ZnO/ZnAl2O4异质结光催化剂的 光催化降解和杀灭大肠杆菌性能
来源期刊:中国有色金属学报(英文版)2014年第3期
论文作者:张 丽 阎建辉 周民杰 余艳萍 刘 晔 刘又年
文章页码:743 - 749
关键词:ZnO/ZnAl2O4;光催化;降解;杀菌;异质结
Key words:ZnO/ZnAl2O4; photocatalysis; degradation; inactivation; heterojunction
摘 要:通过共沉淀法制备ZnO/ZnAl2O4纳米异质结光催化剂,利用HRTEM、TEM、XRD、BET、TG-DTA和UV-Vis DRS测试方法对样品进行表征。在模拟太阳光照射下,通过测定甲基橙溶液的光催化降解率和对大肠杆菌的杀灭率来评价样品的光催化活性。研究催化剂的组成、焙烧温度、催化剂的用量和不同光源对样品光催化活性的影响。结果表明,当原料中Zn与Al摩尔比为1:1.5时,在600 °C焙烧所得的催化剂具有最佳光催化活性。在模拟太阳光照射下,在50 min内1.0 g/L光催化剂对甲基橙的降解率达98.5%;在60 min内,在相同条件下对大肠杆菌(106 CFU/mL)的杀菌率达到99.8%。
Abstract: ZnO/ZnAl2O4 nanocomposites with heteronanostructures were successfully prepared by co-precipitation method. The as-prepared samples were characterized by HRTEM, TEM, XRD, BET, TG-DTA, and UV-Vis spectra techniques. The photocatalytic activities of the as-prepared samples were evaluated by the photocatalytic degradation of methyl orange and inactivation of Escherichia coli in suspension under the irradiation of the simulated sunlight. The effects of compositions, calcination temperatures, concentration of photocatalysts and light source on the photocatalytic activities were systematically studied. The results show that when the concentration of ZnO/ZnAl2O4 photocatalyst with the starting Zn to Al molar ratio of 1:1.5 calcined at 600 °C is 1.0 g/L, the maximum photocatalytic degradation rate of 98.5% can be obtained in 50 min under the irradiation of the simulated sunlight. Under the same conditions, an inactivation rate of 99.8% for E.coli is achieved in 60 min.
Trans. Nonferrous Met. Soc. China 24(2014) 743-749
Li ZHANG1,2,3, Jian-hui YAN1,2,3, Min-jie ZHOU1,3, Yan-ping YU1, Ye LIU1, You-nian LIU2
1. College of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang 414006, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. Hunan Province Key Laboratory of Catalysis and Separation of Speciality Petrochemicals, Hunan Institute of Science and Technology, Yueyang 414006, China
Received 22 October 2012; accepted 13 May 2013
Abstract: ZnO/ZnAl2O4 nanocomposites with heteronanostructures were successfully prepared by co-precipitation method. The as-prepared samples were characterized by HRTEM, TEM, XRD, BET, TG-DTA, and UV-Vis spectra techniques. The photocatalytic activities of the as-prepared samples were evaluated by the photocatalytic degradation of methyl orange and inactivation of Escherichia coli in suspension under the irradiation of the simulated sunlight. The effects of compositions, calcination temperatures, concentration of photocatalysts and light source on the photocatalytic activities were systematically studied. The results show that when the concentration of ZnO/ZnAl2O4 photocatalyst with the starting Zn to Al molar ratio of 1:1.5 calcined at 600 °C is 1.0 g/L, the maximum photocatalytic degradation rate of 98.5% can be obtained in 50 min under the irradiation of the simulated sunlight. Under the same conditions, an inactivation rate of 99.8% for E.coli is achieved in 60 min.
