
Flotation separation of marmatite from pyrrhotite using DMPS as depressant
SUN Wei(孙 伟), LIU Run-qing(刘润清), CAO Xue-feng(曹学锋), HU Yue-hua(胡岳华)
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 18 July 2005; accepted 7 October 2005
Abstract: Mercapto organic compound DMPS was used as the depressant for separation of copper activated marmatite from pyrrhotite in the presence of butyl xanthate. The flotation tests of single mineral show that DMPS has strong depressing effect on pyrrhotite in the absence and presence of copper ion, but has activated effect on marmatite in the presence of copper ion. The floatability of marmatite is improved in the pH range of 2-12. The flotation test of mixture mineral shows that copper-activated marmatite can be separated efficiently from pyrrhotite using DMPS as depressant. Infrared adsorption spectra demonstrate that there are a number of function groups such as —SH and —SO3 in the molecular structure of DMPS. Xanthate and DMPS compete in their adsorption on sulfide minerals.
Key words: flotation; marmatite; pyrrhotite; depressant; separation
1 Introduction
Pyrrhotite and marmatite are found in most sulfide ore bodies together. Their separation becomes an important issue. In the flotation of complex sulfide ores, high pH value is generally used to separate valuable sulfide minerals from pyrrhotite or pyrite with xanthate collectors[1, 2]. About surface oxidation and flotation behavior of pyrrhotite, some scholars revealed the surface reaction and reaction product of pyrrhotite by many kinds of methods such as X-ray photoelectron spectrum(XPS), cyclic voltammetry and chemical analysis[3-6]. It has been indicated that mineral oxidation degree, pulp pH value and potential have great effect on flotation behavior of pyrrhotite. HEYES and TRAHAR[7] showed that the proper oxidization of pyrrhotite could be collectorless flotation under acid condition. CHENG et al[8] carried out the electro- chemical study of pyrrhotite cathode interaction in non-oxygen solution at the natural pH value, the results showed that pyrrhotite was not floatable in the absence of collector. BUSWELL and NICOL[9] studied the flotation electrochemical behavior of pyrrhotite to show that the pyrrhotite interacted with xanthate forming dixanthogen. Although there have been a great deal of reports about flotation mechanism of sphalerite, there are few reports on flotation of marmatite. NAGARAJ[10] indicated that polyacrylamide polymers containing various functional groups could depress iron sulfide minerals. In particular, recently BOULTON et al[11] showed that low molecular mass PAM polymers could be used to separate copper-activated sphalerite efficiently from pyrite by flotation in the presence of isobutyl xanthate. CHEN and XUAN[12] used combined depressant calcium chloride with sodium humate to successfully separate the mixture of marmatite and pyrrhotite which was activated by cupric ions with potassium butyl xanthate as a collector.
The inorganic depressants such as cyanide[13,14], lime were successfully applied in the flotation separate of sulfide ores. Their uses are challenged due to the cost and environmental impact. Compared with inorganic depressants, organic depressants have the characteristic of better selectivity, and low-pollution. In recent years, organic depressant were paid attention by many scholars. Thioglycolic acid[15], diethylenetriamine[16,17] have been used to depress iron sulfide minerals. Polymers are well-known depressants of both sulfide and non-sulfide minerals and have also been used to depress pyrite [18-20]. XU et al[21] found that the new organic depressant RC had a strong depression effect on pytite and pyrrhotite.
In this study, small molecular mercapto organic DMPS were used to separate copper-activated marmatite from pyrrhotite in the presence of butyl xanthate(BX). The effects of DMPS on flotation recoveries of marmatite and pyrrhotite were studied as the functions of pH value and DMPS concentration. Furthermore, research on the mechanisms of mineral-DMPS interaction was investigated by infrared spectroscopy.
2 Experimental
2.1 Materials
Pyrrhotite and marmatite samples used in this study were from Dachang Mine of Guangxi Province. Chemical analysis of two mineral samples indicated that their purity were 93.86% and 96.51%, respectively. These pure mineral lumps were crushed, handpicked and ground, fraction of <100 μm was obtained by screening as flotation samples of single mineral and mixture mineral.
