Photoelectrochemical characteristics of AB5-type hydrogen storage alloy modified with SrTiO3 photocatalyst
ZHANG Wen-kui(张文魁)1, GAN Yong-ping(甘永平)1, HUANG Hui(黄 辉)1, ZHANG Bo(张 博)2,
WANG Gai-tian(王改田)2, TU Jiang-ping(涂江平)2
(1. Department of Applied Chemistry, Zhejiang University of Technology,
Hangzhou 310014, China;
2. Institute of Materials Science and Chemical Engineering, Zhejiang University, Hangzhou 310027, China)
Abstract: Perovskite-type SrTiO3 powders were prepared by using strontium acetate, tetrabutyl titanate and sodium hydroxide via direct hydrolysis-precipitation process. AB5-type hydrogen storage alloy(HSA) electrodes modified with SrTiO3 powders were prepared and the photoelectrochemical characteristics of the as-prepared electrodes were investigated. The results of cyclic voltammograph show that the current of reduction peak increases remarkably under the light irradiation. The obvious photochargeable properties are obtained for the hydrogen storage alloys modified with Perovskite-type SrTiO3 powders. During photocharging process, the potential of the electrode shifts quickly to negative direction and a potential plateau occurs. HSA electrode modified with SrTiO3 powders prepared by direct hydrolysis-precipitation process gives the higher potential of about -0.90V(vs Hg/HgO) under the light irradiation. SEM observation discloses that a large amount of microcracks occur on the surface of the electrode after photocharging process, which is caused by the formation of hydride in the bulk of electrode.
Key words: AB5-type hydrogen storage alloys; SrTiO3 photocatalysts; photoelectrochemical characteristics CLC number: O649.4
Document code: A
1 INTRODUCTION
Solar energy is a kind of clean and unexhaustable energy, the utilization and development of solar energy is becoming very imperative due to the serious energy problems today. Since Fujishima and Honda[1] discovered the effect of photosensitization of TiO2 electrode on the electrolysis of water into H2 and O2, the photocatalysis by TiO2 and other semiconductors has received much attention and has been widely investigated. Many progresses have been made in the visible-light photocatalysis[2-5] and a series of novel photocatalysts including layered titanate[5-7], layered niobates[8,9] and layered perovskite materials and their pillared-products[10-13] were discovered.
Among these photocatalysts, perovskite-type SrTiO3 oxide is one of the most widely used dielectric materials which have widespread applications in electronic devices[14]. On the other hand, SrTiO3 oxide is an efficient photocatalyst. Recently, Akuto and Sakurai[15] performed an interesting photorechargeable metal hydride/air battery. In this battery, SrTiO3 powder was modified on hydrogen storage alloys to form a SrTiO3-HSA photorechargeable electrode, and in combination with air electrode, a SrTiO3-LaNi5-xAlxHn[JB(|]KOH[JB)|]O2 cell system was obtained. The experimental results showed that the above cell system can be recharged by light irradiation. This means that the light energy can be transferred into chemical energy and stored in the cells electrochemically.
In this paper, we prepared the perovskite-type SrTiO3 photocatalysts by direct hydrolysis-precipitation process, and investigated the photoelectrochemical behaviors of the AB5-type hydrogen storage alloy electrodes modified with SrTiO3 powder.
2 EXPERIMENTAL
Perovkite-type SrTiO3 oxide was prepared by a direct hydrolysis-precipitation process, in which the final heat treatment temperature was 700℃. All chemicals are of commercially analytical purity. The procedures of direct hydrolysis-precipitation process are shown in Fig.1.
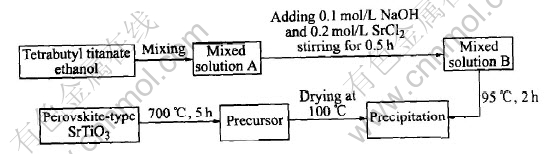
Fig.1 Flow chart of preparing SrTiO3 by direct hydrolysis-precipitation method
A commercial AB5-type hydrogen storage alloy(HSA) with the standard composition of MmMn0.4Co0.7Al0.3Ni3.6 was used to prepare hydrogen storage alloy electrode by a dry powder compression technique without additives. The as-prepared SrTiO3 oxide was dissolved in 10% PTFE suspension and then the HSA electrode was put into the suspension for several seconds. Then the electrode was dried at about 60℃ for about 3min. The above procedures were repeated for 5 times until a thin layer of SrTiO3 oxide was formed on the surface of HSA electrode. Finally the electrode was cool pressed at 10MPa for 1min. The size of the electrode was 1.0cm×1.0cm×0.03cm.
The photoelectrochemical behaviors were measured in a conventional three-electrode electrolysis cells, in which NiOOH was used as the counter electrode, Hg/HgO as the reference electrode, and the electrolyte was 6mol/L KOH solution. All electrochemical measurements were performed by using a EG&G 273A potentiosta/galvanostat. A 500W xenon lamp was used as the light source when measuring the photocharge characteristics. The light intensity can be adjusted by changing the working power of the xenon lamp, and the corresponding light intensity was about 100mW/cm2. To eliminate the influence of corrosion of HSA electrode in KOH solution upon the potential, the as-prepared electrodes were immersed into 6mol/L KOH for 5h before photocharging process. The discharge current was about 6mA/g, and the cut-off potential was set at -0.5V(vs Hg/HgO). All experiments were performed at 25℃.
