Pretreatment of poly (acrylic acid) sodium by continuous diafiltration and time revolution of filtration potential
来源期刊:中南大学学报(英文版)2019年第3期
论文作者:邱运仁 徐静远 汤淑云
文章页码:577 - 586
Key words:pretreatment; diafiltration; ultrafiltration; poly(acrylic acid) sodium; filtration potential
Abstract: The pretreatment for the removal of small molecules from poly(acrylic acid) sodium (PAAS) solution by continuous diafiltration was investigated using ultrafiltration membrane. The effects of PAAS concentration, pH, trans-membrane pressure and pretreatment time on the permeate concentration and permeate flux were studied. The results show that the necessary pretreatment time (NPT) increases with PAAS concentration, decreases with TMP. The change trend of permeate flux with time is affected by pH. The permeate fluxes rapidly decrease from the start, and then increase gradually to stable values at pH 5.0, pH 7.0 and pH 9.3. However, it decreases gradually with time till a state value at pH 3.0 (iso-electric point, IEP). The removal of small molecules is easy at pH greater than iso-electric point (IEP). The change of filtration potential with time indicates the similar trend to that of permeation concentration, but the former is more convenient for indication of NPT.
Cite this article as: XU Jing-yuan, TANG Shu-yun, QIU Yun-ren. Pretreatment of poly (acrylic acid) sodium by continuous diafiltration and time revolution of filtration potential [J]. Journal of Central South University, 2019, 26(3): 577–586. DOI: https://doi.org/10.1007/s11771-019-4029-3.

J. Cent. South Univ. (2019) 26: 577-586
DOI: https://doi.org/10.1007/s11771-019-4029-3
XU Jing-yuan(徐静远), TANG Shu-yun(汤淑云), QIU Yun-ren(邱运仁)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: The pretreatment for the removal of small molecules from poly(acrylic acid) sodium (PAAS) solution by continuous diafiltration was investigated using ultrafiltration membrane. The effects of PAAS concentration, pH, trans-membrane pressure and pretreatment time on the permeate concentration and permeate flux were studied. The results show that the necessary pretreatment time (NPT) increases with PAAS concentration, decreases with TMP. The change trend of permeate flux with time is affected by pH. The permeate fluxes rapidly decrease from the start, and then increase gradually to stable values at pH 5.0, pH 7.0 and pH 9.3. However, it decreases gradually with time till a state value at pH 3.0 (iso-electric point, IEP). The removal of small molecules is easy at pH greater than iso-electric point (IEP). The change of filtration potential with time indicates the similar trend to that of permeation concentration, but the former is more convenient for indication of NPT.
Key words: pretreatment; diafiltration; ultrafiltration; poly(acrylic acid) sodium; filtration potential
Cite this article as: XU Jing-yuan, TANG Shu-yun, QIU Yun-ren. Pretreatment of poly (acrylic acid) sodium by continuous diafiltration and time revolution of filtration potential [J]. Journal of Central South University, 2019, 26(3): 577–586. DOI: https://doi.org/10.1007/s11771-019-4029-3.
1 Introduction
Polymer enhanced ultrafiltration (PEUF), also called complexation-ultrafiltration, is widely used to remove heavy metal ions like copper, lead, cadmium, zinc, nickel, mercury, etc. existing in aqueous effluents as sewage water, underground water or with radio nuclides [1–4]. It uses water soluble polymers, such as chitosan, sodium polycarboxylate, poly (acrylic acid) or poly (acrylic acid) sodium, copolymer of maleic acid and acrylic acid, etc., to bind metal ions from aqueous solution [5, 6]. An ultrafiltration membrane can retain metal- polymer complexes of sufficient molecular size and thus metal ions can be concentrated for recovery. For PEUF process, choosing an appropriate polymer is of great importance. Poly (acrylic acid) sodium (PAAS) is widely used due to its good solubility in water and good complexing ability with heavy metal ions [7–9]. Unlike low molecular weight compound, the polymeric complexing agent, of which the molecular weight is not homogenous. It is necessary to remove small molecules of the complexing agent in PEUF, otherwise, the heavy metal ions combined with small molecules will permeate through the membrane, leading to a decrease in rejection of heavy metal ions. Thus, the polymer solution needs to be pretreated to remove the smaller molecules for improving the removal rate of heavy metal ions in PEUF [8, 9]. However, the pretreatment was only simply mentioned in literatures and lack of systematic study. The variation of electrochemical property in the pretreatment process is also important for filtration. Because of its experimental simplicity, streaming potential measurement has become the most widely used method to study the electrokinetic properties of membranes [10, 11]. But most literatures are based on electrolyte solution and the small molecular solute cannot be rejected by the membrane [12]. Due to molecular weight distribution of PAAS, a part of small molecules can penetrate through the membrane and others not. Thus, in the filtration of polyelectrolyte solution, the potential difference between both sides is different with that of the usual electrolyte solution, streaming potential, caused by pressure difference. Thus, it is necessary to use the more general term of filtration potential instead of streaming potential [13].
