改进电解液中自催化沉淀铜及其特性
来源期刊:中国有色金属学报(英文版)2015年第11期
论文作者:R. SEKAR K. K. JAGADESH G. N. K. RAMESH BAPU
文章页码:3791 - 3801
关键词:化学镀铜;添加剂;动电位极化;电化学阻抗谱
Key words:electroless copper; additives; potentiodynamic polarization; electrochemical impedance spectroscopy
摘 要:研究了以TEA 和 EDTA为络合剂、多聚甲醛为还原剂、二硫基苯并噻唑为稳定剂、凝胶和动物胶为添加剂的镀铜化学沉积过程。化学镀铜溶液的稳定性可通过紫外可见分光光度仪测量溶液的吸光度来监测,在15 h内溶液都相当稳定。采用标准弯曲试验评估在低碳钢箔上铜膜的附着力,显示了很好的吸附性。XRD结果表明,铜膜具有(111)织构,而且,添加剂能够抑制(111)面的优先晶体生长,提高(220)织构的晶体生长速度。利用Scherrer公式从主峰计算铜膜的晶粒尺寸。SEM 和 AFM研究表明,这两种添加剂能够改善铜膜的晶体结构、晶粒尺寸和表面形貌。循环伏安法研究表明,添加剂能够被吸附在电极表面并降低沉积速率。动电位极化和电化学阻抗研究表明,有添加剂时产生的沉淀具有更高的耐腐蚀性。
Abstract: The electroless deposition process of copper plating consisting of TEA and EDTA as complexing agents, paraformaldehyde as reducing agent, and 2-mercaptobenzothiozole as stabilizer and gelatin and animal glue as additives was investigated. The stability of the electroless copper solution was monitored by measuring the absorbance of the solution with a UV-Visible spectrophotometer and the solution was quite stable up to 15 h. The adhesion of copper films on mild steel foil was assessed by standard bend test and exhibited good adhesion. The XRD results indicate that the copper films have a (111) texture. Moreover, the additives suppress the predominant (111) plane crystal growth and increase the rate of (220) texture crystal growth. The crystal size of the copper films was calculated using the Scherrer formula from the predominant peak. SEM and AFM studies reveal that these two additives modify the crystal structure, grain size and surface morphology of the copper films. The cyclic voltammetry studies reveal that the additives are adsorbed on the electrode surface and inhibit the rate of deposition. Potentiodynamic polarization and electrochemical impedance studies reveal that the deposits produced in the presence of additives display higher corrosion resistance.
Trans. Nonferrous Met. Soc. China 25(2015) 3791-3801
R. SEKAR, K. K. JAGADESH, G. N. K. RAMESH BAPU
Electroplating and Metal Finishing Technology Division, Council of Scientific and Industrial Research-Central Electrochemical Research Institute, Karaikudi 630006, Tamil Nadu, India
Received 12 December 2014; accepted 25 May 2015
Abstract: The electroless deposition process of copper plating consisting of TEA and EDTA as complexing agents, paraformaldehyde as reducing agent, and 2-mercaptobenzothiozole as stabilizer and gelatin and animal glue as additives was investigated. The stability of the electroless copper solution was monitored by measuring the absorbance of the solution with a UV-Visible spectrophotometer and the solution was quite stable up to 15 h. The adhesion of copper films on mild steel foil was assessed by standard bend test and exhibited good adhesion. The XRD results indicate that the copper films have a (111) texture. Moreover, the additives suppress the predominant (111) plane crystal growth and increase the rate of (220) texture crystal growth. The crystal size of the copper films was calculated using the Scherrer formula from the predominant peak. SEM and AFM studies reveal that these two additives modify the crystal structure, grain size and surface morphology of the copper films. The cyclic voltammetry studies reveal that the additives are adsorbed on the electrode surface and inhibit the rate of deposition. Potentiodynamic polarization and electrochemical impedance studies reveal that the deposits produced in the presence of additives display higher corrosion resistance.
Key words: electroless copper; additives; potentiodynamic polarization; electrochemical impedance spectroscopy
1 Introduction
Auto catalytic copper plating (ACP) or electroless plating has been a hot area in the electronic industry for decades. Electroless copper plating is mostly used for metallization in the fabrication of printed circuit boards and other electronic devices, super computers, auto bumpers, and jewellary making. It is also employed in other industries such as hetero junction solar cells, starting material for the synthesis of thin films of copper indium gallium selenide (CIGS) films for photovoltaic, filling contact holes in microelectronics devices and delineating semiconductor junctions [1,2]. The electroless copper plating process has several unique advantages such as high throwing power, free from nodular deposit, no excess deposit on high and sharp points, uniform thickness throughout the surface, and plating on non-conductive materials such as ceramics and plastics which would be difficult or impossible to do with conventional electrolytic technique [3]. UZUNLAR et al [4] have studied the electroless copper deposition process on epoxy laminate substrates using a non- palladium catalyst, and without the use of the swell- and-etch process. TIAN and GUO [5] optimized the conditions of electroless copper deposition in order to establish more stable and higher deposition rate electrolyte to produce higher quality deposit [5].