Key words: ZnO/ZnAl2O4; photocatalysis; degradation; inactivation; heterojunction
1 Introduction
Since the first application of TiO2 photocatalyst under UV irradiation for microbial inactivation by MATSUNAGA et al in 1985 [1], much effort has been devoted to developing semiconductor photocatalysts with high photocatalytic activities in air purification, disinfection, and water treatment process [2-4]. Among various semiconductor photocatalysts, the hetero- structures fabricated by the coupling of different semiconductor materials possess significant advantages for promoting the separation of electron-hole pairs and achieving a higher photocatalytic activity compared with single-phase photocatalyst [5,6]. ZnO is well known as a preferable material for a variety of environmental applications due to its high photosensitivity, non-toxic nature, low cost and chemical stability [7,8]. It is even more efficient than TiO2 in the photodegradation [9]. However, the quick recombination of e-and h+ pairs is the major limitation in achieving high photocatalytic efficiency. Studies indicated that the composite between ZnO and another nonsensitive semiconductor (usually having very wide band gap), e.g., In2O3 (Eg=3.6 eV) [10], SnO2 (Eg=3.8 eV) [11], and NiO (Eg=3.5 eV) [12], also has been proved to be an effective method to improve their photocatalytic activity. Spinel ZnAl2O4 with a broad bandgap of 3.8 eV has attracted considerable attention since it can be used as catalyst [13], and catalyst support [14] due to its high thermal stability, low surface acidity, and high specific surface area [15].
In the present work, ZnO/ZnAl2O4 heterostructure photo- catalyst with high photocatalytic activity was synthesized. Subsequently, the composition, morphology and UV-absorbing properties of the resulting nanocomposites were systematically investigated by HRTEM, TEM, XRD and UV-Vis spectra techniques. Finally, the photocatalytic activities of the ZnO/ZnAl2O4 nanocomposites were evaluated by the degradation of methyl orange (MO) and inactivation of Escherichia coli (E. coli).
2 Experimental
2.1 Sample synthesis
Zn(NO3)2.6H2O and Al(NO3)3.9H2O were employed as starting materials. Firstly, a mixture of Zn(NO3)2·6H2O and Al(NO3)3·9H2O with a certain Al to Zn molar ratio (varying from 1.1 to 2) in the starting materials was dissolved into deionized water to form a clear solution with a total cationic concentration of 30 mmol/L. Subsequently, aqueous ammonia was added under constant stirring to the above solution until pH reaching 7-8 to ensure complete precipitation. The gel solution was aged for 24 h before filtration. After filtration and washing with distilled water and ethanol several times, the obtained sample was dried in an oven at 80 °C overnight. Finally, the samples were obtained after caclination at different temperatures (500, 600, 700, 800 and 900 °C).
2.2 Sample characterization
The crystalline phase and the crystal size of the samples were identified by X-ray diffraction (XRD, Bruker D8) using Cu Kα radiation (wavelength 1.5418 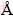 ) at a scan speed of 0.05 (°)/s, a voltage of 40 kV and a current of 300 mA. High resolution transmission electron microscopy (HRTEM) images were recorded on JEOL JEM-2010 high-resolution transmission electron microscope. The accelerating voltage was 200 kV. UV-Vis diffuse reflectance spectra of the samples were obtained using UV-Vis spectrophotometer (UV-2550, Shimadzu, Japan). BaSO4 was used as a reflectance standard in the UV-Vis diffuse reflectance experiment. Brunauer–Emmett–Teller (BET) surface area (SBET) was determined with the nitrogen absorption isotherms apparatus (ST-08 analyzer).
) at a scan speed of 0.05 (°)/s, a voltage of 40 kV and a current of 300 mA. High resolution transmission electron microscopy (HRTEM) images were recorded on JEOL JEM-2010 high-resolution transmission electron microscope. The accelerating voltage was 200 kV. UV-Vis diffuse reflectance spectra of the samples were obtained using UV-Vis spectrophotometer (UV-2550, Shimadzu, Japan). BaSO4 was used as a reflectance standard in the UV-Vis diffuse reflectance experiment. Brunauer–Emmett–Teller (BET) surface area (SBET) was determined with the nitrogen absorption isotherms apparatus (ST-08 analyzer).
2.3 Photocatalytic activity measurement
The photocatalytic reaction was carried out in a self-made tube-shaped quartz reactor including three layers connected with gas collecting devices. The reaction temperature was kept at (25±0.2) °C by controlling the external circulation water in the water jacket of the reactor during the entire experiment. The photocatalyst powder (0.6 g) was dispersed by a magnetic stirrer in 600 mL MO solution with the concentration of 25 mg/L. A 150-W xenon lamp with λ=200-900 nm was used as the simulated sunlight source. The luminous intensity was measured at 100 mW/m2 by the auto-range ST-85 optical radiometer (Photoelectric Instrument Factory of Beijing Normal University). Prior to light illumination, the suspension was strongly magnetically stirred for 30 min in dark for adsorption/desorption equilibrium. During irradiation, the catalyst was kept in suspension state by a magnetic stirrer. Samples for analysis were extracted through pipette every 10 min and centrifuged immediately. Decoloration rate is presented as (ρ0-ρt)/ρ0×100%, where ρ0 is the initial concentration of MO solution and ρt is the concentration of MO solution after the irradiation time t.