In this work, pretreatment of poly(acrylic acid) sodium using diafiltration by hollow fiber ultrafiltration membrane and the time revolution of filtration potential were first studied. The behaviors of diafiltration and the pretreatment time in different operating conditions were investigated, the time revolution of permeate flux and filtration potential at different concentration, pH and trans-membrane pressure (TMP) were discussed. The necessary pretreatment time (NPT) of PAAS, can be expressed not only by the permeate concentration and flux but also by filtration potential in continuous diafiltration, and the latter is more convenient.
2 Experimental
2.1 Chemicals, membrane and apparatus
Poly(acrylic acid) sodium (PAAS) was purchased from Tianjin Guangfu Fine Chemical Research Institute, China, and the relative molecular weight was 60 kDa. 0.1 mol/L of NaOH and HCl solutions were used to regulate the pH of PAAS solution. All the chemicals were used without further purification and all solutions were prepared by distilled water.
The polyvinal butyral (PVB) hollow fiber membranes used in this study were prepared via the thermally induced phase separation (TIPS) method [14, 15]. The molecular weight cut off (MWCO) of membrane is 20 kDa, the inner diameter and outside diameter of the hollow fiber membrane are 0.9 mm and 1.3 mm, respectively. The total available membrane area is 0.2 m2.
2.2 Diafiltration
Diafiltration is a useful membrane filtration technique to separate macro-solutes from micro- solutes based on their molecular size difference [16]. For continuous diafiltration, permeate is continuously removed in the process and meanwhile a comparable amount of distilled water is continuously added into the retentate side. The retentate volume keeps 2.5 L.
2.3 Pretreatment experiment
The pretreatment of poly(acrylic acid) sodium is necessary for its use in complexation- ultrafiltration as a complexing agent. Before pretreatment, the PAAS solution was continuously stirred for 30 min by a magnetic stirrer (DF-101S) and the feed temperature was kept at 25 °C, and then the feed was pumped into the hollow fiber membrane module by a peristaltic pump (YZ1515). The initial concentration of PAAS solution was 200 mg/L, the pH was 7.0, the trans-membrane pressure (TMP) was kept 27.5 kPa if no specific instruction was given. The volume of the liquid was 2.5 L. The flow rate was adjusted to 40 L/h by the inlet and outlet valves. The permeate weight was measured by an electronic balance (PB203-E). The pH value of PAAS solution was measured by pH meter (PHS-25). The necessary pretreatment time (NPT) is defined as the needed time that over 99% of the small molecules are removed from bulk solution or the time when the diafiltration turns from concentration-dependent process into constant concentration process.
2.4 Determination of PAAS concentration
The COD method was used to determine the concentration of PAAS in the solution due to the fact that there is no other matter that produces COD except PAAS in the solution. The method for COD determination consists of oxidizing the organic matter of the sample by adding a known amount of oxidant in an sulphuric acid medium, refluxing at high temperature on an open container and titrating the excess oxidant [17].
2.5 Removal rate of small molecular PAAS
The accumulated removal mass of small molecular PAAS was calculated according to
 (1)
(1)
where Mst is the accumulated removal mass of small molecular PAAS by time t, J is the permeate flux, A is the membrane area, cp is the permeate concentration, and t is the filtration time. The total mass (Ms0) of small molecular PAAS in the feed can be obtained when the filtration time is long enough, or the permeate concentration is near zero, that is:
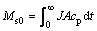 (2)
(2)
Thus, the removal rate (R) of small molecular PAAS can be calculated as:
 (3)
(3)
2.6 Filtration potential measurements
The filtration potential was measured during the process of pretreatment of poly(acrylic acid) sodium. The electrical potential differences across the membrane were measured by a pair of Ag/AgCl electrode placed on the permeate and retentate side, respectively. Meanwhile, the electrode was connected to digital voltmeter to achieve the electrical potential under different operating conditions.
3 Results and discussion
3.1 Effect of initial PAAS concentration
The removal of small molecular fractions of PAAS can be calculated by measuring the permeate concentration. The effects of initial PAAS concentration on pretreatment time and permeate flux were studied in the range of 50–500 mg/L at TMP 27.5 kPa. The necessary pretreatment time (NPT) increases with PAAS concentration, as shown in Figure 1. The permeate concentration of PAAS decreases rapidly from 13.77 to 5.2 mg/L during the first 15 min for the treatment of 50 mg/L PAAS solution, then gradually decreases to about 0.1 mg/L after 80 min, and 0.016 mg/L after 100 min. For the diafiltration of 500 mg/L PAAS solution, it takes 60 min for the permeate concentration decreases from 137.6 to 19.3 mg/L, then gradually decreases to about 2.6 mg/L after 120 min, and 0.082 mg/L after 240 min. As the amount of small molecules in the solution increases with the PAAS concentration, the NPT increases with the increase of initial concentration. The permeate concentration gets very small as diafiltration goes on, but it does not get zero. This indicates that a little amount of small molecular fractions still remains in the retentate. This can be explained by charge repulsion effect between molecules. As the membrane is negatively charged at pH 7.0, and the polymer chain is also negatively charged, the membrane surface has repulsion for the approaching small polymer chain [14].