Sodium hypophosphite has been widely used as a reducing agent for electroless nickel plating. Electroless copper plating process is more complicated than electroless nickel plating, as copper is not as good catalyst as nickel for the oxidation of hypophosphite. Nickel ions can act as mediators in electroless copper plating. A very small amount of nickel acts as a catalyst in the copper deposit and the oxidation of hypophosphite enabling continuous copper deposition [6]. Tartarate [7] and EDTA [8,9] were used as complexing agents in electroless copper bath and gave low deposition rate and high bath stability for short duration. The need of complexing agent is necessary to prevent precipitation of Cu(OH)2 under alkaline condition. In order to improve the deposition rate and bath stability, double complexing agents (EDTA and TEA) were used [10]. Saccharine was reported as complexing agent in electroless copper bath solution by NORKUS et al [11]. SONE et al [12] used Fe(II) as reducing agent in electroless copper plating bath and they first reported that the complexing agents shift the oxidation-reduction potential of the Fe(III)-Fe(II) system to more negative potentials. Xylitol, D-mannitol and D-sorbitol were proposed as environmentally friendly alternate copper(II) ligands for electroless copper plating solutions [13]. YU et al [14] studied the effect of pH on rate of deposition, crystal structures, surface morphology and magnetic properties of cobalt-phosphorus alloy films. LI et al [15] reported that 2,2’-dipridyl was used as additive in electroless copper bath. The color of deposit changes from dark brown to semi-bright with improved uniformity. It also had a small crystalline size and higher (111) plane orientation [15]. Controlling and monitoring the bath stability of electroless copper electrolyte by in-situ transmittance and UV absorbance measurements were studied [16,17]. LI and YANG [18] investigated an alternative acidic electroless copper deposition on aluminum seeded ABS plastics surface. GAN et al [19] studied the influence of PEG on the electroless copper deposition using hypophosphite as reducing agent [19]. The copper(II) reduction by formaldehyde, the autocatalytic behaviour of this process, thermodynamic and kinetic aspects were studied [20].
In this work, an electroless copper plating electrolyte was studied using double complexing agents (TEA and EDTA) to improve the self life of the bath and prevent the formation precipitation of copper complexes. Paraformaldehyde and 2-mercaptobenzothiozole were used as reducing agents and stabilizer, respectively. Gelatin and animal glue were used as grain modifiers. The effect of bath constituents, stability of the solution, crystal structure and surface morphology of the electroless copper coatings and catalytic activity for the oxidation of paraformaldehyde during the process in electroless copper plating bath were characterized. The stability of the electroless copper solution was also monitored by measuring the absorbance of the solution with a UV-Visible spectrophotometer.
2 Experimental
The electroless copper plating bath listed in Table 1 containing copper sulphate, sodium hydroxide, EDTA and TEA (complexing agent), paraformaldehyde (reducing agent), gelatin and animal glue (additives) was prepared by dissolving the required quantity of chemicals in double distilled water. These chemicals of analytical grade (AR) were purchased from Merck and Ranbaxy Company and used for bath preparation. The plating experiments were performed in triplicate with a 400 mL electroless copper plating solution at ambient temperature. Surface preparation prior to deposition is an important factor and can be achieved by mechanical and electrochemical methods [21]. The procedure adapted was removal of surface scale using acid dipping, mechanical polishing to get a smooth surface, degreasing with trichloroethylene and final electro cleaning at 4 A/dm2 in a solution of sodium hydroxide, and sodium carbonate (30 g/L each). The mild steel foils (Mn: 0.23%; S: 0.03%; C: 0.063%; P: 0.011%; Fe: balance) with sizes of 7.5 cm × 5 cm × 0.1 cm were used as substrate, and activated in 0.5 g/L of palladium chloride solution for 30 s and then electroless copper plating was performed for 60 min. The solutions were agitated by magnetic stirrer and temperature was maintained at 50 °C for all experiments to improve the rate of film thickness and quality of the films. The film growth rate was determined by the difference in mass of the substrate before and after deposition.
The rate of copper deposition was determined at pH 12.5 and at room temperature with respect to varying the concentration of the electroless copper electrolyte constituents such as EDTA concentration, which was varied from 5 to 35 g/L. TEA concentration was varied from 10 to 40 g/L, paraformaldehyde concentration was varied from 1 to 9 g/L and CuSO4 concentration was varied from 5 to 15 g/L. From this, the rate of film thickness and stability of the solution was observed and based on the electroless copper electrolyte composition and additives such as gelatin and animal glue concentrations were optimized.
Table 1 Bath compositions and operating parameters

The stability of the electroless copper solution overtime was determined by monitoring the UV-Visible absorbance by taking 1 mL of bath using UNICO UV double beam UV-Visible spectrophotometer with reference to water sample at room temperature. Initially, the peak absorbance of the solution was measured every 3 h intervals up to 15 h which was then measured at 24 h intervals till the intensity of the peak absorbance decreased significantly. The time dependence of its peak absorbance value was recorded.
A glass three-electrode cell arrangement was used for cyclic voltammetry studies. Mild steel, a large platinum foil and saturated calomel electrode were employed as working, counter and reference electrodes, respectively. To minimize IR drop, a finely drawn Luggin capillary positioned very close to the working electrode was used. The solutions were prepared using double distilled water and AnalaR grade chemicals. Experiments were carried out in the absence and in the presence of additives with various concentrations at pH 12.5. Potential sweeps were conducted at 20 mV/s using potentiostat and the response was recorded using Biologic SP-150 instrument with EC-Lab software.
The bend test by ASTM test method B571-84 [22] was followed to evaluate the adhesion of the electroless copper coatings on the steel substrate. X-ray diffraction patterns were obtained using the X-pert pro powder diffraction system PE 3040/60 for electroless copper deposits in various copper electrolytes. The depositions produced with and without additives for 10 μm-thick deposits of copper films were studied. The samples were scanned at 20°-120° (2θ) at the rate of 3° per minute using Cu Kα (λ=1.5404  ) radiation. The peaks due to the different phases were identified and the corresponding lattice parameters were calculated. The crystal size of the electroless copper films were calculated using the Scherrer formula [23,24] from the predominant peak using the following equation:
) radiation. The peaks due to the different phases were identified and the corresponding lattice parameters were calculated. The crystal size of the electroless copper films were calculated using the Scherrer formula [23,24] from the predominant peak using the following equation:

where t is the average size of the crystallites, 0.9 is the Scherrer constant, λ is the wavelength of the radiation, β is the peak width at half maximum and θ corresponds to the peak position.
