
Preparation of flake AgSnO2 composite powders by hydrothermal method
YANG Tian-zu(杨天足)1, DU Zuo-juan(杜作娟)1, GU Ying-ying(古映莹)2,
QIU Xiao-yong(邱晓勇)2, JIANG Ming-xi(江名喜)1, CHU Guang(楚 广)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 8 July 2006; accepted 24 October 2006
Abstract: Silver-tin oxide composite powders and silver powders were synthesized by hydrothermal method using NH3 to complex Ag+, SO2-3 to reduce Ag(NH3)+2 and Na2SnO3 as the source of tin. The powders were characterized by XRD, SEM and EDX. The results show that there are macroscopic and microscopic differences between two kinds of powders. Spherical silver powders are 3 μm in diameter, and silver-tin oxide composite powders are mainly flake of about 0.3 μm in thickness. Silver crystal in silver-tin oxide composite powders is preferentially oriented in the (111) crystallographic direction and its oriented index is 2.581. Crystal lattice parameter of silver crystal of silver tin-oxide composite powders is 0.409 34 nm, larger than 0.408 68 nm of silver powders. The XPS analysis shows that silver in silver-tin oxide composite powders is metallic silver and tin oxide in silver tin-oxide composite powders has the red shift for Sn4+(3d5/2) and O2-(1s).
Key words: composite material; silver-tin oxide powder; silver powder; hydrothermal method; oriented growth
1 Introduction
Silver-tin oxide materials have acceptable arc resistance, sufficient safety with respect to contact welding, comparable low material migration with low contact resistance and good overtemperature behavior, and practical processing and jointing properties and have become the focus of research in contact materials in recent years[1-3]. The air contactors of low voltage/high current equipment, within a switching current range of 100-3 000 A, are primarily made from materials based on silver-tin oxide, in which the oxide content, in practice, lies between about 8% and 12% (mass fraction). At present, there are many preparing techniques for AgSnO2 contact material such as powder metallurgy, alloy internal oxidation, reacting spray[4], chemical plating[5-6], reaction synthesis[7] and reaction ball milling technique[8-9]. Each of these processes has its own advantages, but at the same time, some limitations as well[10]. Further improvements in the processing and contact properties of these AgSnO2 materials are desired, as an increase in their range of applications. Further developments in materials and technology might be involved with other oxide additives and manufacturing technology, particularly the technology aimed at controlling the structure-dependent properties.
In this work the hydrothermal process is applied to prepare silver tin oxide composite powders, with silver ammine complex solution and Na2SnO3 solution as raw materials and sodium sulphite as reductant. The reduction of Ag+ and the crystallization deposit of SnO2 are realized in the hydrothermal process and the obtained silver-tin oxide composite powders are mainly flake structure with homogeneous distribution of tin-oxide. The composite powders are compared with silver powders that are prepared under the same condition.
2 Experimental
2.1 Procedure
Ammonia water was added to 0.1 mol/L aqueous AgNO3 solution for forming silver-ammonia complex. Na2SO3 solution was added to the solution of silver ammine complex as reductant according to stoichiometry. The resulted solution was put into a high pressure autoclave to react for 4 h at 150 ℃. The produced gray powders were washed and filtered, and then dried for 4 h at 100 ℃.
For the preparation of silver-tin oxide powder, Na2SnO3 solution was added to above-mentioned ammonia complex solution, in which the ratio of tin-oxide to silver was 1?9, and then the same processing procedure followed that for silver powder preparation to obtain silver tin-oxide composite powders.
2.2 Characterization
X-ray diffractometer (D/MAX-YA type) was used to measure the crystalline structure of the sample, Cu target, Kα=1.54 nm. SEM (Japanese JSM-5600LV) was adopted to observe the appearance and grain size of the sample. Energy Dispersive X-ray Detector (EDX) (Japanese JSM-5600LV) was used to analyze the elemental composition of the sample. X-ray photoelectron spectrum was measured for analyzing the chemical state of silver and tin in silver tin-oxide composite powders. Chemical analytic methods were also adopted to determine the content of silver[11] and tin[12].
3 Results and discussion
3.1 Co-precipitation of silver and tin oxide
H2SO3 is a weak acid. In alkaline solution, it exits mostly in the form of 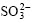 . The standard electrode potentials of
. The standard electrode potentials of 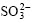 and
and 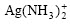 complex ion are as follows:
complex ion are as follows:

Obviously, 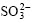 can reduce
can reduce 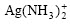 to metallic silver. The experiment indicates that
to metallic silver. The experiment indicates that 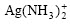 is not reduced significantly by
is not reduced significantly by 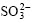 under ambient temperature. However, complete reduction can be realized under the hydrothermal condition of high temperature and high pressure.
under ambient temperature. However, complete reduction can be realized under the hydrothermal condition of high temperature and high pressure.
Na2SnO3 is a compound with unstable chemical properties in the aqueous solution. 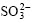 will be hydrolyzed under the hydrothermal condition:
will be hydrolyzed under the hydrothermal condition:
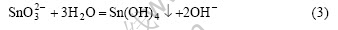
Meanwhile, under the hydrothermal condition, Sn(OH)4 deposit can be further dehydrated to produce SnO2 crystals[13]:
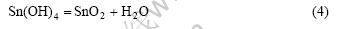
The depositional equilibrium of 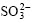 is destroyed by the hydrothermal crystallization of Sn(OH)4, which favors further depositing of Sn(OH)4.
is destroyed by the hydrothermal crystallization of Sn(OH)4, which favors further depositing of Sn(OH)4.
According to reactions (1), (2) and (3), OH- is consumed in reaction (1) and is released in reaction (3). Although no consuming and releasing of OH- occur, NH3, released in reaction (2) will react as
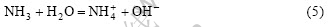
Owing to the presence of OH- in the reaction system, it provides the possibility of coprecipitation of silver and tin oxide. The pH value of the solution shows the final balance of every reaction. When [Ag]T is equal to 0.1 mol/L in the solution, the change of pH before and after hydrothermal reaction, the deposition of tin oxide and silver are shown in Table 1.
Table 1 Changes of pH value before and after hydrothermal reaction and deposition of silver and tin-oxide
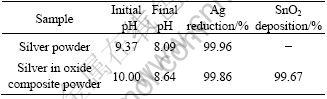
It can be seen from Table 1 that pH value of the silver powder preparation system is decreased by 1.28, while pH value of the silver-tin oxide composite powder preparation system is decreased by 1.31. The pH decrease of the two systems is almost equal. The reason is that NH3 plays buffer on pH to some extent. It also can be seen that the reduction of silver is 99.86% and the deposition rate of SnO2 of silver tin-oxide composite powders is 99.67%. It suggests that silver and tin-oxide can be synchronously coprecipitated.
3.2 SEM analysis
There exists obvious difference in appearance and structure between silver powders and silver tin-oxide powders prepared by hydrothermal process. Figs.1 and 2 show the SEM images of silver powders and silver tin-oxide composite powders. It is observed that the silver powder has similarly spherical structure of about 3 μm in diameter and the silver tin oxide composite powder is mostly of irregular flake structure with about 0.3 μm in thickness. The composition of these spherical particles is difficult to be quantitatively analyzed because of its small grain size. However, a back reflection contrast analysis indicates that these particles are of the same substance as the flakes. The results of an energy dispersive spectrum analysis (as shown in Fig.3) of the composite powders show that the sample contains elements Ag and Sn. In the sample the content of SnO2 is 9.65% (mass fraction), closely conforming with the actual addition amount of 10%. It is also proved by a chemical analysis as well.

Fig.1 SEM image of Ag powders

Fig.2 SEM image of AgSnO2 powders
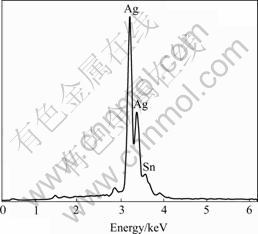
Fig.3 EDX spectrum of AgSnO2 powders
3.3 XRD analysis
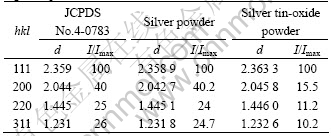
Figs.4 and 5 show the XRD patterns of silver powders and silver tin-oxide composite powders, respectively, each of which has four main peaks assigned to (111), (200), (220), (311) faces, respectively. The peaks are sharp with the position being identical with the standard peaks of Ag of cubic phase, indicating perfectly-grown silver particle with polycrystalline structure. Meanwhile, it is found that there appear heterophase peaks at 2θ=3.359 5?, 2.639 8? and 1.763 3?, which correspond to the diffraction peaks of SnO2 of tetragonal phase. These peaks are less sharp than the diffraction peaks corresponding to silver crystal. That may result from the small diameter of SnO2 crystal particles and the widening of the peaks. Therefore, the silver tin oxide composites powders consist of matrix Ag and SnO2, without other phases.
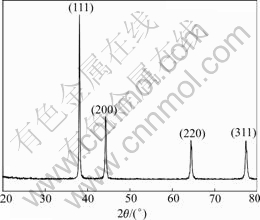
Fig.4 XRD pattern of Ag powders
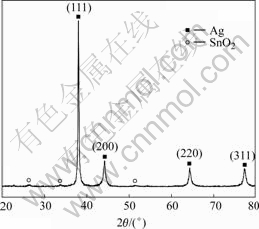
Fig.5 XRD pattern of AgSnO2 powders
When the diffraction intensities of silver powders are compared with those of silver tin-oxide composite, it can be found that the relative diffraction intensities have obvious differences. The Miller indices (hkl), interplanar spacing d(nm) and relative intensities (I/Imax) of X-ray diffraction peaks of silver powders and silver tin oxide powders are listed in Table 2 and are compared with corresponding JCPDS card. Obviously, the silver crystal of silver tin-oxide composite powders is preferentially oriented in the (111) crystal planes. The ratio of intensities can be established by using intensities of the (111) and (200) crystal plane, as described by
R=I(111)/I(200) (6)
where I(111) and I(200) are the diffraction intensities of the (111) and (200) crystal planes, respectively. If there exists no preferential orientation, R value (also named as R0) is 2.5, such as the sample of silver crystal. When there exists preferential orientation the orientation index p can be adopted to characterize its orientation:

When p=1, it shows no orientation; when p>1, it shows preferential orientation. The orientation of crystal grains is more intensive as p increases[14]. In the prepared silver powders, the orientation index is 1.048, which shows almost no preferential orientation, whereas the orientation index of silver tin-oxide powders is 2.581, which shows obvious preferential orientation.
Table 2 Miller indices (hkl), interplanar spacings d and intensities I/Imax of X-ray diffraction peaks of Ag powders and AgSnO2 powders
Since the structure of silver crystal is face centered cubic, its crystal lattice parameter is calculated from the XRD data by using the following formula[15]:

The results show that the lattice parameter of silver crystals of silver powders is 0.408 68 nm and that of the silver tin-oxide powders is 0.409 36 nm, which is obviously larger than the former.
As mentioned above, the differences between silver powders and silver tin-oxide powders, which are prepared hydrothermally under the same conditions, are given in Table 3. It shows the obvious macroscopic and microscopic differences. The  complex ions in the hydrothermal system have efficacious effect on forming flake silver tin-oxide powders. The growth of silver crystal of silver tin-oxide powders is limited by
complex ions in the hydrothermal system have efficacious effect on forming flake silver tin-oxide powders. The growth of silver crystal of silver tin-oxide powders is limited by  complex ion and show preferential orientation in the (111) crystal plane.
complex ion and show preferential orientation in the (111) crystal plane.
Table 3 Differences of Ag powders and AgSnO2 powders prepared by hydrothermal method

3.4 XPS analysis
Fig.6 shows the XPS spectrum of silver tin-oxide, showing clearly the peaks of silver and tin atoms of the silver tin-oxide composite powders. Fig.7 shows the high-resolution XPS spectra of silver tin-oxide composite powders. In the XPS spectrum of Fig.7(a), the peaks at the binding energy of 368.3 eV and 374.3 eV correspond to the Ag3d5/2 and Ag3d3/2, in good agreement with the standard spectrum of silver[16]. It suggests that the silver of silver tin-oxide composite powders exists as metallic silver. While in the 3d spectrum of tin, the binding energy of Sn 3d is 487.4 eV, which shifts 0.7 eV from Sn4+(3d5/2) 486.7 eV[17]. Similarly, the binding energy of O1s shifts 0.7 eV from 530.6 eV to 531.3 eV. The difference between the binding energies of metal and oxygen (ΔEB) shows the electron states of metal-oxygen bonds in metal oxides. In the present case, the ΔEB (O1s-Sn3d5/2) of SnO2 is calculated to be 43.9 eV. The value is consistent with Ref.[18], suggesting that the electron state of SnO2 is not changed. The red shift of Sn4+(3d5/2) and O2-(1s) suggests that silver has effect on SnO2.
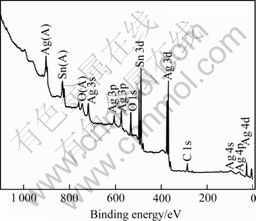
Fig.6 XPS spectrum of silver tin-oxide powders
4 Conclusions
Silver-tin oxide composite powders and silver powders were synthesized successfully by the hydrothermal method using NH3 to complex Ag+, SO32- to reduce Ag(NH3)2+ and Na2SnO3 as the source of tin. Under the same preparing conditions, there are remarkable differences between silver powders and silver-tin oxide composite powders from macroscopic shape to microscopic structure. Analogously spherical silver powders are about 3 μm in diameter, and silver-tin oxide composite powders are mainly flakes of about 0.3 μm in thickness. Silver crystal of silver powder does not show preferential orientation and silver-tin oxide composite powders are preferentially oriented in the (111) crystallographic face and its oriented index is 2.581. From XRD data, crystal lattice parameter of silver crystal of silver tin-oxide composite powders is obtained, which is 0.409 34 nm, larger than 0.408 68 nm of silver powders. The silver in silver tin-oxide composite powders is Ag0, and tin-oxide of silver tin-oxide composite powders has the red shift for Sn4+(3d5/2) and O2- (1s). It suggests that silver has effect on tin-oxide.