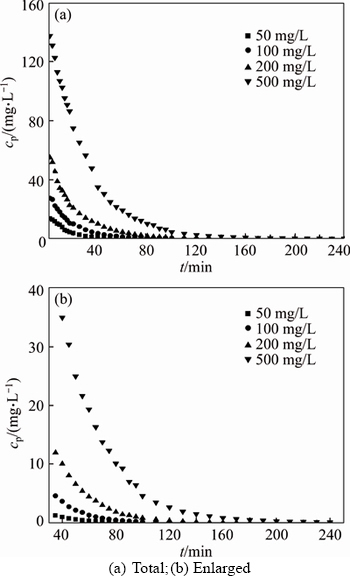
Figure 1 Variation of permeate concentration with time for different concentrations of PAAS solution:(pH=7.0, TMP= 27.5 kPa)
Figure 2 indicates the removal rate of small molecular PAAS with time at various initial PAAS concentrations in diafiltration. The permeate concentrations are both near zero for low concentration of PAAS solutions, such as 50 and 100 mg/L, after 100 and 130 min diafiltration, respectively, and the removal rate of small molecular PAAS can arrive at near 100%. Thus, the mass fraction of small molecular PAAS in original PAAS was calculated by mass balance, which is about 27.4%, as shown in Table 1. Table 1 also indicates if the feed concentration is greater than 200 mg/L, the small molecular PAAS is very difficult to remove completely due to the membrane fouling, while relatively easy to remove completely at low concentration, such as 50 and 100 mg/L.

Figure 2 Removal rate of small molecular PAAS with time in diafiltration (pH=7.0, TMP= 27.5 kPa)
However, the removal rate of small molecular PAAS can arrive at about 95% for 200 mg/L solution after 180 min diafiltration, and just 77% for 500 mg/L solution after 240 min diafiltration. For low concentration solution, the membrane fouling in cross-flow diafiltration at low pressure is very small because the feed concentration gradually decreases with the removal of small molecular fractions. As there exists repulsion force between membrane surface and carboxyl groups, the adsorptive PAAS is very little, and the removal rate of small molecular PAAS is very high. However, when the PAAS concentration is greater than 200 mg/L, although the permeate concentration is very small, only 0.082 mg/L for 500 mg/L PAAS solution after 240 min, the removal rate of small molecular PAAS is not so high. This may be that the adsorptive fouling on the membrane surface becomes severe, and some pores are blocked by the larger molecular PAAS and polyacrylic acid (PAA), as part of PAAS molecules still exist as neutral PAA,which is easily adsorbed on the membrane surface and membrane pores, as can be seen in Table 1. The mass fraction of small molecules in PAAS is about 27.40%, which is calculated by the diafiltration results of PAAS solution results and mass balance, and the removal rate of small molecules can arrive at over 95% at suitable operating conditions.
The permeate flux in continuous diafiltration, slightly declines with time from the start, and then increases up to a pseudo-steady state (changes very little), as shown in Figure 3. The decrease of permeate flux in the initial period of diafiltration results from concentration polarization and membrane fouling. However, as the small PAAS molecules in the retentate continuously move through the membrane pores, the viscosity of PAAS solution decreases gradually up to a pseudo-steady state, and the permeate flux gradually increases up to a pseudo-steady state. Comparing Figures 3 and 2, the permeate flux is not so sensitive as permeate concentration in indicating the pretreatment effect as the viscosity of PAAS solution changes little although there are still some small molecules to be removed. Figure 4 indicates the pseudo-steady state permeate flux and removal rate of the small molecules for different initial concentration of PAAS solution. The permeate flux decreases with the initial concentration of PAAS, because the solution viscosity increases with the PAAS concentration, so the adsorptive fouling of the membrane surface increases. With the continuous removal of small molecules, fewer and fewer small molecules in the retentate are remained, so the feed concentration gets stable (only large molecules are kept in the feed) and the filtration turns into constant concentration process, and the permeate flux also gets stable.