The collector used was potassium butyl xanthate, which was industrial grade product from Reagent Factory of Zhuzhou, Butyl ether alcohol was employed as the frother, and also industrial grade product. The organic depressant was DMPS, which is chemical pure. Hydrochloric acid and sodium hydroxide were used as adjusting reagent. Both of them were of analytical grade. In the flotation test of mixture mineral, sodium silicate was used as the dispersant. Distilled water was used in flotation test.
2.2 Flotation test
In each flotation test, 2.0 g of sample was put into a beaker and treated to clean the surface for 5 min using supersonic cleaner. The sample was settled for 10 min, and upper layer liquid was decanted, flotation of the minerals were carried out in flotation machine with a cell of total pulp volume of 40 mL. After adjusting pH to appropriate value, the mineral pulp was conditioned with CuSO4 , depressant collector and frother in turn. The conditioning time for the CuSO4 was 2 min, that for the depressant was 3 min, that for collector was 2 min, that for frother was 1 min, and the flotation time was 3 min. The floating and non-floating fractions were filtrated, dried and weighed.
In flotation separation test of artificial mixture minerals, 0.8 g marmatite and 1.2 g pyrrhotite were taken, the surface was cleaned using supersonic cleaner, respectively. Then the sample was transferred into 40 mL cell, adding dispersant and agitating for 1 min, other steps are in accord with those in single mineral flotation test. Products were filtrated, dried and weighed to calculate the recovery.
2.3 Infrared spectra
In order to study the mechanism of mineral- depressant interaction, 1.0 g sample was immersed in 25 mL corresponding reagent solutions, using an agate pestle and mortar handground for 30 min, then settled for 30 min, filtrated, flushed 2-3 times using corre- sponding pH buffer solution. The solid obtained was vacuum dried. Infrared spectra were recorded by NEXUS-470 infrared spectrum apparatus.
3 Results and discussion
3.1 Flotation behavior of marmatite and pyrrhotite
The effect of pH on the flotation recovery of pyrrhotite and marmatite in the presence and absence of CuSO4 with butyl xanthate as a collector is shown in Fig.1. Pyrrhotite can be seen to float best at various pH values, and the recovery exceeds 80%. Marmatite floats best under acid condition and the recovery is more than 90%. The recovery drops fast when pH>6. In the presence of cupric ions, the recovery of pyrrhotite does not vary at pH<8. but when pH>8, the recovery decreases by more than 40%. On the other hand, cupric ions promote the flotation of marmatite under tested pH values. The results in Fig.1 also indicate that in the absence and presence of cupric ions, separation of marmatite from pyrrhotite is difficult with butyl xanthate collector.
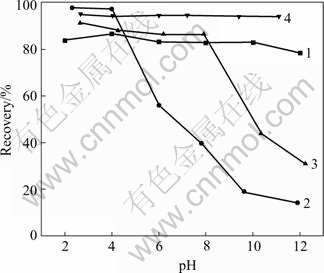
Fig.1 Effect of pH on flotation recovery of pyrrhotite and marmatite using 10-4mol/L KBX as collector: 1 Pyrrhotite; 2 Marmatite; 3 Pyrrhotite+10-4 mol/L CuSO4; 4 Marmatite+ 10-4 mol/L CuSO4
The effect of DMPS on the flotation recovery of pyrrhotite and marmatite in the presence and absence of CuSO4 with butyl xanthate is shown in Fig.2. This follows that the flotation of pyrrhotite and marmatite is greatly affected by DMPS addition. In absence of cupric ion the recovery of pyrrhotite hardly exceeds 40%. Only at pH=2, the recovery of marmatite is more than 90%, but the recovery continuously sharply decreases to below 20% with increasing pH value. The results show that pyrrhotite and marmatite can not be separated in absence of cupric ion with DMPS as s depressant and xanthate as a collector. In the presence of cupric ions, marmatite flotation is improved under the wide pH condition. The flotation of pyrrhotite is activated around pH 2. The results demonstrate that flotation separation of copper-activated marmatite from pyrrhotite is possible in the presence of butyl xanthate and DMPS.