The copper films obtained from the different electroless copper baths were observed by scanning electron microscopy using the Model TESCAN at 25 kV. Molecular imaging atomic force microscopy (AFM) was used in a contact mode with the silicon nitride tip to reveal the 3D surface topography of the deposits.
The corrosion characteristics were studied in 3.5% NaCl test solution for electroless copper films of 10 μm in thickness. The electroless copper film samples were masked to expose 1 cm2 area on one side. A platinum foil (2.5 cm × 2.5 cm) counter electrode and saturated calomel electrode were used as auxiliary and reference electrode, respectively. The working electrode was introduced into the test solution and was allowed to attain a steady potential value. Anodic and cathodic polarizations were performed up to ±200 mV away from the OCP at a scan rate of 1 mV/s. φcorr and Jcorr values were obtained from the plot of φ versus lg J by the Tafel extrapolation method using a Biologic model SP-150 with EC-Lab software. The same three-electrode cell arrangement and instrument were used for the AC impedance measurements. The electrochemical impedance measurements were performed at OCP in the frequency range from 10 mHz to 100 kHz. The values of solution resistance (Rs), double layer capacitance (Cdl) and charge transfer resistance (Rct) were generated from the Nyquist plots of real (Zre) versus imaginary (-Zim) components of the impedance.
3 Results and discussion
3.1 Effect of pH and rate of film thickness
The depositing electrolyte and additive concentration were optimized by preliminary experiments and are listed in Table 1. The electroless copper deposition was performed at various pH (7.5-13) with the addition of sodium hydroxide and the result is shown in Fig. 1. At pH 7.5-11.0, a galvanic displacement reaction takes place between copper and steel substrate and loosely bonded copper films are obtained. When the pH was raised to 12, the displacement reaction was completely arrested and the copper films were well adherent to the substrate and quality of the copper film is improved at pH 12, and the rate of film thickness increases significantly, and beyond pH 12.5, rate of film thickness decreases. Hence, pH plays a vital role in electroless plating. It affects both anodic and cathodic reactions as well as the nature of the complex species. It is well known that the oxidation of paraformaldehyde is increased by the presence of hydroxyl ions. Electroless copper plating electrolytes are characterized by the deposition rate that first increases then decreases as a function of pH. Similar results were reported by NUZZI [25] who proposed the following mechanism for electroless copper deposition.
1) Firstly, the increase of the rate of deposition up to pH 12 is due to the effect of OH- ions as reactants in the total reaction as indicated by
Cu2++2HCHO+4OH-→Cu0+H2↑+2H2O+2HCOO-(1)
2) The decrease in the rate of deposition at pH above 12.75 is due to the consumption of OH- ions by hydrolysis of formaldehyde to methylene glycol anions, followed by oxidation to formate ions as
HCHO+OH-→CH2(OH)O- (2)
CH(OH)O-+OH-→CHOO-+H2O+e (3)
One important side reaction in electroless copper deposition is disproportionation of formaldehyde (Cannizzaro reaction):
2HCHO+OH-→HCOO-+CH3OH (4)
In this reaction, between two molecules of formaldehyde, one molecule is oxidized into formic acid and the other is reduced into methanol.

Fig. 1 Effect of pH in Bath A of electroless copper plating at 30 °C
When the pH was raised to 13, the quality, stability and rate of deposition were significantly affected. Hence, it is suggested that pH 12 is optimum for producing a good quality with high rate of deposition and the bath is also stable.
Based on the above studies, the optimized electroless copper solution (Bath A) contained 10 g/L CuSO4, 20 g/L NaOH, 5 g/L paraformaldehyde, 5 g/L EDTA, 30 g/L TEA and 6 mg/L MBT.
3.2 Effect of additives and rate of film thickness
Figure 2 shows the effect of concentration of gelatin (curve a) and animal glue (curve b) from 0.5-2.0 g/L in Bath A. In Fig. 2, curve (a) shows the copper film yielded from the gelatin containing electrolyte and it clearly indicates that the rate of film thickness decreases with the increase of concentration of gelatin. The similar result was observed from copper film obtained from the animal glue containing electrolyte (curve (b)). However, the brightness of the deposit increases with the increasing concentration. The reduction in deposition rate with increasing concentration of additives could be attributed to additives which inhibit the deposition rate depending on their concentration in the plating bath. Low metal ion (cupric ion) concentration led to low rate of deposition in the absence of forced convection, and the effect of this concentration difference approaches to mass transport control process.
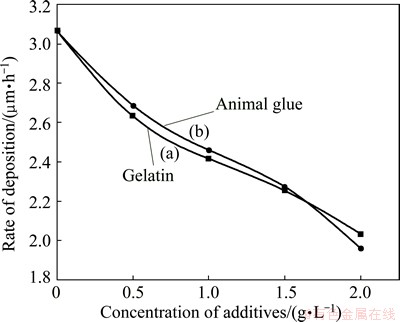
Fig. 2 Effect of additives in Bath A of electroless copper plating at 30 °C
3.3 UV-Visible absorbance spectra analysis
Figure 3 shows the UV-Visible absorbance spectra of samples taken from the electroless copper plating solution over a period of 15 h for every 3 h intervals. For the as-prepared sample, i.e., before starting the experiment, the solution exhibited a broad absorption peak at 725 nm with peak intensity value of 1.8. The maximum absorption observed is the characteristic of the absorption spectra of copper(II) coordination complexes where copper(II) is attached to 4 oxygen atoms. These absorption spectra are characterized by large band of low intensity, a typical d-d transition. After 3, 6, 9, 12 and 15 h intervals of measurement, the absorbance almost overlapped and maintained the peak position steadily till 15 h. The observed overlapping spectral lines and no significant change of the peak intensity implied that the electroless bath solution is quite stable for 15 h and the bath is not decomposed or disproportionated significantly over a period of 15 h.