3.2 Effect of solution pH
The effect of pH on pretreatment time and permeate flux are shown in Figures 5 and 6 at the initial PAAS concentration 200 mg/L and TMP 27.5 kPa. Figure 5 shows that the permeate flux decreases in the first 5–8 min and then increases up to a pseudo-steady value after 50–60 min at pH 5.0, 7.0 and 9.3, while the permeate flux gradually decreases all the way during the first 60 min at pH 3.0, then increases very little with time. The permeate flux increases with pH when pH is not greater than 7.0. At pH 3.0, the majority of carboxylic groups are combined with H+, exist as the form of poly(acrylic acid), which is not charged. Furthermore, pH 3.0 is just the iso-electric point (IEP) of PVB membrane [18], and the membrane is easily fouled at IEP. As there are no intramolecular and intermolecular repulsion forces at IEP, chain flexibility favors cluster conformation of the polymer, and the absence of intermolecular repulsion forces provokes aggregation of polymer molecules by hydrogen bond which promotes fouling and decreasing membrane flux. So it takes more time to remove all the small molecules at IEP. As the pH increases, the permeate flux increases up to a steady value at pH 7.0, as shown in Figure 6. After filtering for 70 min, the permeate concentration reaches 1.46 mg/L at pH 3.0, and it remains 2.49, 2.99 and 2.69 mg/L–1 at pH 5.0, 7.0 and 9.3, respectively. The permeate concentration reaches 0.14 mg/L after 180 min at pH 3.0, and it decreases to 0.051 mg/L, 0.028 and 0.023 mg/L, respectively, after 180 min at pH 5.0, 7.0 and 9.3. Thus, the complete removal of small molecules can be done at pH greater than IEP by diafiltration for 180 min, while it is difficult to remove all the small molecules at IEP due to easy formation of adsorptive fouling. Although the permeate concentration reaches 0.059 mg/L after 220 min at pH 3.0, it seems high removal rate of small molecules, the real removal rate of small PAAS is only 60.84%, which is calculated by mass balance, and the removal rate increases to 86.75% at pH 5.0, and near 100% at pH 7.0 and 9.3, as shown in Figure 6.
Table 1 Mass balance of PAAS at various feed concentration in dialfiltration at TMP 27.5 kPa

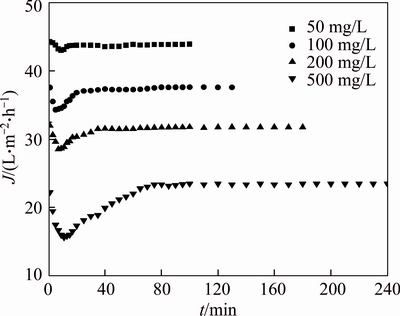
Figure 3 Variation of permeate flux with time for different original PAAS concentration in diafiltration (pH=7.0, TMP=27.5 kPa)
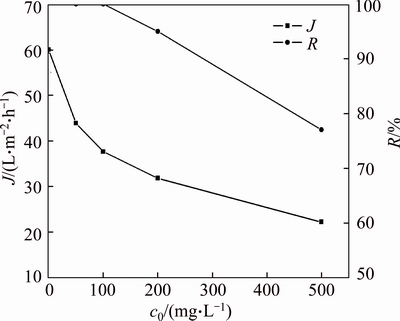
Figure 4 Steady state permeate flux and removal rate of small molecular PAAS for different initial concentrations of PAAS solution (pH=7.0, TMP=27.5 kPa)
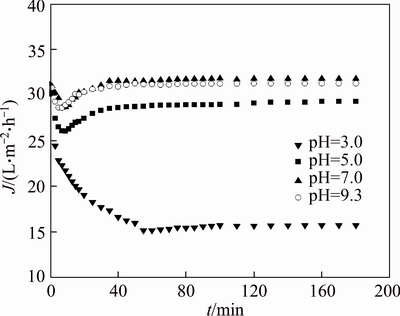
Figure 5 Variation of permeate flux with time at different pH values (TMP=27.5 kPa, c0=200 mg/L)
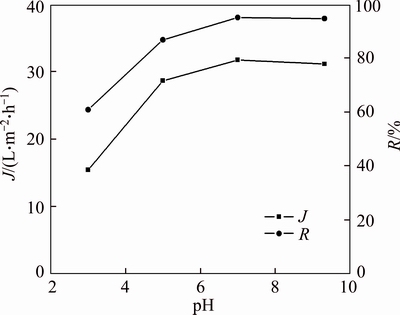
Figure 6 Steady state permeate flux and removal rate of small molecular PAAS at various pH (TMP=27.5 kPa, c0=200 mg/L)
Figure 6 indicates the steady (pseudo-steady) state permeate flux at different pH conditions and the removal rate of small molecular PAAS molecules. The permeate flux increases from 15.4 L/(m2·h) to 31.7 L/(m2·h) with the increase of pH from 3.0 to 7.0. The permeate fluxes at various pH values are affected not only by the characteristics of the membrane but also by the properties of the solution [19]. The negative charge of PAAS increases with the increase of pH, and the intramolecular and intermolecular repulsion forces of PAAS increase with pH, the negative charge of PVB membrane surface also increases with pH, so less membrane fouling is suffered with the increase of pH. The higher the pH, the more negative charged, the less adsorptive polymer, and the adsorptive polymer on the membrane surface becomes more “open” due to the inter-foulant repulsion, and accordingly the permeate flux increases.
