Phase evolution and room temperature ferroelectric and magnetic properties of Fe-doped BaTiO3 ceramics
来源期刊:中国有色金属学报(英文版)2010年第10期
论文作者:邱深玉 李旺 刘宇 刘桂华 吴毅强 陈楠
文章页码:1911 - 1915
Key words:Fe-doped BaTiO3 ceramics; phase evolution; ferroelectricity; magnetism
Abstract: To make the ferroelectric BaTiO3 possess ferromagnetism simultaneously, magnetic Fe was doped into BaTiO3 ceramics at doping levels up to 10% (molar fraction). Both tetragonal and hexagonal phases coexisted in the Fe-doped BaTiO3 ceramics except at 1% doping level. X-ray diffraction analysis indicated that higher doping level of Fe, higher sintering temperature and longer sintering time promoted the formation of hexagonal phases in Fe-doped BaTiO3 ceramics. Ferroelectricity was observed in all samples at room temperature, but it was greatly depressed by Fe doping. Except at doping level of 1%, room-temperature ferromagnetism was observed in the BaTiO3 ceramics. The dependence of the saturation magnetization and coercivities of the Fe-doped BaTiO3 ceramics on doping level was systematically studied. Both the saturation magnetization and magnetic coercivities were found to be dependent on the doping level as well as the fraction of the hexagonal phase in the ceramics.

QIU Shen-yu(邱深玉)1, LI Wang(李 旺)2, LIU Yu(刘 宇)2, LIU Gui-hua(刘桂华)2,
WU Yi-qiang(吴毅强)3, CHEN Nan(陈 楠)2
1. Department of Science, Nanchang Institute of Technology, Nanchang 330099, China;
2. School of Materials Science and Engineering, Nanchang University, Nanchang 330031, China;
3. Department of Electronics, Nanchang University, Nanchang 330031, China
Received 11 November 2009; accepted 7 June 2010
Abstract: To make the ferroelectric BaTiO3 possess ferromagnetism simultaneously, magnetic Fe was doped into BaTiO3 ceramics at doping levels up to 10% (molar fraction). Both tetragonal and hexagonal phases coexisted in the Fe-doped BaTiO3 ceramics except at 1% doping level. X-ray diffraction analysis indicated that higher doping level of Fe, higher sintering temperature and longer sintering time promoted the formation of hexagonal phases in Fe-doped BaTiO3 ceramics. Ferroelectricity was observed in all samples at room temperature, but it was greatly depressed by Fe doping. Except at doping level of 1%, room-temperature ferromagnetism was observed in the BaTiO3 ceramics. The dependence of the saturation magnetization and coercivities of the Fe-doped BaTiO3 ceramics on doping level was systematically studied. Both the saturation magnetization and magnetic coercivities were found to be dependent on the doping level as well as the fraction of the hexagonal phase in the ceramics.
Key words: Fe-doped BaTiO3 ceramics; phase evolution; ferroelectricity; magnetism
1 Introduction
Multiferroic materials have received intensive attention in recent years[1-5]. This is mainly due to their potential applications based on the magnetoelectric coupling effect. A multiferroic material possesses two or more ferroic properties, which usually refer to ferroelectricity and ferromagnetism (or antiferro- magnetism)[5]. To introduce a ferromagnetic property into a ferroelectric material originally with no ferromagnetism by itself, one of the common approaches is to dope magnetic impurities into the ferroelectric host material[4]. The other approach is to combine a ferroelectric material and a ferromagnetic material together into a composite material, which subsequently shows multiferroic properties. Tetragonal BaTiO3, a well-known ferroelectric material with a perovskite structure[6-10], is a good candidate for this purpose. This is because the B-site Ti can be easily substituted by other transition metal ions including the magnetic ions, such as Fe ions[11-14].
