating combining ceramic layer, copper layer and benzotriazole layer on magnesium alloy
JIANG Yong-feng(蒋永锋), BAO Ye-feng(包晔峰), ZHANG Guo-wei(张国伟)
College of Mechanical and Electrical Engineering, Hohai University, Changzhou 213022, China
Received 23 September 2009; accepted 30 January 2010
Abstract: A novel composite coating was fabricated on AZ91 magnesium alloy by applying a composite surface treatment which combined the methods of plasma electrolytic oxidation (PEO) pre-treatment, electroless copper and benzotriazole (BTA) passivation. The cross-section microstructures and chemical compositions of coating were examined using scanning electron microscopy (SEM) equipped with energy dispersive analysis of X-rays (EDX). Potentiodynamic polarization curves and salt spray tests were employed to evaluate corrosion protection of the coating to substrate in 5% NaCl solution. It is indicated that electroless copper produces a rough interface between the electroless copper layer and the ceramic layer. The corrosion potential shifts to the positive direction significantly and the current density decreases by more than one order of magnitude. There is no visible galvanic corrosion pits on the surface of the composite coating combination of PEO and electroless copper after 168 h neutral salt spray testing. The color of copper after BTA immersion could be held more than 60 d.
Key words: plasma electrolytic oxidation; magnesium; electroless copper; corrosion protection
1 Introduction
Magnesium and its alloys possess high specific strength and excellent mechanical properties, good damping capacity and electromagnetic shielding, which can be applied to communication and automotive for environmental protection and energy saving[1-2]. However, corrosion protection is one of the main obstacles to the applications of magnesium alloys in atmosphere environments.
Fortunately, a variety of surface treatments, such as conversion coating, anodizing, plating, laser surface alloying and plasma electrolyte oxidation, were attempted to improve the corrosion resistance of magnesium alloys in previous decades[1-4]. Among various surface treatments, plasma electrolytic oxidation (PEO) has been used for surface treatment of magnesium alloys[5-13]. Previous works[5, 8, 13] revealed that typical porosity level in ceramic coating is below 3%. However, CURRAN and LLYNE[14] established that the high magnification images showed evidence of a network of fine, largely surface-connected pores and the coatings are approximately 20% porous. Thus, an adverse effect of pores in ceramic coating on magnesium alloy is the low corrosion protection. In order to improve corrosion protection, a novel process of electroless Ni-P plating with plasma electrolytic oxidation pretreatment is attempted to modify the ceramic coating with sealing Ni-P coating[15]. However, this method is costly due to high nickel price and heating in electroless Ni-P process. Therefore, a study of electroless copper coatings on magnesium alloys attracted much attention because of such advantages as uniform deposition, good corrosion resistance, good electrical and thermal conductivity, good solderability and decoration for 3C (commu- nication, computer and consumption) products.
A novel composite coating was fabricated to produce pore free copper coatings which provide better corrosion resistance on AZ91 magnesium alloy by applying a composite surface treatment. It combined the methods of plasma electrolytic oxidation (PEO) pre-treatment, electroless copper plating and benzotriazole (BAT) passivation.
2 Experimental
Rectangular specimens with dimensions of 15 mm×10 mm×2 mm were cut from die cast AZ91 magnesium alloy and were mechanically polished by Al2O3 paper up to 1 000 grits. The procedure of the processes was as follows: PEO, activation, electroless copper plating and BTA passivation. Finally, the specimens was rinsed thoroughly with water and dried in hot air. The detailed operation conditions of above processes could be found in Refs.[16-17].
The potentiodynamic polarizations of the magnesium alloy were measured by an electrometer coupled with an amplifier in which the specimen acted as a working electrode. A saturated calomel electrode which acted as reference electrode and a platinum counter electrode are included in the electrochemical cell. In this cell, a 5% NaCl solution was used and the testing was carried out at ambient temperature to obtain polarized specimens data. The salt spray test was carried out for 168 h according to ASTM B117—97 standard.
The cross-section morphologies and chemical compositions of composite coating were examined using an optical microscopy and a XL-30 (PHILIPS) scanning electron microscopy (SEM) equipped with energy dispersive analysis of X-rays (EDX).
3 Results and discussion
3.1 Potentiodynamic polarization
The potentiodynamic polarization curves of the substrate, the substrates with PEO layer and novel composite coating combination of PEO layer, electroless copper layer and BTA passivation layer are shown in Fig.1. It is shown that the substrate with PEO layer exhibits a lower corrosion potential than the substrate only, namely -1 504.8 mV (vs SCE) compared with -1 576 mV (vs SCE) for the latter. An activated controlled cathodic process occurs in the cathodic branch, and the main reaction is hydrogen evolution. Although corrosion potential (φcorr) presents little shift to the
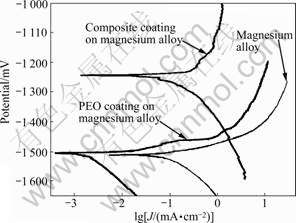
Fig.1 Potentiodynamic polarization curves for AZ91 substrate, PEO coating, composite coatings
positive direction, the polarization current density decreased by three orders of magnitudes under the same corrosion potential for the specimen treated by PEO. This is perhaps that a network of fine, largely surface-connected pores is presented in PEO coating [14].