Fig. 3 UV-Visible absorbance spectra of Bath A of electroless copper during 15 h
The variation of the UV-Visible absorbance of the electroless solution measured over a period of 8 d at regular interval of 1 d is shown in Fig. 4. Over the period of measurement, although the peak position is centered at 725 nm, the peak intensity decreased steadily from 1.8 to 0.2. As absorbance is directly proportional to the concentration of the metal ions in the solution, the decrease in observed peak intensity implied that the concentration of the complex Cu ions decreased in the electroless solution after a period of 1 d. The observed change in the intensity of the blue colored complex solution turns to colorless with respect to time. At the end of the 8th day, the solution becomes completely colourless which confirms the decrease of the Cu ion concentration in the electrolyte.

Fig. 4 UV-Visible absorbance spectra of Bath A of electroless copper during 1-8 d
3.4 Cyclic voltammetry studies
According to the mixed potential theory of electroless plating, the overall reaction of the electroless process is measured by the two-half reaction at the electrode-electrolyte interface, i.e., the oxidation of the reducing agent and the reduction of cupric ions. Consequently, it is useful to understand the effects of gelatin and animal glue on the half-reaction in the electroless copper plating bath using paraformaldehyde as reducing agent. Cyclic voltammeter behaviors of the electroless copper plating solution containing 0.04 mol/L CuSO4, 0.01 mol/L EDTA, 0.2 mol/L TEA, 0.5 mol/L NaOH, 4×10-4 mol/L MBT and 0.16 mol/L para- formaldehyde in the absence and presence of additives were examined at 20 mV/s between 0.5 and -1.3 V (vs SCE) using a mild steel working electrode, as shown in Fig. 5. Figure 5(a) shows the cyclic voltammogram of the electroless copper plating solution without any additive. The forward scan exhibited two cathodic peaks at -0.8 V and -1.05 V, respectively. The first cathodic peak is due to the reduction of Cu(II) to Cu(I) and the second cathodic peak is for the reduction of Cu(I) to Cu(0) [26]. These two main steps would be attributed to the occurrence of potential difference between the two peaks at 250 mV. This suggests that: 1) stepwise electronation of the divalent copper complex takes place and the reduction of monovalent copper complex becomes increasingly difficult; 2) electronation may take place via hydroxyl complex and also via copper (II) hydroxyl complexes [27]. The reverse scan shows two anodic peaks at -0.05 V and -0.6 V, where the former is the oxidation of copper metallic copper to cuprous ions and the latter is the oxidation of cuprous to cupric ions.

Fig. 5 Cyclic voltammograms obtained in electroless copper solution containing 0.04 mol/L CuSO4 + 0.01 mol/L EDTA + 0.2 mol/L TEA + 0.5 mol/L NaOH + 0.16 mol/L PF + 4×10-4 mol/L MBT at sweep rate of 20 mV/s
Figures 5(b)-(d) represent the cyclic voltammo- grams of electroless copper plating solution containing 0.04 mol/L CuSO4, 0.01 mol/L EDTA, 0.2 mol/L TEA, 0.5 mol/L NaOH, 4×10-4 mol/L MBT and 0.16 mol/L paraformaldehyde with different concentrations of gelatin at a sweep rate of 20 mV/s. When polarized from 0.5 V to -1.3 V, the forward scan showed two cathodic peaks at -0.8 V and -1.05 V, respectively. The cathodic peak potentials become more cathodic with increasing concentration of gelatin. On the other hand, cathodic peak height and current decrease with increasing concentration of gelatin. Hence, it is worth mentioning that additives are adsorbed at the electrode surface thereby blocking the surface area. During the reverse scan, two anodic peaks appear at -0.05 V and -0.6 V respectively but the peak potentials become active. The anodic peak height is decreased with increasing concentration of additives. This is because gelatin gets preferentially adsorbed on the copper electrode surface and inhibits copper deposition.
Figure 6 shows the cyclic voltammograms of the electroless copper plating solution containing 0.04 mol/L CuSO4, 0.01 mol/L EDTA, 0.2 mol/L TEA, 0.5 mol/L NaOH, 4×10-4 mol/L MBT and 0.16 mol/L para- formaldehyde without and with animal glue as additive for different concentrations at a sweep rate of 20 mV/s. Figure 6(a) represents the cyclic voltammogram of the electroless copper plating without any additive. The forward scan shows that two well defined cathodic peaks occur at -0.8 and -1.05 V, respectively. During the reverse scan, two anodic peaks were observed at -0.05 V and -0.6 V and potentials are shifted towards negative direction with the increasing concentration of additive (Figs. 6(b)-(d)). Cathodic as well as anodic peak current still decreases with increasing the concentration of animal glue. Hence, the rate of deposition is affected by the addition of animal glue in electroless plating bath. In general, additives inhibit the nucleation growth of the deposits.
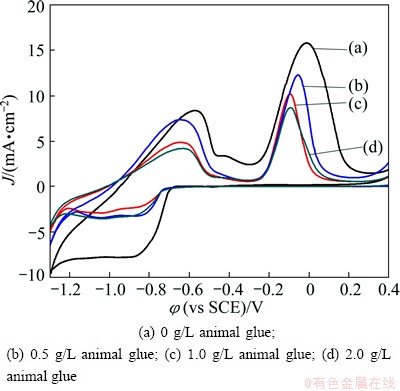
Fig. 6 Cyclic voltammograms obtained in electroless copper solution containing 0.04 mol/L CuSO4 + 0.01 mol/L EDTA + 0.2 mol/L TEA + 0.5 mol/L NaOH + 0.16 mol/L PF + 4×10-4 mol/L MBT at sweep rate of 20 mV/s
3.5 Characterization of deposits
3.5.1 Adhesion
Adhesion of the electroless copper films on the steel substrate obtained in the presence and absence of additives was tested by subjecting the deposited specimens to standard bend tests. No flaking or peeling of the deposit occurred during the bend test. The deposits prepared under various experimental conditions were found to withstand the bend test, showing that the keying and adhesion of the copper films to the base were very good in all cases.