In recent years, phase transformations of Fe-doped BaTiO3 ceramics were studied[11-15], and it was found that the B-site Ti can be substituted by Fe up to 70% (molar fraction) or higher. At high doping levels, such as 20% and up to 70% (molar fraction), it has been generally agreed[11-12] that the phase structure of Fe-doped BaTiO3 ceramics is hexagonal when it is sintered at 1 200 °C. However, for doping levels less than 10% (molar fraction), there existed some conflicting reports in the literature. Pure hexagonal structure was reported for Fe-doped BaTiO3 single crystals grown by the floating zone technique[13] with a doping level of 7% (molar fraction). On the contrary, pure tetragonal phase of Fe-doped BaTiO3 ceramics with doping level up to 2% (molar fraction) when sintered at 1 200 °C was reported by other researchers[14]. It is well-known that tetragonal BaTiO3 ceramics possesses good ferroelectricty, and this suggests that the Fe-doped BaTiO3 ceramics would be expected to have multiferroic properties if the Fe dopants can introduce ferromagnetism into the ferroelectric ceramics.
Ferromagnetism has been observed in hexagonal Fe-doped BaTiO3 ceramics at low doping levels[13]. For the doping levels at 0.5% and 1% (molar fraction), it has been shown that the hexagonal Fe-doped BaTiO3 single crystal does not show room-temperature ferromagnetism except at low temperatures around 2 K[13], while room-temperature ferromagnetism has been observed for other doping levels up to 7% (molar fraction) in Fe-doped BaTiO3 ceramics with pure hexagonal structure[13].
It is the motivation of this work to investigate the ferroelectric and ferromagnetic properties of the Fe-doped BaTiO3 ceramics at room temperature. To our knowledge, there is few research on the ferroelectric properties of Fe-doped BaTiO3 ceramics[15]. This could be due to the fact that hexagonal structure of Fe-doped BaTiO3 ceramics has been mainly reported in Refs.[11-13], while the typical ferroelectric BaTiO3 ceramic is of tetragonal structure. In addition, the room-temperature ferromagnetism of Fe-doped BaTiO3 ceramics at low doping levels has hardly been investigated systematically[13]. In this work, the solid-state reaction method was used to investigate the phase evolution of Fe-doped BaTiO3 ceramics at doping levels from 1% to 10% (molar fraction). The influence of the doping level of Fe on the evolution of tetragonal and hexagonal phases in Fe-doped BaTiO3 ceramics was studied.
2 Experimental
High-purity BaCO3 (99.99%), TiO2 (99.99%), and Fe2O3 (99.99%) powders were used as the source materials for synthesizing Fe-doped BaTiO3 ceramics. Appropriate amounts of BaCO3, TiO2 and Fe2O3 powders were weighed and mixed according to the formulae of BaTi1-xFexO3, where the doping level x = 0, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8% and 10% (molar fraction). The mixed powders were thoroughly ground in an agate mortar, and were then placed in a box furnace for calcination in air at 1 200 °C. Two cycles of calcination (each cycle in a period of 10 h) were conducted for each sample, and powders were ground once more between the two cycles of calcination processes. The calcined powders were pressed into pellets (two pellets for each sample) with a diameter of 10 mm and a thickness of 2-3 mm, which were then sintered in air for 2 h at 1 250 °C. Samples processed under different conditions were specified in the corresponding context of the next section. The phase structures of the sintered Fe-doped BaTiO3 ceramics were analyzed with the X-ray diffraction (XRD, Bruker D8 Focus) method. The ferroelectric properties were tested with TF Analyzer 2000, while the ferromagnetic properties were measured with a vibrating sample magnetometer (VSM, LakeShore Model 7404).