3 RESULTS AND DISCUSSION
3.1 Crystal structure and morphology
Fig.2 shows the XRD pattern of the as-prepared perovkite-type SrTiO3 oxide. As shown in Fig.2, the sample is composed of a single phase without other impurities. The lattice parameters calculated according to the diffraction data is a=0.3916nm, which is in accordance with the standard value, indicating that the heat treatment condition is suitable.
Fig.3 shows the SEM image of as-prepared SrTiO3 oxide. The mean size of SrTiO3 oxide is 100-200nm. However, the aggregation of particles is also observed.
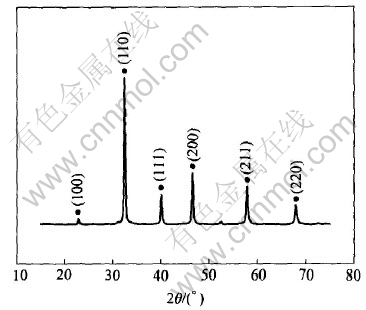
Fig.2 XRD pattern of as-prepared perovkite-type SrTiO3 oxide

Fig.3 SEM image of as-prepared SrTiO3 oxide
3.2 Photoelectrochemical properties
Fig.4 shows the cyclic voltammogram curves of SrTiO3/HSA electrode. The scanning rate was 5mV/s and the HSA electrode was not activated by electrochemical charging/discharging cycle before light irradiation. As shown in Fig.4, the cyclic voltammogram behavior exhibits great difference under the light irradiation. When the xenon light irradiates on the surface of electrode, the cathodic reduction peak current increases fast as the potential shifts to negative direction, indicating that the light irradiation can remarkably improve the reduction current of water. Considering the electrode reaction:
M+xe+xH2O→MHx+nH2O(1)
the additional part of the reduction current may be ascribed to the photogenerated electrons which react with water to produce hydrogen atom.
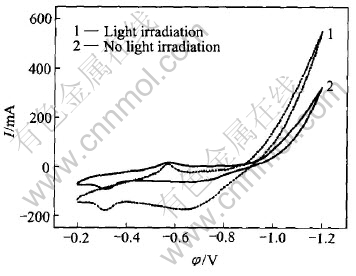
Fig.4 Cyclic voltammogram curves of SrTiO3/HSA electrode
Fig.5 shows the potential change as a function of the photocharging time for SrTiO3/HSA electrode. For comparison, the result of the HSA electrode modified with SrTiO3 prepared by sol-gel method was also presented. The results indicate that the potential of SrTiO3/HSA electrode all rises quickly with the increase of photocharging time, and then tends to a constant value. The potential plateaus of about -0.9V and -0.8V(vs Hg/HgO) appear for the two electrodes separately. The initial potential is about -0.52V and -0.45V for the two electrodes respectively. At the first stage of light irradiation, the potential of SrTiO3/HSA electrode modified with SrTiO3 prepared by sol-gel method rises more quickly, however, the potential plateau of SrTiO3/HSA electrode modified with SrTiO3 prepared by direct hydrolysis-precipitation method is higher.
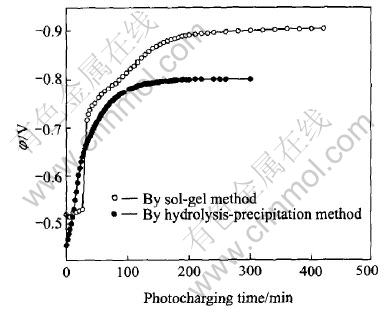
Fig.5 Change of electrode potential with photocharging time for SrTiO3/HSA electrode

Fig.6 Discharge curves of SrTiO3/HSA electrodes
The discharge curves for the two SrTiO3/HSA electrodes are shown in Fig.6. The discharge capacity of the SrTiO3/HSA electrode modified with SrTiO3 prepared by hydrolysis-precipitation method is about 54min, but that of the electrode modified with SrTiO3 prepared by sol-gel method is only about 10min. This result indicates that the higher the photocharging potential plateau, the larger the discharge capacity. Compared with the results of Akuto et al[10], the electrodes prepared by hydrosis-precipitation method can obtain higher charging potential. The effect of the photocatalyst on the photoelectrochemical properties is very remarkable and worth further investigation.
3.3 Surface observation
Fig.7 depicts the morphologies of SrTiO3/HSA electrodes modified with SrTiO3 prepared by hydrolysis-precipitation method after photocharging process. Fig.7(a) shows the morphology of the HSA electrodes prepared by dry powder cool compression technique without additives, in which the traces observed on the surface of HSA electrode represent the rolling direction. After 400min photocharging and electrochemical discharge process, a large amount of crevice and microcracks with the mean size of 200nm occur on the surface of HSA electrode. Especially, these cracks usually distribute near the SrTiO3 powder.