3.5.2 X-ray diffraction analysis
Table 2 shows the grain size of copper, calculated from the Scherrer equation from XRD data, in the presence and absence of additives. Figure 7 shows the X-ray diffraction patterns of electroless copper obtained from various baths at 30 °C. The deposit is crystalline and has FCC structure. The observed d values are in good agreement with the standard values of copper. Figure 7(a) represents the deposits obtained without additive at 30 °C. It can be seen that the intensity of (111) copper plane is more predominant as compared with the other peaks, copper oxide peak (002) plane was detected, and the crystal size was about 50 nm. Figure 7(b) indicates that the deposits are produced from the bath containing 0.5 g/L gelatin, which shows that the (111) copper peak intensity is slightly reduced and those of (220), (002) and (313) planes are slightly increased and the crystal size was about 12 nm. This indicates that the formation of copper oxide inhibits the crystal growth of the copper deposit. However, the intensities of copper (220) and (313) planes increased with increasing the intensity of copper oxide (002). Figure 7(c) represents the deposits obtained from 1 g/L gelatin as additive, and the intensity of the copper (111) plane decreased and the intensity of copper oxide (002) plane significantly increased. Figures 7(d) and (e) indicated that the deposits were produced from 1.5 g/L and 2 g/L gelatin, respectively and the same behavior was observed. In general, additive concentration increases with decreasing intensity of the copper (111) plane and the intensity of the copper oxide (002) plane increases. Hence, it clearly indicates that the rate of deposition decreases with addition of additive. It is concluded that the additives may be adsorbed on the cathode surface thereby blocking the surface area of the electrode and there is no significant change in the crystal size with increasing concentration of additive.
Table 2 Crystal size for electroless copper deposits produced from different baths
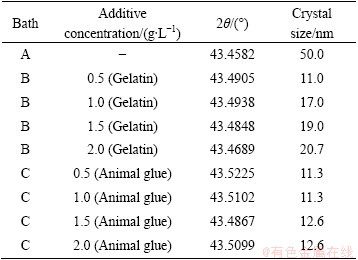
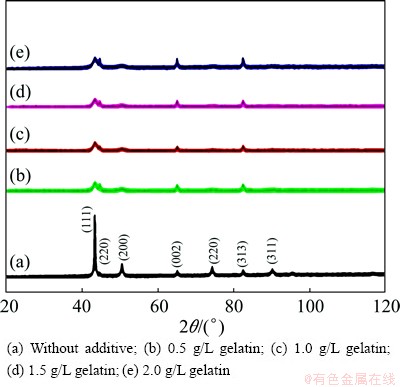
Fig 7 XRD patterns for electroless copper deposits at 30 °C
Figure 8(a) shows the X-ray diffraction patterns of electroless copper obtained from Bath A without additive at 30 °C. Figure 8(b) represents the deposits obtained from the electroless copper bath containing 0.5 g/L animal glue as additive. It is seen that the intensity of the copper (111) plane is predominated as shown in Fig. 8(b). With animal glue, the intensity of copper oxide (002) plane also increases and the crystal size is about 11 nm. When the concentration of animal glue increases to 1.0, 1.5 and 2.0 g/L, there is no significant change in the grain size, as shown in Figs. 8(c)-(e).

Fig. 8 XRD patterns for electroless copper deposits at 30 °C
3.5.3 Surface morphology analysis
Figure 9 shows the SEM images of electroless copper deposits with and without gelatin. Figure 9(a) represents the deposits produced without any additive at 30 °C. Eventually, seeds like spherical particles are unevenly distributed in the crystal lattice. Figures 9(b)-(e) show the SEM images with the addition of 0.5, 1.0, 1.5 and 2 g/L gelatin, respectively. The deposit obtained from 0.5 g/L gelatin exhibited high crystal refinement with dense and void-free structure. When the concentration of gelatin increases above 0.5 g/L, there is no significant change in grain size of the deposit. But the surface morphology was slightly changed. Hence, gelatin is expected to serve as grain refiner.
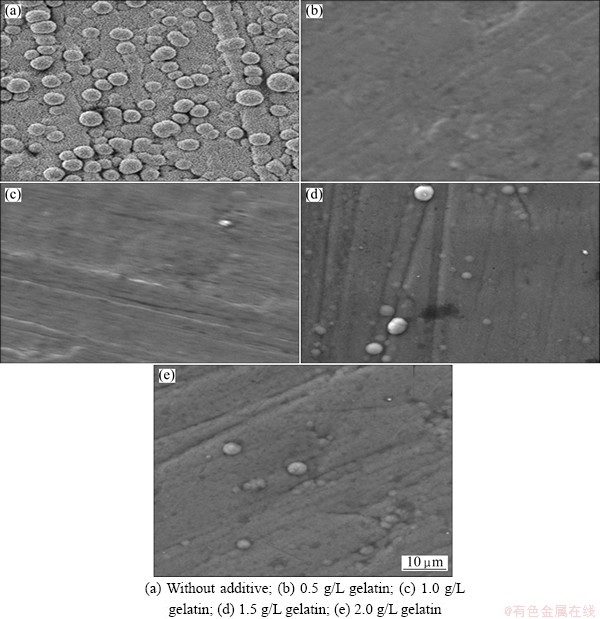
Fig. 9 SEM images obtained for various electroless copper deposits at 30 °C
Figure 10 shows the SEM micrographs of electroless copper deposits obtained with and without additives. Figure 10(a) represents the deposits obtained from Bath C containing 0.5 g/L animal glue that shows a smooth uniform fine grained surface morphology. This can be attributed to adsorption effect. The surface activity of the electrode decreases which in turn should suppress the growth of nuclei. Hence, coatings with smooth and fine structures are expected to be obtained. When the concentrations of animal glue increase to 1, 1.5, and 2 g/L, respectively, the unevenness of the crystals increases and there is no remarkable change in the surface morphology, which are shown in Figs. 10(b)-(d).