Fig.7 XPS spectra of Ag 3d (a), Sn 3d (b) and O1s (c) levels of AgSnO2 powders
References
[1] CHANG H. Novel method for preparation of silver-tin oxide electrical contacts [J]. Journal of Materials Engineering and Performance, 1992(1): 255-260.
[2] ROLAND M. Metallurgical aspects of silver-based contact materials for air-break switching devices for power engineering [J]. IEEE Transactions on Components, Hybrids, and Manufacturing Technology, 1989, 12(1): 71-81.
[3] LIU H Y, WANG Y P, DING B J. Preparation and microstructure analysis of nanostructured silver tin oxide contact materials [J]. Rare Metal Materials and Engineering, 2002, 31(2): 122-124. (in Chinese)
[4] BEHRENS V, HONIG Th, KRAUS A, MICHAL R, SEAGER K E, SCHMIDBERGER R, STANEFF Th. Advanced silver/tin oxide contact material [A]. Proceedings of the Annual Holm Conference on Electrical Contacts [C]. Pittsburgh: IEEE, 1993: 19-25.
[5] ERNEST M J, KIRK M. Electrically conductive material and method for making [P]. US 5846288, 1998.
[6] ROGER W, MECHTHILD M, FRANK H, DIETRICH R, DAN G. Method for producing composite powders based on silver-tin oxide, the composite powders so produced, and the use of such powders to produce electrical contact materials by powder metallurgy techniques [P]. US 0051102, 2001.
[7] CHEN J C, SUN J L, DU Y, ZHOU X L, GAN G Y. Investigation of electrical conductivity of silver tin oxide electrical contact materials fabricated by reactive synthesis [J]. Rare Metal Materials and Engineering, 2003, 32(12): 1053-1056. (in Chinese)
[8] ZOZ H, REN H, SP?TH N. Improved Ag-SnO2 electrical contact material produced by mechanical alloying [J]. Metall, 1999, 53(8): 423-428.
[9] LORRAIN N, CHAFFRON L, CARRY C, DEICROIX P, LECA?R G. Kinetics and formation mechanisms of the nanocomposite powder Ag-SnO2 prepared by reactive milling [J]. Materials Science and Engineering A, 2004, A367: 1-8.
[10] DU Z J, YANG T Z, GU Y Y, QIU X Y. The advances of preparing methods and application of AgSnO2 contact material [J]. Materials Review, 2005, 19(2): 39-42. (in Chinese)
[11] Zhuzhou Smelting Plant. Separation and Determination of Elements in Nonferrous Metallurgy [M]. Beijing: Metallurgical Industry Press, 1979. (in Chinese)
[12] JB/T 7777.1-1995. Chemical analysis procedures for silver tin oxide, indium oxide electrical contact materials: determination of tin by iodimetry [S]. 1995. (in Chinese)
[13] LI W J, SHI E W, ZHONG W Z, ZHENG Y Q, YIN Z W. Growth unit model of anion coordination polyhedron and the particle size of powders in the hydrothermal formation [J]. Journal of the Chinese Ceramic Society, 1999, 27(2): 164-171. (in Chinese)
[14] QI J Y. X-Ray Structure Analysis [M]. Shanghai: Tongji University Press, 2003. (in Chinese)
[15] QIAN Y T. Crystal Chemical Review [M]. 2nd ed. Hefei: Science and Technology University of China Press, 1999.
[16] MOULDER J F, STICKLE W F, SOBOL P E, BOMBEN K D. Handbook of X-Ray Photoelectron Spectroscopy [M]. Minnesota: Perkin-Elmer Corporation, 1992: 127.
[17] SONG S K, CHOI W K, JUNG H J, BAIK H K, KOH S K. Comparison of properties of tin oxide films deposited by reactive-partially ionized beam, ion assisted, and hybrid ion beam methods [J]. Nanostrucrured Materials, 1997, 8(4): 477-488.
[18] CHIKA N, KENJI T, EIJI S. Synthesis and characterization of homogeneous germanium-substituted tin oxide by using sol-gel method [J]. Journal of Solid State Chemistry, 2000, 154: 579-583.
Corresponding author: YANG Tian-zu; Tel: +86-731-8836791; E-mail: tianzuyang@163.com
(Edited by YUAN Sai-qian)