Under the same conditions, photocatalytic activity was measured by inactivation of E. coli (DH 5a) provided by the Biology Laboratory of Hunan Institute of Science and Technology, China. E. coli was in Luria–Bertani (LB) nutrient solution at 37 °C for 18 h with shaking, and then washed by centrifugation at 4000 r/min. The treated cells were then re-suspended and diluted to 109 colony-forming units (CFU/mL) with 0.9% saline. All materials used in the experiments were autoclaved at 121 °C for 25 min to ensure sterility. The diluted cell suspension and photocatalyst were added to a 600-mL beaker with a cover. The final photocatalyst concentration was adjusted to 0.2-1.0 g/L, and the final bacterial cell concentration was 1×106 CFU/mL. The reaction mixture was stirred with a magnetic stirrer throughout the experiment. The light source for photocatalysis was the 150-W Xe arc lamp. For comparison, the disinfection under natural sunlight was made with identical solar irradiance, and the solar irradiance was monitored with a Daystar solar meter (USA). A bacterial suspension without photocatalyst was irradiated as a control and the reaction mixture with no light irradiation was a dark control. Before and during the light irradiation, aliquots of the reaction solution were immediately diluted with saline and the samples with the appropriate dilution were incubated at 37 °C for 24 h in nutrient agar medium. Then the colonies were counted to determine the number of viable cells. Survival ratio of E. coli was calculated as the ratio of the number of viable colonies remaining after exposure to the number of viable colonies the present initially experimental conditions to.
3 Results and discussion
3.1 Characterization
3.1.1 TG-DTA analysis
Figure 1 shows the TG-DTA curves of ZnO/ZnAl2O4 sample with the starting Zn to Al molar ratio of 1:1.5. The total mass loss occurs in the temperature range of 20-500 °C. The first mass loss before 200 °C may be attributed to the evaporation of absorbed water, meanwhile the corresponding endothermic peak is observed from the thermo- gravimetric curve. The second mass loss occurs at 200-320 °C, and the corresponding exothermic peak at about 280 °C can be ascribed to the decomposition of the residual organic compounds. The third mass loss at 320-500 °C is due to the decomposition of the nitrate and synthesis of the ZnAl2O4 compounds, and then the mass loss becomes almost constant when the temperature is more than 600 °C. The corresponding endothermic peak at about 700 °C is perhaps owing to the forming of ZnO.
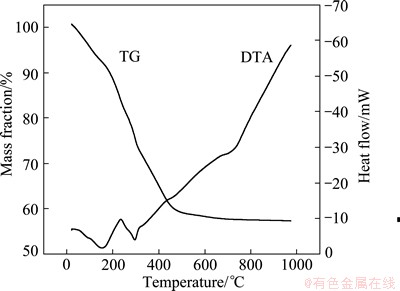
Fig. 1 TG-DTA curves for precursor ZnO/ZnAl2O4 sample
3.1.2 XRD analysis
Figure 2 shows the XRD patterns of the ZnO/ZnAl2O4 nanocomposites with the starting Zn to Al molar ratio of 1:1.5 calcined at different temperatures. We can clearly see that the sample calcined at 500 °C shows a pure phase of spine ZnAl2O4 without any other compositions. The diffraction peaks at 2θ=31.22°, 36.81°, 44.73°, 55.63°, 59.33° and 65.25° are ascribed to the typical spine structure of ZnAl2O4 (JCPDS card No. 05-0669). This is mainly because of the poor crystallization of the as-grown sample at the low calcination temperature. When the temperature is increased to 600 °C, a phase transition appears. The sample shows a mixed phase of ZnAl2O4 and ZnO. When the calcination temperature is higher than 600 °C, the peaks become sharp (the peak width at half-height decreased) as the calcination temperature increases. This means that the crystallinity of samples is improved, and the crystal grains grow bigger when the calcination temperature increases.