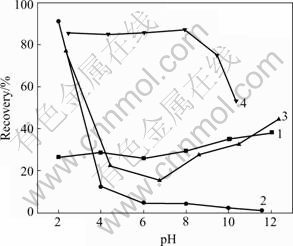
Fig.2 Flotation recovery of mineral as function of pH using 10-4 mol/L KBX as collector and 2×10-4 mol/L DMPS as depressant: 1 Pyrrhotite; 2 Marmatite; 3 Pyrrhotite+10-4 mol/L CuSO4+2×10-4 mol/L DMPS; 4 Marmatite+10-4 mol/L CuSO4 +2×10-4 mol/L DMPS
The flotation response of pyrrhotite and marmatite against DMPS concentration at pH 6 is presented in Fig.3. DMPS has a strong depression effect on pyrrhotite. The recovery is not more than 40% even at around 1×10-4 mol/L depressant. The minimum recovery is obtained at 2×10-4 mol/L depressant. While the concentration of DMPS increases continuously, the recovery does not drop distinctly. For marmatite, when the DMPS concentration is 1×10-4 mol/L, the effect of depression is optimal; the recovery is only 10.50%, beyond this point, the depressant effect is deteriorated.
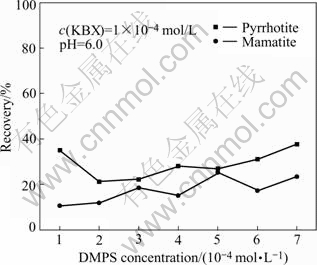
Fig.3 Effect of DMPS concentration on flotation recovery of mineral
3.2 Separation of artificial mixture mineral
In the light of single mineral flotation test, hydrochloric acid and sodium hydroxide were used to adjust pulp pH value, the separation test of manual mixture mineral was performed under the condition of various pH, DMPS concentration was 2×10-4 mol /L, butyl xanthate 1×10-4 mol/L, butyl ether alcohol concen- tration was 16 mg/L, dispersant concentration was 20 g/L. The results are shown in Table 1.
Table 1 Result of flotation separation of copper-activated marmatite-pyrrhotite manual mixture mineral
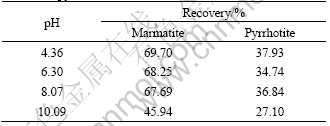
From Table 1, it can be seen that in the range of pH 4-8 the recovery of marmatite is approximately 70%, while pyrrhotite is less than 38%. On the other hand, when pH=10.09, the recovery of marmatite and pyrrhotite drops, the separation of marmatite and pyrrhotite becomes difficult. From these results it is indicated that the separation of marmatite and pyrrhotite is possible in the region of pH 4-8 with DMPS as a depressant and xanthate as a collector.
3.3 Mechanism of mineral-DMPS interaction
The result of the IR spectra of DMPS presented in Fig.4 shows the —SOH and —SH stretching vibrations corresponding to 3 454 cm-1 and 1 636 cm-1, respec- tively. 1 360 cm-1 is —CH2 inner vibration which connects with —SH and outside twist vibration of —SH. 1 190 cm-1 comes from CH—CH outside twist vibration, it may be asymmetrical stretching vibration of —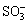 group. 1 029.65 cm-1 comes from symmetrical stretching vibration of —
group. 1 029.65 cm-1 comes from symmetrical stretching vibration of —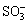 group, and 524 cm-1 is inner winding vibration of —SO3.
group, and 524 cm-1 is inner winding vibration of —SO3.
Fig.5 shows the IR spectra of pyrrhotite in mixture solution of butyl xanthate and DMPS. Butyl xanthate has characteristic IR peaks at 3 407.42, 2 961.28, 2 873.73, 1 462.22, 1 374.84 and 1 112.21 cm-1, however, no trace of butyl xanthate is shown in the spectra of curve 3 given in Fig.5. Only the adsorptive peak of DMPS is shown on the mineral surface. This indicates that DMPS is easier to adsorb than butyl xanthate on the mineral surface.