3 Results and discussion
3.1 Phase evolution
Figure 1 shows the XRD patterns of the Fe-doped BaTiO3 ceramics at doping levels of x= 0, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, and 10%. As expected, the BaTiO3 ceramics without Fe doping showed only tetragonal structure. At doping level of 1%, only tetragonal structure was observed, without detectable presence of any other phases. As the Fe doping level was increased from 2% up to 10%, the presence of hexagonal phases was clearly observed, and the XRD diffraction peaks for the hexagonal phases became more significant with the increase of doping level (Fig.1). To have a better view on the evolution behaviors of the tetragonal and hexagonal phases in the Fe-doped BaTiO3 ceramics, the strongest XRD peaks (101) for the tetragonal phase and (104) for the hexagonal phase are shown in the inset of Fig.1. At doping levels larger than 6%, the hexagonal phases became dominant in the Fe-doped BaTiO3 ceramics. The diffraction peaks for the hexagonal phases became stronger with the increase of Fe doping levels, and this indicates that Fe doping promotes the formation of hexagonal phase in Fe-doped BaTiO3 ceramics.
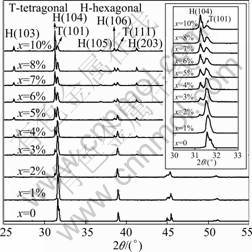
Fig.1 XRD patterns of BaTi1-xFexO3 ceramics
The presence of mixed phases (tetragonal and hexagonal) in Fe-doped BaTiO3 ceramics at doping levels from 2% to 10% (Fig.1) is different from the results reported in Refs.[13-14], in which either pure hexagonal phases or tetragonal phases were observed. This could be due to different processing conditions used in Refs.[13-15]. In order to verify this point, the effects of temperature and processing time on the phase structures in Fe-doped BaTiO3 ceramics were studied. It was found that higher temperature promoted the formation of the hexagonal phases in Fe-doped BaTiO3 ceramics. Fig.2(a) shows that for x = 10%, the XRD peak (104) of the hexagonal phases became stronger compared with the peak (101) of the tetragonal phases as the sintering temperatures were increased from 1 250 °C to 1 400 °C, each for a sintering time of 10 h. At 1 400℃, the Fe-doped BaTiO3 at 10% doping level was mostly hexagonal with very little tetragonal phases (Fig.2(a)). At the same temperature, longer processing time was found to promote the formation of hexagonal phases in Fe-doped BaTiO3 ceramics (Fig.2(b)). In Fig.2(b), for 5% Fe-doped BaTiO3 processed at 1 400 °C for 20 h, the tetragonal phase can hardly be detected by XRD. Thus, higher temperature and longer processing time will be necessary to synthesis of Fe-doped BaTiO3 ceramics with hexagonal structure, while lower temperature will be required to obtain Fe-doped BaTiO3 ceramics with tetragonal phases.
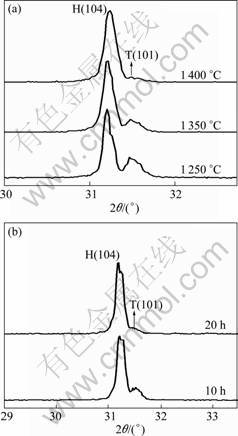
Fig.2 XRD patterns of BaTi1-xFexO3 ceramics: (a) x = 10%, sintered at different temperatures for 10 h; (b) x = 5%, sintered at 1 400 °C for 10 and 20 h
3.2 Ferroelectric and magnetic properties
The room-temperature ferroelectric and ferromagnetic properties of the Fe-doped BaTiO3 ceramics are shown in Fig.3. Compared with the undoped BaTiO3 ceramics, the ferroelectricity of the Fe-doped BaTiO3 ceramics was greatly suppressed by Fe doping (Fig.3(a)). At 1% Fe doping level, the remanent polarization (Pr) is 1.4 μC/cm2, which is about 1/6 that of the undoped BaTiO3 ceramics (8.2 μC/cm2, see Fig.3(a)). This indicates that impurities like Fe can be highly detrimental to the ferroelectric properties of BaTiO3 ceramics even at low doping levels. On the other hand, the strong suppression of ferroelectricity at 1% Fe doping level also implies that Fe ions were uniformly distributed in the BaTiO3 host.