3.5.4 AFM measurements
AFM imaging gives a perspective of the Z direction with three dimensional images [28-31]. Figure 11 shows the representative AFM imaged scanned over an area of 2 μm × 2 μm. It indicates that the topography of the copper deposit obtained from Bath A in the absence of additive is rough and shows flat hills like structures without defined grain boundaries and average grain size of 50 nm and the average roughness is 40 nm whereas with the introduction of 0.5 g/L gelatin as additive in the electroless copper bath (Bath B), the deposit becomes smooth, dense and compact with fine grained surface morphology and the grained size is 12 nm and the average roughness drastically changes to 0.65 nm. With addition of 0.5 g/L animal glue as additive in the electroless copper bath (Bath C), the topography was slightly changed as compared with the Bath B and the grain size is also marginally reduced to 11 nm and the average roughness value is 0.48 nm. A corresponding improvement in colour of the deposit also occurred. The bright appearance of the deposit generally indicates better mechanical and physical properties.
3.5.5 Potentiodynamic polarization studies
The data derived from φ-lgJ curves for various electroless copper deposits of 10 μm in thickness in 3.5% sodium chloride solution are given in Table 3. Figure 12 (curve (a)) illustrates the potentiodynamic polarization curves obtained from Bath A without additive, which shows a corrosion potential less active and high corrosion current. Curve (b) corresponds to the deposit produced from Bath B, the corrosion potential shifts to more active direction and the corrosion current decreases as compared with Bath A. Curve (c) is for the deposit obtained from Bath C containing animal glue, which exhibited that the corrosion potential and corrosion current still decreased. This increased corrosion resistance can be attributed to the dense, compact and fine grained structure of the deposit. The morphology of the deposit analyzed with SEM and AFM is in good agreement with the obtained corrosion data.
3.5.6 Electrochemical impedance spectroscopy (EIS)
The Nyquist impedance study performed in 3.5% sodium chloride solution is exhibited in Fig. 13. Curve (a) represents the EIS spectrum for the deposit obtained from Bath A, curves (b) and (c) show the deposits from Baths B and C, respectively.
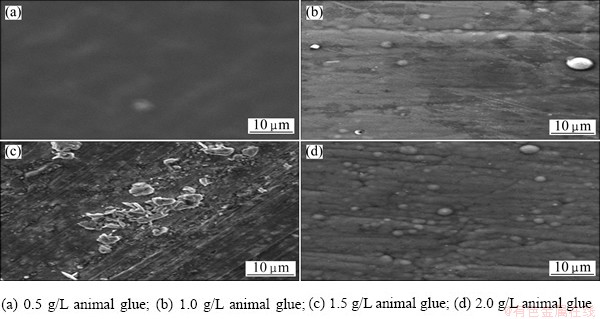
Fig. 10 SEM images obtained for various electroless copper deposits at 30 °C
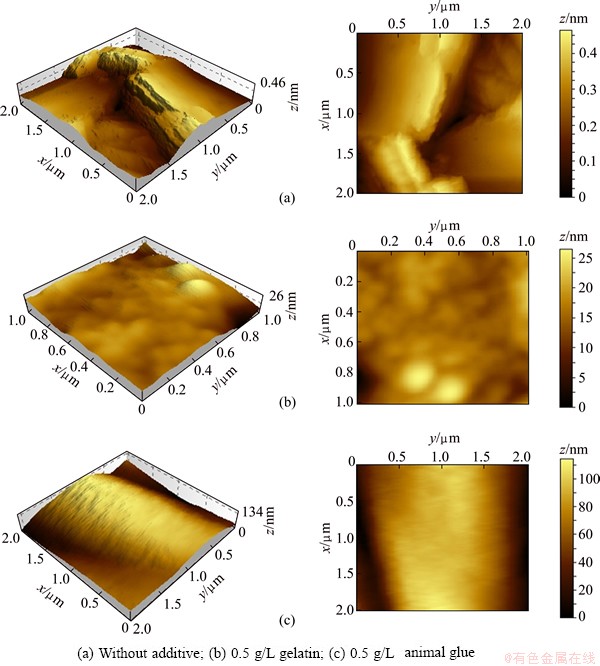
Fig. 11 AFM images obtained for various electroless copper deposits at 30 °C
Table 3 Parameters derived from φ-lgJ curves for electroless copper deposits in 3.5% NaCl solution

The impedance plots relevant to the electroless copper deposit obtained from Bath A with no additive showed a well defined capacitive loop and there was no evidence of other inductive or capacitive loop at lower frequencies. The occurrence of single semicircle or a semicircle in the high frequency region followed by a low frequency loop is typical metallic coatings. Moreover, the curves in the Nyquist plot seem to be the same with respect to their shape, and they vary considerably in their sizes. The semicircle at the high frequency region indicates the coating response, while the loop at low frequency region is associated with simultaneous physicochemical phenomena at the coating/solution interface [32,33]. The corrosion of copper coatings purely depends on charge transfer controlled, as revealed from the impedance spectrum of perfect semicircle shape [34]. The shape of the impedance spectra supports the assumption that the polarization resistance (Rp) is the same as the charge transfer resistance (Rct) which is easily estimated on the real impedance axis by extrapolating the impedance trend at the lowest frequencies. The data generated from EIS spectra are listed in Table 3. The Rct value of deposits obtained from Baths A, B and C are 466, 603 and 1257 Ω·cm2, respectively. The occurrence of a single semicircle in the Nyquist plots indicates that the corrosion process of electroless copper deposits involves a single time constant. The protective characteristics of coatings increase with increasing diameter of the semicircle. On the other hand, the difference in corrosion resistance among electroless copper coatings is due to the difference in their structure [35]. The deposit produced from the bath containing animal glue (Bath C) showed that the deposits are more compact, dense and fine grained structure as compare to the other two deposits. Hence, the deposits produced from bath containing animal glue (Bath C) showed that larger semicircle with the highest Rct value on the real axis. In general the diameter of the semicircle increases with increases of the coating resistance (protective value increases). This is in clear agreement with SEM and AFM microstructural studies, indicating that animal glue acts as the best grain refiner for the copper deposits from electroless bath.