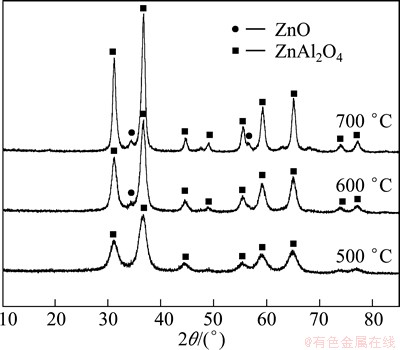
Fig. 2 XRD patterns of ZnO/ZnAl2O4 samples calcined at different temperatures
3.1.3 BET surface area
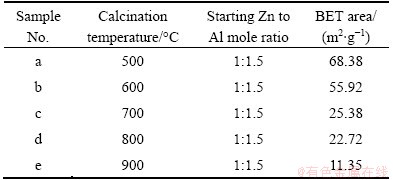
The ZnO/ZnAl2O4 nanocomposites with the starting Zn to Al molar ratio of 1:1.5 calcined at different temperatures varied a lot in the BET surface areas, and their values are listed in Table 1. The BET surface areas of samples decrease when the calcination temperature increases. The sample calcined at 500 °C shows the highest BET surface area of 68.38 m2/g. The BET surface area of sample calcined at 600 °C decreases a little (55.92 m2/g). And the values for the samples calcined at 700 and 800 °C become smaller (25.38 and 22.72 m2/g, respectively). When the sample was calcined at 900 °C, the BET surface area is even smaller (e.g., 11.35 m2/g), only about 1/5 of the value of the sample calcined at 600 °C. The sample calcined at 500 °C has a large BET surface area, but poor crystallinity, which may result in relatively low photocatalytic activity. On the basis of the above analysis, 600°C is regarded as the optimum calcination temperature in our experiments.
Table 1 BET surface Areas of ZnO/ZnAl2O4 samples
3.1.4 TEM and HRTEM images
Figure 3 shows the TEM and HRTEM images of ZnO/ZnAl2O4 nanocomposites with the starting Zn to Al molar ratio of 1:1.5 calcined at 600 °C. Figure 3(a) shows that the sample is irregularly spherical and its particle size is relatively identical. The particle size of sample is 15-20 nm. More detailed morphology about the ZnO/ZnAl2O4 nanocomposites is indicated by HRTEM (Fig. 3(b)). HRTEM image reveals that both ZnO and ZnAl2O4 form a heterojunction nanostructure proven by well-defined lattice fringes: the spacing of 0.26 nm represents the lattice-resolved (001) crystalline plane of ZnO phase, and the spacing values of 0.28 nm and 0.46 nm correspond to the (220) and (111) facets of ZnAl2O4 phase, respectively, which are in good agreement with the report in Ref. [16].
3.1.5 UV-Vis DRS analysis
The diffuse reflectance spectrum for the synthesized ZnO/ZnAl2O4 nanocomposites with the starting Zn to Al molar ratio of 1:1.5 calcined at 600 °C is presented in Fig. 4. For comparison, the spectra of pure ZnO and ZnAl2O4 synthesized are also plotted. The ZnO/ZnAl2O4 sample shows a certain absorption in the visible light region (the wavelength ranging from 400 to 800 nm), while no absorption can be observed for pure ZnO. and ZnAl2O4. For ZnO, the CB bottom and the VB top lie at -4.19 and -7.39 eV, respectively, with respect to absolute vacuum scale (AVS). While for ZnAl2O4, the CB bottom and VB top lie at -3.36 and -7.16 eV vs AVS, respectively. Both the CB bottom and the VB top of ZnO lie below the CB bottom and VB top of ZnAl2O4, respectively. When they are coupled together, the middle band gap (2.97 eV) between the CB bottom of ZnO and the VB top of ZnAl2O4 forms, which may be the main reason for the absorption in the visible light region.