With the increase of Fe doping level, the ferroelectricity of the Fe-doped BaTiO3 ceramics was
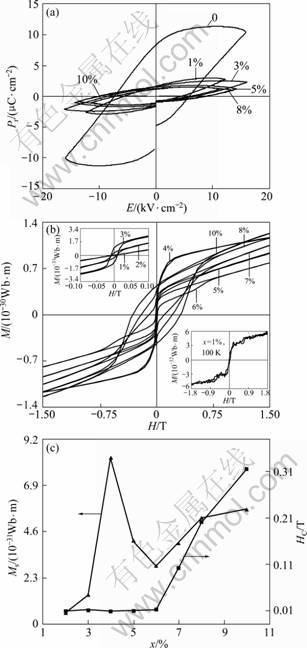
Fig.3 Room-temperature ferroelectric hysteresis loops of BaTi1-xFexO3 ceramics (a), room-temperature M-H curves of BaTi1-xFexO3 ceramics (b) and dependence of MS and HC on Fe doping level (c)
further reduced (Fig.3(a)). This is understandable because Fe doping promotes the formation of hexagonal phases (see Fig.1) that are not ferroelectric.
Room-temperature ferromagnetism was observed in the Fe-doped BaTiO3 ceramics at doping levels of 2%-10% (Fig.3(b)). However, as shown in the upper left inset of Fig.3(b), the 1% Fe-doped BaTiO3 ceramic did not show ferromagnetic behavior at room temperature, but rather paramagnetic. The undoped BaTiO3 ceramic also exhibited paramagnetic behavior (not shown here). The paramagnetic behavior of the 1% Fe-doped BaTiO3 ceramic in this work is consistent with the Ref.[8] which showed that 1% Fe-doped BaTiO3 single crystal was paramagnetic at room temperature. The reason for this similarity is probably because, at low doping level such as 1%, the average distance between neighboring Fe ions within the BaTiO3 matrix is so large that they are not be able to establish magnetic exchange interactions. On the other hand, the crystal structure for the 1% Fe-doped BaTiO3 ceramic was tetragonal in the current work (see Fig.1), while the 1% Fe-doped BaTiO3 single crystal was hexagonal in Ref.[13]. It is noted in Ref.[13] that the Fe-doped BaTiO3 single crystal was grown from molten Fe-doped BaTiO3. The higher temperature used in Ref.[13] is believed to be the reason why the 1% Fe-doped BaTiO3 single crystal in their work was hexagonal. This, in fact, is supported by the results (see Fig.2(a)) presented in previous paragraphs. When measured at 100 K, however, ferromagnetism exhibited in the 1% Fe-doped BaTiO3 ceramics (see the lower right inset of Fig.3(b)). It is noted that BaTiO3 changes from tetragonal to orthorhombic crystal structures at about 0 °C. However, the average distance between neighboring Fe ions should be little changed, and the chemical states should remain to be the same. This indicates that at low temperatures there existed magnetic exchange interactions among Fe ions distributed within the BaTiO3 host lattice at a doping level as low as 1%.
It is noted in Fig.3(b) that the magnetization M of the Fe-doped BaTiO3 ceramics at doping levels of 2% - 10% did not reach a complete saturation at room temperature. This suggests that there exist some paramagnetic and/or antiferromagnetic components within the ceramics. The presence of paramagnetic components in Fe-doped BaTiO3 ceramics was also reported in Ref.[13]. The magnetic coercivities (HC) remained very low and almost unchanged at about 1×10-2 T from x = 2% to 6%, and then suddenly jumped much higher to about 0.1 T at x = 7% and over 0.3 T at x = 10% (Fig.3(c)). It is known that HC has a large dependence on the microstructure of materials. Thus, we suppose that the dependence behavior of HC on the doping content should be a result of the changes in the microstructures of the Fe-doped BaTiO3 ceramics for
different x values. As shown in Fig.3(c), the saturation magnetization (MS) increased with x increasing to reach a maximum value at about 8.2×10-31Wb·m per Fe atom when x = 4%, followed by a decrease, and then increased again. It is noted that Fe3+ ions substituting for Ti4+ ions will introduce oxygen vacancies into BaTiO3. Such oxygen vacancies may act as a kind of medium through which superexchange interactions between neighboring Fe3+ ions occur. This explains the increasing behavior of MS with x until x = 4%. As x increases, some portion of Fe3+ ions may start to substitute for Ba2+ ions, and this will decrease the concentration of oxygen vacancies, i.e. MS will decrease. As x further increases, the average distance between neighboring Fe ions will decrease, and in the meantime, some small clusters of Fe ions may start to be present. This explains why MS increases again with x.