Fig.7 Morphologies of SrTiO3/HSA electrodes after photocharging
Considering the photocharging process, the following reactions can take place on the surface of SrTiO3/HSA electrode:
M+xe+xH2O→MHx+nOH-(2)
SrTiO3+hν(≥3.2eV)→2e-+2p+(3)
During the photocharging process, the photogenerated electrons excited into the conduction band transfer to the hydrogen storage alloy. As a result, the electrons react with water to produce hydrogen atom, and the produced H atom can be absorbed by the electrode to form hydride. Thus, a large amount of microcracks occur on the surface of the electrode, which are caused by the formation of hydride in the bulk of electrode.
4 CONCLUSIONS
Perovskite-type SrTiO3 powders were prepared by using strontium acetate, tetrabutyl titanate and sodium hydroxide via direct hydrolysis-precipitation process. AB5-type hydrogen storage alloy(HSA) electrodes modified with SrTiO3 powders were prepared and the photoelectrochemical characteristics of the as-prepared electrodes were investigated.
1) The current of reduction peak increases remarkably under the light irradiation. The obvious photochargeable properties are obtained for the hydrogen storage alloy modified with Perovskite-type SrTiO3 powders.
2) During photocharging process, the potential of the electrode quickly shifts to negative direction and a potential plateau appears. HSA electrode modified with SrTiO3 powders prepared by direct hydrolysis-precipitation process causes a higher potential of about -0.90V(vs Hg/HgO) by the light irradiation.
3) SEM observation discloses that a large amount of microcracks occur on the surface of the electrode after photocharging process, which are caused by the formation of hydride in the bulk of electrode.
REFERENCES
[1]Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 37(1): 237-238.
[2]Izawa H, Kikkawa S, Koizumi S. Ion exchange and dehydration of layered titannates Na2Ti3O7 and K2Ti4O9 [J]. J Phys Chem, 1982, 86: 5023-5026.
[3]Asahi R, Morikawa T, Ohwaki T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides [J]. Science, 2001, 294: 269-671.
[4]Shahed U M K, Mofareh A S, William B. Efficient photochemical water splitting by a chemically modified n-TiO2 [J]. Science, 2002, 297: 2243-2245.
[5]Zou Z G, Ye J H, Sayama K, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst [J]. Nature, 2001, 414: 625-627.
[6]Oguawa M, Takizawa Y. Intercalation of alkylammonium cations into a layered titanate in the presence of macrocyclic compounds [J]. Chem Mater, 2000, 12(11): 3253-3255.
[7]Machida M, Ma X W, Taniguchi H. Pillaring and photocatalytic property of partially substituted layered titanates, Na2Ti3-xMxO7 and K2Ti4-xMxO9(M=Mn, Fe, Co, Ni, Cu) [J]. J Mole Cata A, 2000, 155: 131-142.
[8]Uchida S, Yamamoto Y, Fujishiro Y. Intercalation of titanium oxide in layered H2Ti4O9 and H4Nb6O17 and photocatalytic water leavage with H2Ti4O9/(TiO2, Pt) and H4Nb6O17(TiO2, Pt) nano-composites [J]. J Chem Soc, Faraday Trans, 1997, 93(17): 3229-3234.
[9]Sato T, Yamamoto Y, Fujishiro Y. Intercalation of iron oxide in layered H2Ti4O9 and H4Nb6O17: visible-light induced photocatalytic properties [J]. J Chem Soc, Faraday Trans, 1996, 92: 5089-5092.
[10]Ishii T, Kato H, Kudo A. H2 evolution from an aqueous methanol solution on SrTiO3 photocatalysts codoped with chromium and tantalum ions under visible light irradiation [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2004, 163(1-2): 181-186.
[11]Kato H, Kudo A. Photocatalytic water splitting into H2 and O2 over various tantalate photocatalysts [J]. Catalysis Today, 2003, 78( 1-4): 561-569.
[12]Kato H, Kudo A. Energy structure and photocatalytic activity for water splitting of Sr2(Ta1-xNbx)2O7 solid solution [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2001, 145(1-2): 129-133.
[13]Harada H, Hosoki C, Kudo A. Overall water splitting by sonophotocatalytic reaction: the role of powdered photocatalyst and an attempt to decompose water using a visible-light sensitive photocatalyst [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2001, 141(2-3): 219-224.
[14]ZHANG S C, YE H, CHEN B C, et al. The influence of TiO2·H2O gel on hydrothermal synthesis of SrTiO3 powders [J]. Materials Letter, 2001, 51: 368.
[15]Akuto K, Sakurai Y. A photorechargeable metal hydride/air battery [J]. J Electrochem Soc, 2001, 148(2): A121-125.
(Edited by YUAN Sai-qian)
Foundation item: Project(50201016) supported by the National Natural Science Foundation of China; Project(Y404044) supported by the Natural Science Foundation of Zhejiang Province
Received date: 2004-11-08; Accepted date: 2005-02-25
Correspondence: ZHANG Wen-kui, Professor, PhD; E-mail: echem@zjtu.edu.cn