Regarding the AZ91 alloy with the novel composite coating combination of PEO layer, electroless copper layer and BTA passivation layer, polarization curves shift towards lower corrosion current densities (Jcorr) and exhibit a short period of passive behavior on the anodic branch, indicating that the new electroless copper layer has less porosity and better corrosion resistance than the traditional one. This is further demonstrated by the fact that the corrosion potential shifts to the positive direction by 300 mV. Particularly, there is no any pitting corrosion for the AZ91 alloy with the composite coating combination of PEO layer, electroless copper layer and BTA passivation layer. It is indicated that pores are sealed with the copper layer and BTA passivation layer or the copper is implanted in the pores of PEO layer.
3.2 Salt spray test
There is no visible galvanic corrosion pits on the surface of the composite coating combination of PEO layer, electroless copper layer and BTA passivation layer after 168 h neutral salt spray test, demonstrating that the novel composite coating combining PEO layer, electroless copper layer and BTA passivation layer has less pores than that of the PEO coating. Electroless copper layer is definitely considered superior to magnesium alloy substrate in almost all aqueous media.
3.3 BTA passivation
In order to maintain the color of copper after electroless copper process, passivation was carried out in a bath containing BTA at atmosphere temperature. Specimens with passivation were exposed in ambient atmosphere for 60 d, there was no invisible change in the color of copper on the surface of magnesium alloy combining PEO, electroless copper and BTA passivation, as shown in Fig.2. There is also no pitting corrosion on the surface, indicating that copper layer is covered by BTA layer.
3.4 SEM and EDX analysis
Figs.3 and 4 show cross-section microstructure of composite coatings. It is clearly seen that the traditional electroless copper process produces a rough interface between the electroless copper layer and the ceramic layer due to the very porous outer sublayer of PEO [10-11]. The interface between the ceramic layer and the substrate is relatively smooth and the coating has a more uniform thickness, because the metallurgical intermixing

Fig.2 Macrostructures of copper before passivation (a) and after passivation for 60 d (b)
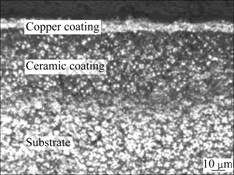
Fig.3 OM images of cross-section in composite coating
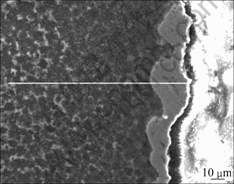
Fig.4 SEM images of cross-section in composite coating
and inter-diffusional bonding generated by the micro- arcing process create a high degree of interfacial adhesion between coating and substrate[13]. The relatively dense inner sublayer is presented.
The EDX spectra collected from polished cross-sections of composite coatings is shown in Fig.5. It is indicated a considerable increase in oxygen content from the copper layer to the ceramic layer, and decrease from outer sublayer to inner sublayer of the ceramic layer, suggesting the ceramic layer should be a combination of aluminum oxides and magnesium oxides modified by other oxide constituents created from components of electrolyte media, such as phosphate. The EDX spectra collected from the underlying magnesium substrate indicate that the inward diffusion of oxygen and copper occurs under the high local temperatures produced by the PEO process and electroless copper process, respectively.
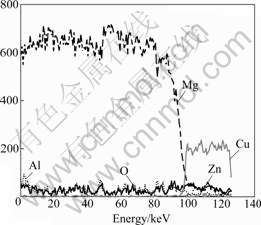
Fig.5 EDX spectra of composite coatings
3.5 Adhesion
Thermal shock test and drawing test were used to evaluate the adhesion of composite coating between substrate and each layer. The specimens were heated to 200 ?C and held for 1 h, then quenched in water at room temperature, which is repeated for 10 times. It is shown that there is no blister or spalling, indicating that the layers are bonded well to each other. It is 0.5 MPa for adhesion between PEO layer and copper layer in the drawing test, implying that the tensile strength for implantation of copper in PEO layer is low.
3.6 Illustration
The formation of clutch and interlocking joint through the implantation of copper in micropores between ceramic layer and copper layer of ceramic coating on magnesium alloy by electroless copper is illustrated in Fig.6. Along with the metallurgical intermixing and inter-diffusional bonding, a high degree of interfacial adhesion between coating and substrate is obtained. Then, the surface of copper layer is passivated in BTA bath. Thus, a composite coating combining PEO layer, copper layer and BTA layer, which exploits the particular advantages of each layer, improves the corrosion resistance and decoration of substrate.