As shown in Fig.1, the Fe-doped BaTiO3 ceramics at doping levels of 2%-10% consisted of two phase components, tetragonal and hexagonal. Hexagonal phase component was shown by other researchers[13] to be ferromagnetic. It is, however, not possible in this work to individually measure the ferromagnetism originated from either of the two phase components, because they basically formed a composite material. But, as shown in Fig.2(b), it is possible to increase the volume fraction of the hexagonal phase component in the Fe-doped BaTiO3 ceramics by prolonging the sintering time, and then monitor the variation in ferromagnetism.
Figure 4 shows the room-temperature M-H curves of the 10% Fe-doped BaTiO3 ceramics sintered at 1 250 °C for a duration of 5, 10 and 15 h, respectively, while the upper left inset is the corresponding normalized XRD patterns, and the lower right inset is the dependence of MS and HC on the sintering time. With the increase of the sintering time, MS became greater in a monotonic manner, but HC loops decreased consistently. From the XRD pattern, it is clear that the intensity of the strongest XRD peak for tetragonal phase decreased consistently as the sintering duration was prolonged from 5 to 15 h, suggesting that the volume fraction of hexagonal phase component increased. This indicates that, at a fixed doping level of Fe, higher volume fraction of hexagonal phase component in the Fe-doped BaTiO3 ceramics will lead to greater MS and smaller HC. The decrease of HC with the increase of the hexagonal phase component could be due to less tetragonal/hexagonal phase interfaces, which may act as pinning centers for magnetic domains. The increase of MS may imply that the exchange interaction within the hexagonal phase component was stronger than that in the tetragonal phase component, but further evidence will be necessary.

Fig.4 Room-temperature M-H hysteresis loops for x = 10% sintered at 1 250 °C for 5, 10, and 15 h (Upper left inset: normalized XRD patterns; lower right inset: dependence of MS and HC on sintering time)
4 Conclusions
Fe-doped BaTiO3 ceramics with low doping levels of 1%-10% (molar fraction) were fabricated by the conventional solid state reaction method. Both tetragonal and hexagonal phases coexisted in the Fe-doped BaTiO3 ceramics except at doping level of 1%, at which hexagonal phase was not detected by XRD. The formation of hexagonal phases in the Fe-doped BaTiO3 ceramics was promoted by higher doping levels of Fe, higher sintering temperature, and longer sintering time. Room-temperature ferroelectricity was exhibited in all Fe-doped BaTiO3 ceramic samples, but it was greatly depressed by Fe doping. The ferroelectricity decreased with the increase of Fe doping level. Except at doping level of 1%, room-temperature ferromagnetism exhibited in the Fe-doped BaTiO3 ceramics. The saturation magnetization MS did not show a monotonic trend with the Fe doping level increasing. MS increased with doping level to reach a maximum value at 8.2×10-31Wb·m per Fe atom at doping level of 4%, followed by a decrease, and then increased again. The magnetic coercivities HC remained very low and almost unchanged at about 0.01 T at doping levels of 2%-6%, and then suddenly jumped much higher to about 0.1 T at doping level of 7% and over 0.3 T at doping level of 10%. At the same doping level, the increase of hexagonal phase component resulted in a higher MS and lower HC.