Fig. 12 Potentiodynamic polarization curves for 10 μm electroless copper deposits in 3.5% NaCl solution

Fig. 13 EIS spectra obtained for electroless copper deposits (10 μm) in 3.5% NaCl solution
4 Conclusions
Smooth, uniform, adherent coating was produced from the electroless copper plating bath, and in the presence of additives, film thickness decreases. This is attributed to the additive adsorbed on the electrode surface, which inhibits the nucleation growth of the deposition. The UV-Visible absorption spectra of the electroless copper solution over a period of time show that the solution is stable for 15 h, which thereafter undergoes chemical reaction and shows a decrease in peak intensity from 1.8 to 0.2 over a period of 8 d due to consumption of Cu ions. Cyclic voltammetry studies reveal that with the introduction of additives, the cathodic current decreases with increasing the concentration of additives and anodic peaks are shifted to negative direction. X-ray diffraction studies reveal that the deposits produced from all the three baths, the (111) plane is more predominant and the introduction of additives refined the grains. SEM studies show that without additive, the deposit exhibits seed-like structure and the crystals are randomly distributed. AFM studies indicate that the animal glue is the best grain refiner. Potentiodynamic polarization studies reveal higher corrosion current for deposits produced in the absence of additives compared with additive containing baths. Among these baths studied, deposits obtained from the animal glue containing bath have the highest corrosion resistance.
References
[1] JEONG H B, JANG W K. Novel electroless copper deposition on carbon fibers with environmentally friendly processes [J]. Journal of Colloid and Interface Science, 2010, 348: 649-653.
[2] TOUIR R, LARHZIL R H, EBNTOUHAMI M, CHERKAOUI M, CHASSAING E. Electroless deposition of copper in acidic solutions using hypophosphite reducing agent [J]. Journal of Applied Electrochemistry, 2006, 36: 69-75.
[3] EE Y C, CHEN Z, CHAN L, SEE A K H, LAW S B, TEE K C. Effect of processing parameters on electroless Cu seed layer properties [J]. Thin Solid Films, 2004, 462-463: 197-201.
[4] UZUNLAR E, WILSON Z, KOHL P A. Electroless copper deposition using Sn-Ag catalyst on epoxy laminates [J]. Journal of the Electrochemical Society, 2013, 160: D3237-D3246.
[5] TIAN Qing-hua, GUO xue-yi. Electroless copper plating on microcellular polyurethane foam [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s283-s287.
[6] CHU S Z, SAKAIRI M, TAKAHASHI H. Copper electroless plating at selected areas on alumimium with pulsed Nd-YAG laser [J]. Journal of the Electrochemical Society, 2000, 147: 1423-1434.
[7] DECKERT C A. Electroless copper plating: A review, Part II [J]. Plating and Surface Finishing, 1995, 82: 58-64.
[8] LOWENHEIM F A. Modern electroplating [M]. 3rd ed. New York: Wiley, 1974.
[9] NADIIA K, SERHIY C, CHUNG C H. Copper electroless plating in weakly alkaline electrolytes using DMAB as a reducing agent for metallization on polymer films [J]. Electrochimica Acta, 2012, 59: 179-185.
[10] LI J, KOHL P A. Complex chemistry and the electroless copper plating process [J]. Plating and Surface Finishing, 2004, 91: 2-7.
[11] NORKUS E, 
 I, MACALADY D L. Application of saccharose as copper(II) ligand for electroless copper plating solutions [J]. Carbohydrate Research, 2007, 342: 71-78.
I, MACALADY D L. Application of saccharose as copper(II) ligand for electroless copper plating solutions [J]. Carbohydrate Research, 2007, 342: 71-78.
[12] SONE M, KOBAYAKAWA K, SAITOU M, SATO Y. Electroless copper plating using FeII as a reducing agent [J]. Electrochimica Acta, 2004, 49: 233-238.
[13] NORKUS E,  GAIDAMAUSKAS E, MACALADY D L. Environmentally friendly natural polyhydroxylic compounds in electroless copper plating baths: Application of xylitol, D-mannitol and D-sorbitol as copper(II) ligands [J]. Journal of Applied Electrochemistry, 2005, 35: 41-47.
GAIDAMAUSKAS E, MACALADY D L. Environmentally friendly natural polyhydroxylic compounds in electroless copper plating baths: Application of xylitol, D-mannitol and D-sorbitol as copper(II) ligands [J]. Journal of Applied Electrochemistry, 2005, 35: 41-47.
[14] YU Y D, LI M G, WEI G Y, GE H L. Effects of pH values on electroless deposition of CoP films [J]. Surface Engineering, 2013, 29: 767-771.
[15] LI J, HAYDEN H, KOHL P A. The influence of 2,2‘dipyridyl on non-formaldehyde electroless copper plating [J]. Electrochimica Acta, 2004 , 49: 1789-1795.
[16] KYUNG J P, HYO-CHOL K, TAEHO L, MYUNG J K, OH J K, JAE J K. Evaluation of stability and reactivity of Cu electroless deposition solution by in-situ transmittance measurement [J]. Journal of the Electrochemical Society, 2011, 158(9): D541-D545.