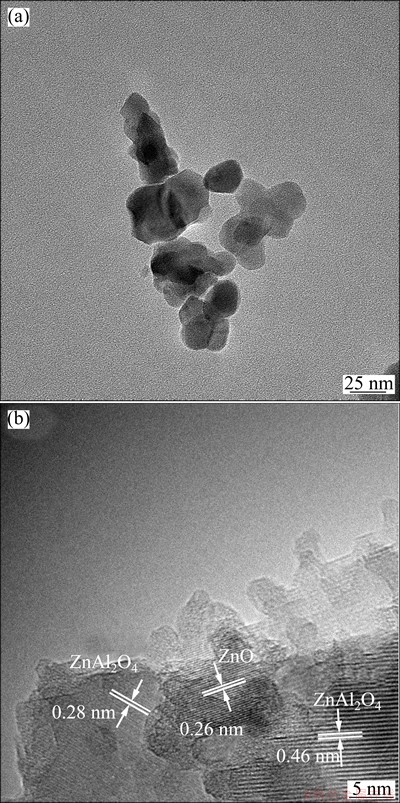
Fig. 3 TEM (a) and HRTEM (b) images of ZnO/ZnAl2O4 nanocomposites
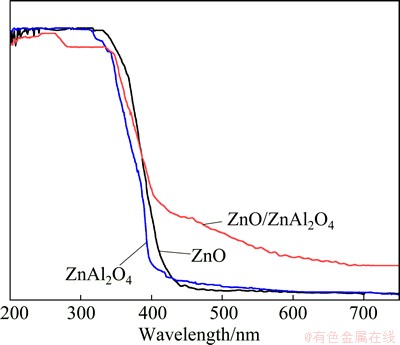
Fig. 4 UV-Vis DRS of samples
3.2 Photocatalytic activity
3.2.1 Effect of ZnO/ZnAl2O4 composition on photo- catalytic degradation activity
Figure 5 shows the degradation of MO in the presence of ZnO/ZnAl2O4 nanocomposites with different starting Zn to Al molar ratios calcined at 600 °C under the irradiation of the simulated sunlight. It can be seen that the photocatalytic activities of ZnO/ZnAl2O4 samples first increase with the molar ratio of Zn to Al decreasing to 1:1.5, and then slightly decrease when the molar ratio exceeds 1:1.5. ZnO/ZnAl2O4 sample with Zn to Al molar ratio of 1:1.5 is found to be the most active, with a photocatalytic activity about 3.5 times higher than that of pure ZnO sample. The reason is attributed to the existing of heterojuction and the content of ZnO in the ZnO/ZnAl2O4 nanocomposite. When they are coupled together to form a heterostructure, photons may be absorbed in both ZnO and ZnAl2O4 and form the e- and h+ pairs. The electrons at the CB bottom of ZnAl2O4 would migrate to the ZnO; whereas holes at the VB top of ZnAl2O4 would remain there. On the other hand, the holes at the VB top of the ZnO would migrate to ZnAl2O4, and electrons at the CB bottom of ZnO remain there. Such process is energetically favorable and the photogenerated e- and h+ pairs can be efficiently separated, which is regarded as the key factor for the enhancement of photocatalytic activities of the ZnO/ ZnAl2O4 sample [16]. Furthermore, the heterojunction photocatalysts where one component is grown or deposited on the other, and one must control over the coverage because of complete and sparse coverage of one component on the other will reduce the catalytic efficiency [17]. Thus, the optimum content of ZnO is proposed to contribute the enhancement of utilization of light, which can favorably improve the photocatalytic activities [18,19]. Therefore, Zn to Al molar ratio of 1:1.5 in the starting materials is found to be the optimum composition for ZnO/ZnAl2O4 samples.
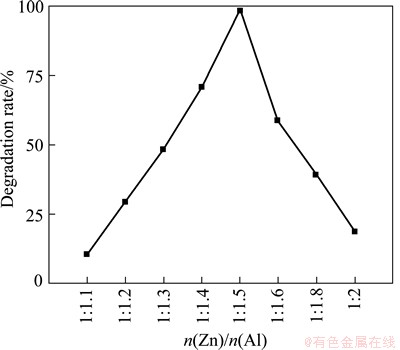
Fig. 5 Photocatalytic activities of ZnO/ZnAl2O4 with different starting Zn to Al molar ratios
3.2.2 Effect of calcination temperature on photocatalytic degradation activity
Figure 6 reveals the photocatalytic activities of the ZnO/ZnAl2O4 nanocomposites with the starting Zn to Al molar ratio of 1:1.5 calcined at different temperatures. From Fig. 6, the photocatalytic activities vary greatly for the samples. The sample calcined at 600°C shows the highest photocatalytic activity. The maximum photocatalytic degradation rate of 98.5% in 50 min is obtained. For the other samples calcined at 500, 700, 800, and 900 °C, the degradation rates of MO within 50 min are estimated to be 89.3%, 94.2%, 93.9%, and 91.4%, respectively. The difference in photocatalytic activity may be ascribed to the BET surface area and the crystalline quality of the nanocomposition. Although the sample calcined at 500°C possesses a larger specific surface area, the photocatalytic activity is lower, which may be ascribed to the relatively poor crystallinity. The sample calcined at 600 °C shows a better crystalline quality. The photocatalyst with good crystallization can provide a shorter migration distance for electrons and holes and reduce the chances of their recombination. Therefore, the photocatalytic reaction efficiency is accelerated and the catalytic activity is improved [20,21]. The high photocatalytic activity of sample calcined at 600 °C is also attributed to the higher specific surface area than that of the sample calcined at 700, 800, or 900 °C (shown in Table 1). A larger surface area provides more surface active sites for the adsorption of reactants molecules, making the photocatalytic process more efficient [22].