3.3 Effect of TMP
The pH is transferred to 7.0 at the initial concentration of PAAS 200 mg/L. The effects of TMP on pretreatment time and permeate flux are discussed. Figure 7 indicates that for different TMP, the change of permeate concentration with time is similar. The permeate concentrations at 20 min are 20.89, 16.22, 10.99 and 8.96 mg/L, respectively, at TMP 27.5, 32.0, 38.0 and 44.0 kPa. The removal rate of small molecules increases with TMP at the same pretreatment time. The NPT decreases with TMP. It takes 70 min to remove 90 % of the small molecules at 27.5 kPa and only 40 min is needed at 44.0 kPa. Because the permeate flux increases with TMP, as shown in Figures 8 and 9, the small molecules are easily washed from the feed if permeate flux increases in continues diafiltration. Only 95% of small molecular PAAS was removed by 180 min of diafiltration at pH 7.0 and 27.5 kPa, while 100% was removed at 44.0 kPa. Comparing Figure 9 with Figure 8, the permeate flux is not so sensitive as permeate concentration in indicating the pretreatment effect. Figure 9 indicates that the permeate flux increases with TMP, but not in linear. This is a consequence of membrane fouling and concentration polarization, which is more serious as TMP increases [20, 21].
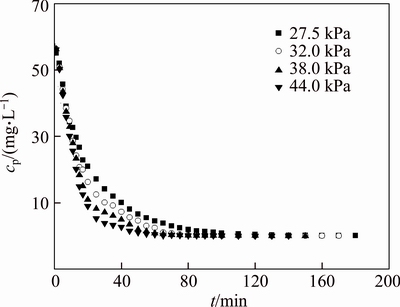
Figure 7 Variation of permeate concentration of PAAS with time at different TMP (pH=7.0, c0=200 mg/L)
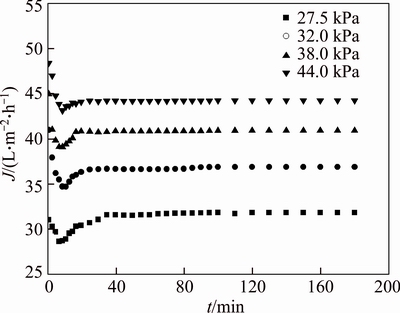
Figure 8 Variation of permeate flux with time at different TMP (pH=7.0, c0=200 mg/L)
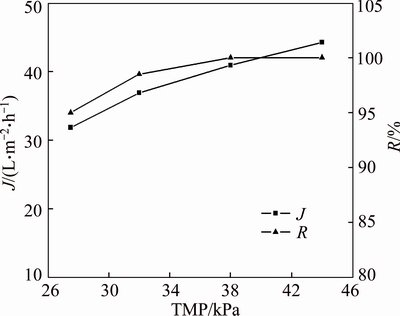
Figure 9 Permeate flux and removal rate of small molecular PAAS at various TMP (pH=7.0, c0= 200 mg/L)
3.4 Filtration potential
During pretreatment process, electrical potential across a membrane is studied, and it can no longer be considered as streaming potential but as a filtration potential [13, 21]. As the membrane rejects PAAS, electrical potential appears when a pressure gradient is applied across the membrane, as shown by Eq.(4) [21]:
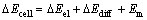 (4)
(4)
where △ECell is the electrical potential measured across the membrane during the filtration (mV), that is the filtration potential, △Eel is the electrode potential (mV) which depends only on the concentration of soluble compounds on the feed and permeate sides of the membrane, △Ediff is the electrical potential induced by the retrodiffusion of rejected solutes in the feed side, △Em is the membrane filtration potential. If the membrane is fouled by the solute, the filtration potential should be corrected as:
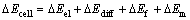 (5)
(5)
where △Ef is the electrical potential induced by the fouling layer of the membrane. The time revolutions of filtration potential in diafiltration are measured, as shown in Figures 10–12.Figure 10 shows the variations of filtration potential with time for different initial PAAS concentrations at TMP 27.5 kPa and pH 7.0. The filtration potentials decline rapidly from the start and then decrease slowly up to steady states. It takes different time for the filtration potential to get stable value for different initial concentration of PAAS solutions. For example, it takes about 50 min to get –45.4 mV and it takes about 90 min to get stable value –46.2 mV for the initial concentration of 50 mg/L PAAS solution, and it takes about 110 min to get –42.1 mV and 180 min to get stable value –42.5 mV for the initial concentration of 200 mg/L PAAS solution. Comparing Figure 10 with Figure 1, for the same initial PAAS concentration, the variation trend of filtration potential with time is similar to that of permeate concentration with time. Thus, the filtration potential can indicate the pretreatment effect in continuous diafiltration.