Fig.6 Schematic illustration of composite coating combining ceramic layer, copper layer on magnesium alloy
4 Conclusions
A novel composite process was established with a combination of plasma electrolytic oxidation (PEO), electroless copper coating and BTA passivation. The PEO layer between the electroless copper layer and the substrate acts as an effective barrier and catalytic layer, and provides high density nucleation sites for the subsequent electroless copper layer. The composite coatings combination of PEO layer and electroless copper layer present superior comprehensive properties to PEO coating.
References
[1] CHEN Zhen-hua. Magnesium alloys [M]. Beijing: Chemical Industry Press, 2004: 385. (in Chinese)
[2] AVEDSIAN M M, BAKER H S. Magnesium and magnesium alloys [M]. New York: ASM International, 1999: 138.
[3] EMLEY E F. Principle of magnesium technology [M]. London: Pergamon Press, 1966: 297.
[4] GRAY J E, LUAN B. Protective coating on magnesium and its alloys-A critical review [J]. Journal of Alloys and Compounds, 2002, 336(1/2): 88-113.
[5] ARRABAL R, MATYKINA E, VIEJO F, SKELDON P, THOMPSON G E. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coatings [J]. Corrosion Science, 2008, 50(6): 1744-1752.
[6] LUO Hai-he, CAI Qi-zhou, WEI Bo-kang, YU Bo, LI Ding-jun, HE Jian, LIU Ze. Effect of (NaPO3)6 concentrations on corrosion resistance of plasma electrolytic oxidation coatings formed on AZ91D magnesium alloy [J]. Journal of Alloys and Compounds, 2008, 464(1/2): 537-543.
[7] DUAN Hong-ping, YAN Chuan-wei, WANG Fu-hui. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D [J]. Electrochimica Acta, 2007, 52(11): 3785-3793.
[8] GUPTA P, TENHUNDFELD G, DAIGLE E O, RYABKOV D. Electrolytic plasma technology: Science and engineering—An overview [J]. Surface and Coatings Technology, 2007, 201(21): 8746- 8760.
[9] CHANG Li-min. Growth regularity of ceramic coating on magnesium alloy by plasma electrolytic oxidation [J]. Journal of Alloys and Compounds, 2009, 468(1/2): 462-465.
[10] BLAWERT C, HEITMANN V, DIETZEL W, NYKYFORCHYN H M, KLAPKIV M D. Influence of electrolyte on corrosion properties of plasma electrolytic conversion coated magnesium alloys [J]. Surface and Coatings Technology, 2007, 201(21): 8709-8714.
[11] L? Guo-hua, CHEN Huan, LI Li, NIU Er-wu, PANG Huan, ZOU Bin, YANG Si-ze. Investigation of plasma electrolytic oxidation process on AZ91D magnesium alloy [J]. Current Applied Physics, 2009, 9(1): 126-130.
[12] YEROKHIN A L, NIE X, LEYLAND A, MATTHEWS A, DOWEY S J. Plasma electrolysis for surface engineering [J]. Surface and Coatings Technology, 1999, 122(2/3): 73-93.
[13] GHASEMI A, RAJA V S, BLAWERT C, DIETZEL W, KAINER K U. Study of the structure and corrosion behavior of PEO coatings on AM50 magnesium alloy by electrochemical impedance spectroscopy [J]. Surface and Coatings Technology, 2008, 202(15): 3513-3518.
[14] CURRAN J A, CLYNE T W. Porosity in plasma electrolytic oxide coatings [J]. Acta Materialia, 2006, 54(7): 1985-1993.
[15] LIU Zhen-Min, GAO Wei. A novel process of electroless Ni-P plating with plasma electrolytic oxidation pretreatment [J]. Applied Surface Science, 2006, 253(5): 2988-2991.
[16] LIU Zhen-min, GAO Wei. Electroless nickel plating on AZ91 Mg alloy substrate [J]. Surface and Coatings Technology, 2006, 200(16/17): 5087-5093.
[17] TOSHINOBU O, CHIYOKO E, YUJI S. Plating method of magnesium and magnesium alloy: Japan, JP61067770 [P]. 1986.
(Edited by FANG Jing-hua)
Foundation item: Project(20070420821) supported by the China Postdoctoral Science Foundation; Project(CQ200801) supported by Young Talents Foundation of Changzhou, Jiangsu Province, China
Corresponding author: JIANG Yong-feng; Tel: +86-519-85191894; E-mail: sjtujyf001@sohu.com