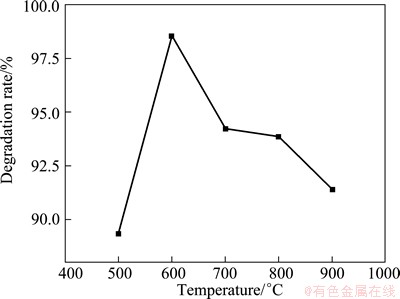
Fig. 6 Photocatalytic activity of ZnO/ZnAl2O4 sample calcined at different temperatures
3.2.3 Effect of ZnO/ZnAl2O4 concentration on photocatalytic inactivation activity
The photocatalytic activities of four different concentrations of ZnO/ZnAl2O4 (0.2-1.0 g/L) under simulated sunlight are shown in Fig. 7. It is clear that with the increase of concentration of ZnO/ZnAl2O4, the photocatalytic inactivation activity is correspondingly increased, reaching a plateau at 1.0 g/L. The photocatalytic inactivation rate of E. coli achieves 99.8% in 60 min. While the inactivation rate of 95.9% (inactivation rate=1-ρ/ρ0) is obtained using 0.5 g/L photocatalyst, which has been obviously attributed to the increase in the number of photons that can be absorbed by ZnO/ZnAl2O4 before reaching a maximum corresponding to the total absorption of the incident radiation [23,24].
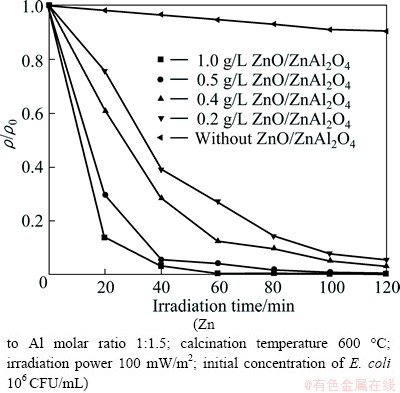
Fig. 7 Photocatalytic inactivation of E. coli with different concentrations of ZnO/ZnAl2O4 under simulated sunlight
3.2.4 Effect of light source on photocatalytic inactivation activity
Figure 8 displays the photocatalytic inactivation of E. coli by ZnO/ZnAl2O4 under different light sources. The survival ratio of E. coli falls initially, followed by a slow diminishing in the presence of ZnO/ZnAl2O4 under simulated sunlight and natural sunlight illumination. It clearly demonstrates the effective photocatalytic inactivation of E. coli by ZnO/ZnAl2O4 under sunlight irradiation. The inactivation response is probably due to the DNA damage to the bacteria caused by the reactive oxygen species (ROS) produced by irradiation [25]. The corresponding photocatalytic inactivation of E. coli by photolysis (without catalyst) is also shown for comparison. No significant inactivation is observed for E. coli both under simulated sunlight irradiation and natural sunlight. However, when the system in the presence of ZnO/ZnAl2O4 is in the dark, a significant decrease in the number of viable E. coli bacteria is observed, although the inactivation of the E. coli by ZnO/ZnAl2O4 in the dark is small compared with the photocatalytic inactivation, which is consistent with the previous reports [26]. The results show that the osmotic stress may change the permeability of the cell wall, allowing the transfer of the smaller ZnO/ZnAl2O4 particles through the cell wall, as suggested by the results of HUANG et al [27], although the outer membrane of Gram-negative bacteria have been reported to limit the permeability to many chemical compounds [28,29].

Fig. 8 Photocatalytic inactivation of E. coli under different light source
4 Conclusions
1) The ZnO/ZnAl2O4 nanocomposites exhibit excellent photocatalytic activity for degradation of MO and inactivation of E. coli under the irradiation of the simulated sunlight, which may be attributed to the fact that ZnO and ZnAl2O4 can form heterojunction structure.