Figure 10 Variation of filtration potential with time for different initial PAAS concentrations (pH=7.0, TMP= 27.5 kPa)
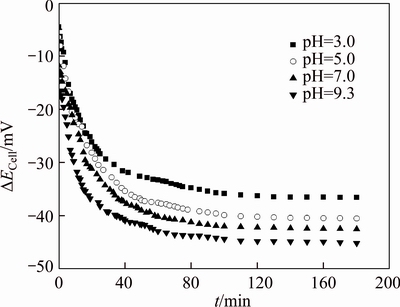
Figure 11 Variation of filtration potential with time at different pH (TMP=27.5 kPa, c0=200 mg/L)
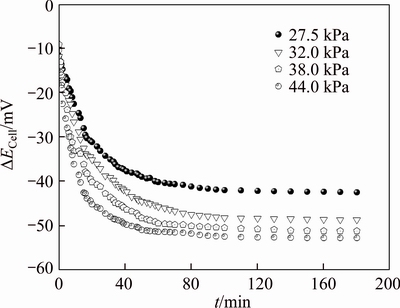
Figure 12 Variation of filtration potential with time at different TMPs (pH=7.0, c0=200 mg/L)
Figure 11 shows the variation of filtration potential with time for the initial concentration of 200 mg/L PAAS solution at TMP 27.5 kPa and different solution pHs. The filtration potentials decline rapidly from the start and then decrease slowly till steady states. The filtration potentials decrease very slowly after 120 min of diafiltration, which decreases from –36.3 to –36.6 mV at pH 3.0, –40.2 to –40.5 mV at pH 5.0, –42.2 to –42.5 mV at pH 7.0, –44.8 to –45.2 mV at pH 9.3, respectively, from 120 to 180 min. Comparing Figure 11 with Figure 7, at the same pH, the variation of filtration potential with time is similar to the variation of permeate concentration with time. Thus, the NPT is consistent with the needed time when filtration potential reaches a stable value.
Figure 12 indicates the variation of filtration potential with time at pH 7.0 and different TMPs for the initial concentration of 200 mg/L PAAS solution. The variations of filtration potential with time at different TMPs show similar change, and the steady state filtration potential decreases with the increase of TMP. The steady state filtration potentials at TMP 27.5, 32.0, 38.0 and 44.0 kPa are about –42.2, –48.5, –51.2 and –52.8 mV, respectively. Comparing Figure 12 with Figure 7, at the same TMP, the variation of filtration potential with time is similar to that of permeate concentration with time. Thus, the filtration potential can indicate the pretreatment effect in continuous diafiltration.
In diafiltration, the bulk solution volume keeps constant, as diafiltration goes on, the small PAAS molecules in the retentate continually pass through the membrane, causing the concentration of small PAAS molecules on both sides of the membrane decreases. This change of concentration will cause a change in △Eel.
It is certain that the PAAS concentration in permeate is zero when all the small PAAS molecules in the retentate are removed completely. That is to say, the concentration of PAAS in bulk solution is unchanged, only large molecules remain. This is the turning point that the diafiltration turns from concentration-dependent process into constant concentration process. △Eel reaches stability as the feed concentration keeps constant and permeate concentration gets near zero, at constant process, and △Ediff keeps constant due to the concentrate boundary layer becomes constant, △Ef also keeps constant as the adsorptive amount of PAAS molecules on the membrane surface is certain under a certain hydrodynamic condition at constant concentration diafiltration, and △Em is certain at a certain operating condition. Therefore, with small PAAS molecules continuous removing from feed fluid, the filtration potential △Ecell gradually gets stable. Thus, the filtration potential method may indicate the end of the pretreatment process more quickly and sensitively.
3.5 Comparison of pretreated and unpretreated PAAS on rejection of copper ions by PEUF
The polymer enhanced ultrafiltration (PEUF) experiments in laboratory-scale are carried out for the removal of copper ions from aqueous solution [22]. An initial feed of 2.5 L is introduced to the feed tank and was circulated through the apparatus, and the initial concentration of copper ions is 10 mg/L, and the pH is kept 6.0 by adjusting with 0.1 mol/L HCl and 0.1 mol/L NaOH. Flow rate of 40 L/h is controlled and operative pressure is set at 40 kPa. The temperature is kept at 25 °C with the help of a thermostatic water batch. In the total recycle process, the permeate stream is returned to the feed tank so that the concentration of feed is constant.