Fig.6 shows the IR spectra of marmatite interaction with reagent. From curve 3 we can see that there is no trace of butyl xanthate adsorption, but there are characteristic peaks of DMPS (3 450.88 cm-1, 1 360.61 cm-1). After cupric ion activation, surface adsorptive products of marmatite are xanthic acid cuprous. According to Ref.[12], dixanthogen has characteristic IR band in the 1 024-1 269 cm-1 region, the xanthic acid heavy metal salt has characteristic bands in the 1 190- 1 200 cm-1 region. By analysis IR bands in the 1 000- 1 300 cm-1 region, a few of dixanthogen adsorption peaks are found in Figs.5 and 6, The result indicates that in the presence of cupric ion and DMPS, metal xanthate forms on marmatite surface but does not form on pyrrhotite surface accounting for good flotation of copper activated marmatite in the presence of DMPS.
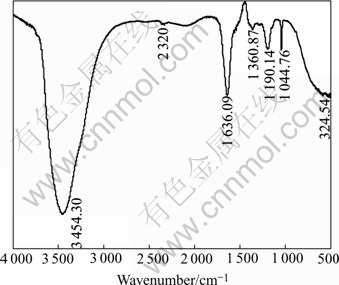
Fig.4 IR spectral analysis of DMPS
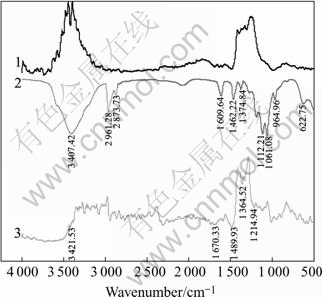
Fig.5 IR spectra of pyrrhotite: 1 Pyrrhotite; 2 Butyl xanthate; 3 Pyrrhotite+butyl xanthate+DMPS
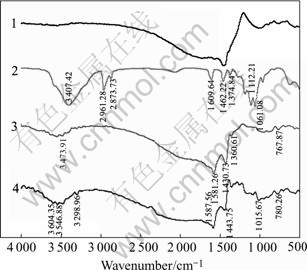
Fig.6 IR spectra of marmatite: 1 Marmatite; 2 Butyl xanthate; 3 Marmatite+butyl xanthate+DMPS; 4 Marmatite+CuSO4+ butyl xanthate+DMPS
4 Conclusions
1) The small molecular mercapto organic depressant DMPS has strong depressing effect on both pyrrhotite and marmatite in the absence of copper ion. Copper ion can activate marmatite flotation and has not improved pyrrhotite flotation in the presence of DMPS.
2) The separation of manual mixture mineral show that DMPS can be used to separate efficiently copper-activated marmatite from pyrrhotite by flotation in the pH range of 4-8, DMPS concentration is 2×10-4 mol/L, butyl xanthate is 1×10-4 mol/L, butyl ether alcohol concentration is 16 mg/L, dispersant concentra- tion is 20 g/L.
3) Infrared adsorption spectra demonstrate that there are a number of functional groups such as —SH, —SO3 in the molecular structure of DMPS. DMPS is easier to adsorb on the mineral surface than butyl xanthate.
References
[1] SHEN W Z, FORNASIERO D, RALSTON J. Effect of collectors conditioning pH and gases in the separation of sphalerite from pyrite [J]. Miner Eng, 1998, 112, 145-158.
[2] BALL B, RICKARD R S. The chemistry of pyrite flotation and depression [A]. FUERSTENAU M C, FLOTATION A M. Gaudin Memorial (Volume 1) [C]. NEW York: AIME, 1976. 458-484.
[3] BUCKLEY A N, WOODS R. X-ray photoelectron spectroscopy of oxidized pyrrhotite surfaces (Ⅰ) exposure to air [J] . Appl Surf Sci, 1985, 22/23: 280-287.
[4] BUCKLEY A N, WOODS R. X-ray photoelectron spectroscopy of oxidized pyrrhotite surfaces (Ⅱ) exposure to aqueousolutions[J]. Appl Surf Sci, 1985, 20: 472-480.