References
[1] BELIK A A, AZUMA M, SAITO T, SHIMAKAWA Y, TAKANO M. Crystallographic features and tetragonal phase stability of PbVO3, a new member of PbTiO3 family [J]. Chemistry of Materials, 2005, 17(1): 269-273.
[2] ZHANG H F, OR S W, CHAN H L. Fine-grained multiferroic BaTiO3/(Ni0.5Zn0.5)Fe2O4 composite ceramics synthesized by novel powder-in-sol precursor hybrid processing route [J]. Materials Research Bulletin, 2009, 44(6): 1339-1346.
[3] NAN C W, CAI N, LIU L, ZHAI J, YE Y, LIN Y. Coupled magnetic–electric properties and critical behavior in multiferroic particulate composites [J]. Journal of Applied Physics, 2003, 94(9): 5930-5936.
[4] SINGH M P, PRELLIER W, MECHIN L, SIMON C, RAVEAU B. Correlation between structure and properties in multiferroic La0.7Ca0.3MnO3/BaTiO3 superlattices [J]. Journal of Applied Physics, 2006, 99(2): 024105.
[5] ZURBUCHEN M A, WU T, SAHA S, MITCHELL J, STREIFFER S K. Multiferroic composite ferroelectric-ferromagnetic films [J]. Applied Physics Letters, 2005, 87(23): 232908.
[6] KOLODIAZHNYI T. Insulator-metal transition and anomalous sign reversal of the dominant charge carriers in perovskite BaTiO3?[J]. Physical Review B, 2008, 78(4): 045107.
[7] RAVEL B, STERN E A, VEDRINSKII R I, KRAISMAN V. Local structure and the phase transitions of BaTiO3 [J]. Ferroelectrics, 1998, 206(1): 407-430.
[8] MATTHEISS L F. Effect of the 110 K phase transition on the SrTiO3 conduction bands [J]. Physical Review B, 1972, 6(12): 4740-4753.
[9] HEIFETS E, KOTOMIN E, TREPAKO V A. Calculations for antiferrodistortive phase of SrTiO3 perovskite: Hybrid density functional study [J]. Journal of Physics: Condensed Matter, 2006, 18(20): 4845-4851.
[10] ITIE J P, VOUZINET B, POLIAN A, FLANK A M, LAGARDE P. Pressure-induced disappearance of the local rhombohedral distortion in BaTiO3 [J]. Europhysics Letters, 2006, 74(4): 706-711.
[11] GREY I E, LI C, CRANSWICK L M D, ROTH R S, VANDERAH T A. Structure analysis of the 6H–Ba(Ti, Fe3+, Fe4+)O3?solid solution [J]. Journal of Solid State Chemistry, 1998, 135(2): 312-321.
[12] MASHKINA E, McCAMMON C, SEIFERT F. A Mossbauer study of oxygen vacancy and cation distribution in 6H-BaTi1-xFexO3-x/2 [J]. Journal of Solid State Chemistry, 2004, 177(1): 262-267.
[13] RAY S, MAHADEVAN P, MANDAL S, KRISHNAKUMAR S R, KURODA C S, SASAKI T, TANIYAMA T, ITOH M. High temperature ferromagnetism in single crystalline dilute Fe-doped BaTiO3 [J]. Physical Review B, 2008, 77(10): 104416.
[14] ZHANG N, FAN J F, RONG X F, CAO H X, WEI J. Magnetoelectric effect in laminate composites of Tb1-xDyxFe2-y and Fe-doped BaTiO3 [J]. Journal of Applied Physics, 2007, 101(6): 063907.
[15] REN X, OTSUKA K. Universal symmetry property of point defects in crystals [J]. Physical Review Letters, 2000, 85(5): 1016-1019.
(Edited by YANG Bing)
Foundation item: Project(60661001) supported by the National Natural Science Foundation of China
Corresponding author: CHEN Nan; Tel: +86-13755789807; Email: nanchen@ncu.edu.cn
DOI: 10.1016/S1003-6326(09)60394-0