As copper ions easily form hydroxide precipitation in the alkaline solution, pH 6.0 is chosen to compare the rejection rates of copper ions using pretreated and unpretreated PAAS as complexing agent. Effect of P/M on the rejection of copper ions is studied, as shown in Figure 13, where P/M is the mass ratio of PAAS to metal ions, RCu is the rejection rate of copper ions. The rejection rate of copper ions increases from 50.7% to 100% when P/M increases from 4 to 25 for pretreated PAAS, a large mass ratio of PAAS to metal ions is necessary for complete rejection of Cu2+. However, the rejection rate of copper ions can arrive at only 74.4% even if the P/M increases up to 30 for unpretreated PAAS, because the small molecular fractions of PAAS can permeate the membrane along with the binding copper ions, causing the decrease of rejection. Thus, the pretreatment of PAAS is necessary for its application in PEUF.
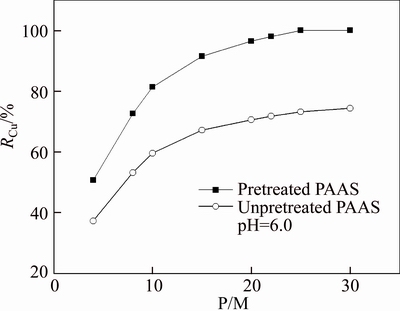
Figure 13 Comparison of P/M on rejection rate of copper ions by PEUF at pH 6.0, TMP=40.0 kPa, [Cu2+]=10 mg/L
4 Conclusions
A continuous diafiltration for the pretreatment of PAAS solution is adopted. The mass fraction of small molecules in PAAS is about 27.40%, and the removal rate of small molecules can arrive at over 95% at suitable operating conditions.
The effects of various operating parameters on pretreatment time, permeate flux and filtration potential are studied. The change trend of permeate flux with time is different at different pH conditions in diafiltration. The permeate flux decreases gradually with time up to a state value at pH 3.0 for PAAS solution, while it rapidly decreases from the start and then increases gradually up to stable value at pH>3.0. The NPT decreases with TMP. With all small molecules removing from the bulk solution, the filtration turns from concentration-dependent process into constant concentration process, and the filtration potential also gets stable. The time for the filtration potential to reach a stable state is consistent with the needed time of complete removal of small molecules. Thus, the filtration potential can indicate the pretreatment effect, while it is not enough for the permeate flux to indicate the pretreatment effect without permeate concentration in continuous diafiltration.
References
[1] CAMARILLO R, P REZ
REZ , CA
, CA IZARES P, de LUCAS A. Removal of heavy metal ions by polymer enhanced ultrafiltration [J]. Desalination, 2012, 286: 193–199.
IZARES P, de LUCAS A. Removal of heavy metal ions by polymer enhanced ultrafiltration [J]. Desalination, 2012, 286: 193–199.
[2] HUANG Y, WU D, WANG X, HUANG W, LAWLESS D, FENG X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation [J]. Sep Purif Technol, 2016, 158: 124–136.
[3] CHAKRABORTY S, DASGUPTA J, FAROOQ U, SIKDER J, DRIOLI E, CURCIO S. Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration [J]. J Membr Sci, 2014, 456: 139–154.
[4] MOLINARI R, ARGURIO P. Arsenic removal from water by coupling photocatalysis and complexation-ultrafiltration processes: A preliminary study [J]. Water Res, 2017, 109: 327–336.
[5] QIU Yun-ren, MAO Lian-jun, WANG Wei-hua. Removal of manganese from waste water by complexation-ultrafiltration using copolymer of maleic acid and acrylic acid [J]. Transactions of Nonferrous Metal Society of China, 2014, 24(4): 1196-1201.
[6] GAO Guo-ying, WEI Yu-qing, QIU Yun-ren. Treatment of wastewater containing nickel ions by polymer enhanced ultrafiltration with copolymer of acrylic acid-maleic acid [J]. Journal of Central South University: Sicence & Technology, 2012, 43(1): 54–58. (in Chinese)
[7] ZHAO L, ZHAO H, NGUYEN P, LI A, JIANG L, XIA Q, RONG Y, QIU Y, ZHOU J. Separation performance of multi-components solution by membrane technology in continual diafiltration mode [J]. Desalination, 2013, 322: 113–120.
[8] GAO Jing, QIU Yun-ren, HOU Ben, ZHANG Qiang, ZHANG Xiao-dong. Treatment of wastewater containing nickel by complexation-ultrafiltration using sodium polyacrylate and the stability of PAA-Ni complex in the shear field [J]. Chem Eng J, 2018, 334: 1878–1885.
[9] TANG Shu-yun, QIU Yun-ren. Removal of copper(II) ions from aqueous solutions by complexation– ultrafiltration using rotating disk membrane and the shear stability of PAA–Cu [J]. Chem Eng Res Des, 2018, 136: 712–720.