[17] FUMIHIRO I, HAROLD P, ALEX R, SILVIA A, YANN C, SHOSO S, PETER L. Electroless copper bath stability monitoring with UV-VIS spectroscopy, pH and mixed potential measurement [J]. Journal of the Electrochemical Society, 2012, 159(7): D437-D441.
[18] LI Da-peng, YANG Chen-lu. Acidic electroless copper deposition on aluminium seeded ABS plastics[J]. Surface and Coatings Technology, 2009, 203: 3559-3568.
[19] GAN Xue-ping, ZHOU Ke-chao, HU Wen-bin, DOU Zhang. Role of additives in electroless copper plating using hypophosphite as reducing agent [J]. Surface and Coatings Technology, 2012, 206: 3405-3409.
[20] MALLORY G O, HAJDU J B. Electroless plating, fundamentals and applications [M]. Orlando: American Electroplaters and surface Finishers Society, 1990.
[21] SEKAR R, JEYAKRISHNAN S. Effect of sulphonic acids on electrodeposition of nickel and its structural and corrosion behaviour [J]. Trasactions of the Institute of Metal Finishing, 2012, 90(60): 324-329.
[22] ASTM Test Method B571-84: Adhesion of Metallic Coatings, 1995 [S].
[23] CULLITY B D. Elements of X-ray diffraction [M]. USA: Addesion Wesley Publishing Co. Inc, 1967.
[24] CLUG H P, ALEXANDER L. X-ray diffraction procedures for polycrystalline and amorphous materials [M]. New york: Wiley, 1980.
[25] NUZZI F J. Accelerating the rate of electroless copper plating [J]. Plating and Surface Finishing, 1983, 70: 51-54.
[26] GRUJICIC D, PESIC B. Reaction and nucleation mechanisms of copper electrodeposition from ammoniacal solutions on vitreous carbon [J]. Electrochimica Acta, 2005, 50: 4426-4443.
[27] ARAVINDA C L, MAYANNA S M, MURALIDHARAN V S. Electrochemical behaviour of alkaline copper complexes [J]. Proceeding of Indian Academy of Sciences (Chemical Science), 2000, 112(5): 543-550.
[28] SMITH J R, BREAKSPEAR S, CAMPELL S A. AFM in surface finishing, Part I: An introduction [J]. Trasactions of the Institute of metal Finishing, 2003, 81(2): B26-B29.
[29] SMITH J R, BREAKSPEAR S, CAMPELL S A. AFM in surface finishing, Part II: Surface roughness [J]. Trasactions of the Institute of metal Finishing, 2003, 81(3): B55-B58.
[30] MARTYAK N M, SEEFELDT R. Additive-effects during plating in acid tin methanesulfonate electrolytes [J]. Electrochimica Acta, 2004, 49: 4303-4311.
[31] BARRERA E,  P, BATINA N,
P, BATINA N,  I. Formation mechanisms of black and white cobalt electrodeposition onto stainless steel [J]. Journal of the Electrochemical Society, 2000, 147(5): 1787-1796.
I. Formation mechanisms of black and white cobalt electrodeposition onto stainless steel [J]. Journal of the Electrochemical Society, 2000, 147(5): 1787-1796.
[32] SEKAR R, EGAMMAI C, JEYAKRISHNAN S. Effect of additives on electrodeposition of tin and its structural and corrosion behaviour [J]. Journal of Applied Electrochemistry, 2010, 40: 49-57.
[33] KRISHNAVENI A K, SANKARA.NARAYANAN T S N, SESHADRI S K. Corrosion resistance of electrodeposited Ni-B and Ni-B-Si3N4 composite coatings [J]. Journal of Alloys and Componds, 2009, 480: 765-770.
[34] MANSFLED F, KENDIG M W, TASI S. Evaluation of corrosion behaviour of coated metals with AC impedance measurements [J]. Corrosion, 1982, 38: 478-485.
[35] SURVILLINE S, CESUNIENE A, JUSKENAS R. Effect of carbide particles on chromium electrodeposition and protective properties of chromium [J]. Trasactions of the Institute of Metal Finishing, 2004, 82(5-6): 185-188.
R. SEKAR, K. K. JAGADESH, G. N. K. RAMESH BAPU
Electroplating and Metal Finishing Technology Division, Council of Scientific and Industrial Research-Central Electrochemical Research Institute, Karaikudi 630006, Tamil Nadu, India
摘 要:研究了以TEA 和 EDTA为络合剂、多聚甲醛为还原剂、二硫基苯并噻唑为稳定剂、凝胶和动物胶为添加剂的镀铜化学沉积过程。化学镀铜溶液的稳定性可通过紫外可见分光光度仪测量溶液的吸光度来监测,在15 h内溶液都相当稳定。采用标准弯曲试验评估在低碳钢箔上铜膜的附着力,显示了很好的吸附性。XRD结果表明,铜膜具有(111)织构,而且,添加剂能够抑制(111)面的优先晶体生长,提高(220)织构的晶体生长速度。利用Scherrer公式从主峰计算铜膜的晶粒尺寸。SEM 和 AFM研究表明,这两种添加剂能够改善铜膜的晶体结构、晶粒尺寸和表面形貌。循环伏安法研究表明,添加剂能够被吸附在电极表面并降低沉积速率。动电位极化和电化学阻抗研究表明,有添加剂时产生的沉淀具有更高的耐腐蚀性。
关键词:化学镀铜;添加剂;动电位极化;电化学阻抗谱
(Edited by Xiang-qun LI)
Corresponding author: R. SEKAR; Tel: +91-4565-241572; Mobile: +91-9940874641; Fax: +91-4565-227779; E-mail: grsek2004@yahoo.com
DOI: 10.1016/S1003-6326(15)64023-7