[5] HAMILTON I C, WOODS R. An investigation of surface oxidation of pyrite and pyrrhotite by linear potential sweep voltammetry [J]. J Electroanal Chem, 1981, 118: 327-343.
[6] STEGER H F. Oxidation of sulfide minerals, VII effect of temperature and relative humidity on the oxidation in of pyrrhotite [J]. Chem Geol, 1982, 35: 281-295.
[7] HEYES G W, TRAHAR W J. Proceedings of the international symposiumon electro-chemistry in mineral and metal processing [J]. Electrochem Soc Inc, 1984, 10: 219-287.
[8] CHENG X, IWASAKI I, SMITH K A. An electrochemical study on cathodic decomposition behavior of pyrrhotite in deoxygenated solutions [J]. Mineral & Metallurgical Processing, 1994, 1(3): 160-167.
[9] BUSWELL A M, NICOL M J. Some aspects of applied electro- chemistry of the flotation of pyrrhotite [J]. Journal of Applied Electrochemistry, 2002, 32(12): 1321-1329.
[10] NAGARAJ D R. Development of new flotation chemicals [J]. Trans Indian Inst Met, 1997, 50: 355-363.
[11] BOULTON A, FORNASIERO D, RALSTON J. Selective depression of pyrite with polyacrylamide polymers [J]. Int J Miner Process, 2001, 61: 13-22.
[12] CHEN Jin-zhong, XUAN Dao-zhong. A new method for separation of marmatite from pyrrhotite by flotation [J]. Nonferrous Metals, 1994, 46(1): 34-39.
[13] GHIANI M, SATTA F, BARBARO M, PASSARIELLO B. Flotation of sphalerite from pyrite by use of copper sulphate and sodium cyanide [A]. JONES M J, OBLATT R. Reagents in the Mineral Industry [C]. London: The Institution of Mining and Metallurgy, 1984. 89-93.
[14] BALL B, RICKARD R S. The chemistry of pyrite flotation and depression [A]. FUERSTENAU M C, FLOTATION. A M. Gaudin Memorial [C]. New York: AIME, 1976. 458-484.
[15] CHMIELEWSKI T, WHEELOCK T D. Thioglycolic acid as a flotation depressant for pyrite [A]. DUGAN P R, QUIGLEY D R, ATTIA Y A. Processing and Utilisation of High Sulfur Coals IV [C]. Amsterdam: Elsevier, 1991. 295-307.
[16] BASILIO C I, MARTICORENA M A, KERR A N, STRATTON- CRAWLEY R. Studies of pyrrhotite depression with diethylene- triamine [A]. Proc XIX Int Miner Process Congr [C]. Littleton: SME, 1995. 275-279.
[17] KELEBEK S, FEKETE S O, WELLS P F. Selective depression of pyrrhotite using sulphur dioxide–diethylenetriamine reagent com- bination [A]. Proc XIX Int Miner Process Congr [C]. Littleton: SME, 1995. 181-187.
[18] BOGUSZ E, BRIENNE S R, BUTLER I, RAO S R, FINCH J A. Metal ions and dextrin adsorption on pyrite [J]. Miner Eng, 1997, 10(4): 441-445.
[19] BOLIN N J, LASKOWSKI J S. Polysaccharides in flotation of sulfides (Part II): Copperrlead separation with dextrin and sodium hydroxide [J]. Int J Miner Process, 1991, 33: 235-241.
[20] KYDROS K A, GALLIOS G P, MATIS K A. Modification of pyrite and sphalerite flotation by dextrin [J]. Sep Sci Technol, 1994, 29-17: 2263-2275.
[21] XU Jing, SUN Wei, ZHANG Qin, LIU Hui, HU Yue-hua. Research on depression mechanism of pyrite and pyrrhotite by new organic depressant RC [J]. Mining and Metallurgical Engineering, 2003, 23(6): 27-29.
Foundation item: Project(50234010) supported by the National Natural Science Foundation of China
Corresponding author: HU Yue-hua; Tel: +86-731-8879815; E-mail: HYH@mail.csu.edu.cn
(Edited by LI Xiang-qun)