[10] SOFFER Y, GILRON J, ADIN A. Streaming potential and SEM-EDX study of UF membranes fouled by colloidal iron [J]. Desalination, 2002, 146: 115–121.
[11] LANTERI Y, SZYMCZYK A, FIEVET P. Membrane potential in multi-ionic mixtures [J]. Journal of Physical Chemistry B, 2009, 113: 9197–9204.
[12] CHIU T Y, JAMES A E. Electrokinetic characterisation techniques on asymmetric microfiltration membranes [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 301: 281–288.
[13] YAROSHCHUK A E, BOIKO Y P, MAKOVETSKIY A L. Filtration potential across membranes containing selective layers [J]. Langmuir, 2002, 18: 5154–5162.
[14] QIU Yun-ren, MATSUYAMA H. Preparation and characterization of poly(vinylbutyral) hollow fiber membrane via thermally induced phase separation with diluent polyethylene glycol 200 [J]. Desalination, 2010, 257: 117–123.
[15] QIU yun-ren, QI Jing,WEI Yu-qing. Synergistic action of non-solvent induced phase separation in preparation of poly(vinyl butyral) hollow fiber membrane via thermally induced phase separation [J]. Journal of Central South University,2014, 21(6): 2184–2190.
[16] PAULEN R, FIKAR M, KOV CS Z, CZERMAK P. Process optimization of diafiltration with time-dependent water adding for albumin production [J]. Chem Eng Process, 2011, 50: 815–821.
CS Z, CZERMAK P. Process optimization of diafiltration with time-dependent water adding for albumin production [J]. Chem Eng Process, 2011, 50: 815–821.
[17] RAPOSO F, DELARUBIA M, BORJA R, ALAIZ M. Assessment of a modified and optimised method for determining chemical oxygen demand of solid substrates and solutions with high suspended solid content [J]. Talanta, 2008, 76: 448–453.
[18] QIU Yun-ren, QI Jing. Electrokinetic characterization of poly(vinyl butyral) hollow fiber membranes by streaming potential and electroviscous effect [J]. J Membr Sci, 2013, 425/426: 71–76.
[19] SADRZADEH M, HAJINASIRI J, BHATTACHARJEE S, PERNITSKY D. Nanofiltration of oil sands boiler feed water: Effect of pH on water flux and organic and dissolved solid rejection [J]. Sep Purif Technol, 2015, 141: 339–353.
[20] VELASCO C, CALVO J, PALACIO L, CARMONA J, PR DANOS P, HERN
DANOS P, HERN NDEZ A. Flux kinetics, limit and critical fluxes for low pressure dead-end microfiltration. The case of BSA filtration through a positively charged membrane [J]. Chem Eng Sci, 2015, 129: 58–68.
NDEZ A. Flux kinetics, limit and critical fluxes for low pressure dead-end microfiltration. The case of BSA filtration through a positively charged membrane [J]. Chem Eng Sci, 2015, 129: 58–68.
[21] TEYCHENE B, LOULERGUE P, GUIGUI C, CABASSUD C. Development and use of a novel method for in line characterisation of fouling layers electrokinetic properties and for fouling monitoring [J]. J Membr Sci, 2011, 370: 45–57.
[22] QIU Yun-ren, MAO Lian-jun. Removal of heavy metal ions from aqueous solution by ultrafiltration assisted with copolymer of maleic acid and acrylic acid [J]. Desalination,2013, 329: 78–85.
(Edited by FANG Jing-hua)
中文导读
连续渗滤法预处理聚丙烯酸钠以及过滤电势随时间的变化
摘要:用超滤膜采用连续渗滤法对聚丙烯酸钠(PAAS)进行预处理以去除PAAS溶液中的小分子。研究了PAAS浓度、pH值、跨膜压差(TMP)和预处理时间对透过液浓度和渗透通量的影响。结果表明,所需预处理时间(NPT)随着PAAS浓度的增加而增加,随着TMP的增加而减小。渗透通量随时间的变化趋势受pH值的影响,在pH为5.0、7.0和9.3时,渗透通量开始迅速下降,然后随着渗滤时间的延长逐渐增加直至稳定值;然而,在等电点pH值为3.0时,渗透通量随着渗滤时间的延长逐渐减小。在pH大于等电点(IEP)进行渗滤时,小分子比较容易去除。过滤电势随时间的变化趋势与渗透浓度的变化趋势相似,但前者对预处理效果的指示更便捷。
关键词:预处理;渗滤;超滤;聚丙烯酸钠;过滤电势
Foundation item: Projects(21176264, 21476265) supported by the National Natural Science Foundation of China
Received date: 2018-07-03; Accepted date: 2018-09-10
Corresponding author: QIU Yun-ren, PhD, Professor; Tel: +86-731-88876616; E-mail: csu_tian@csu.edu.cn; ORCID: 0000-0003- 1705-5051