2) The photocatalytic degradation activity of the ZnO/ZnAl2O4 nanocomposites can be improved by varying the concentration of ZnO/ZnAl2O4, the molar ratio of Zn to Al and calcination temperature. When the concentration of ZnO/ZnAl2O4 is 1.0 g/L, the maximum activity is obtained at the molar ratio of Zn to Al of 1:1.5 and calcination temperature of 600 °C.
3) For comparison, the photocatalytic inactivation of E. coli was performed under the same conditions. A 99.8% inactivation rate of E. coli was obtained in 60 min.
References
[1] MATSUNAGA T, TOMODA T R, NAKAJIMA T, WAKE H. Photoelectrochemical sterilization of microbial cells by semiconductor powders [J]. FEMS Microbiol Lett, 1985, 29: 211-214.
[2] HOFFMANN M R, MARTIN S T, CHOI W, BAHNEMANN D W. Environmental applications of semiconductor photocatalysis [J]. Chem Rev, 1995, 95: 69-96.
[3] YAN Jian-hui, CHEN Hao, ZHANG Li, JIANG Jin-zhi. Inactivation of escherichia coil on lmmobilized CuO/CoFe2O4-TiO2 thin-film under simulated sunlight irradiation [J]. Chinese Journal of Chemistry, 2011, 29(6): 1133-1138.
[4] DANESHVAR N, SALARI D, KHATAEE A R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2 [J]. J Photochem Photobiol A, 2004, 162: 317-322.
[5] WU De-zhi, FAN Xi-mei, TIAN Ke, DAI Jia, LIU Hua-rong. Fabrication and photocatalytic properties of Cu2S/T-ZnOw heterostructures via simple polyol process [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1620-1628.
[6] YANG D, PARK S E, LEE J K, LEE S W. Sonochemical deposition of nanosized Au on titanium oxides with different surface coverage and their photocatalytic activity [J]. Journal of Crystal Growth, 2009, 311: 508-511.
[7] STROYUK A L, SHVALAGIN V V, KUCHMII S Y. Photochemical synthesis and optical properties of binary and ternary metal semiconductor composites based on zinc oxide nanoparticles [J]. J Photochem Photobiol A, 2005, 173: 185-191.
[8] JIA Zhi-gang, PENG Kuan-kuan, LI Yan-hua, ZHU Rong-sun. Preparation and photocatalytic performance of porous ZnO microrods loaded with Ag [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 873-878.
[9] SAKTHIVEL S, NEPPOLIAN B, SHANKAR M V, ARABINDOO B, PALANICHAMY M, MURUGESAN V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2 [J]. Sol Energy Mater Sol Cells, 2003, 77: 65-68.
[10] WANG Z Y, HUANG B B, DAI Y, QIN X Y, ZHANG X Y, LIU H X, YU J X. Highly photocatalytic ZnO/In2O3 heteronanostructures synthesized by a copercipitation method [J]. J Phys Chem C, 2009, 113: 4612-4617.
[11] ZHENG L R, ZHENG Y H, CHEN C Q, ZHAN Y Y, LIN X Y, ZHENG Q, WEI K M, ZHU J F. Network structured SnO2/ZnO heterojunction nanocatalyst with high photocatalytic activity [J]. Inorg Chem, 2009, 48: 1819-1825.
[12] HAMEED A, MONTINI T, GOMBAC V, FORNASIERO P. Photocatalytic decolourization of dyes on NiO-ZnO nanocomposites [J]. Photochem Photobiol Sci, 2009, 8: 677-682.
[13] FARHADI S, PANAHANDEHJOO S. Spinel-type zinc aluminate (ZnAl2O4) nanoparticles prepared by the co-precipitation method: A novel, green and recyclable heterogeneous catalyst for the acetylation of amines, alcohols and phenols under solvent-free conditions [J]. Appl Catal A, 2010, 382: 293-302.
[14] BALLARINI A D, BOCANEGRAS A, CASTRO A A, DEMIGUEL S R, SCELZA O A. Characterization of ZnAl2O4 obtained by different methods and used as catalytic support of Pt [J]. Catal Lett, 2009, 129: 293-302.
[15] CONRAD F , MASSUE C, KUHL S, KUNKES E, GIRGSDIES F, KASTKIN I, ZHANG B, FRIEDRICH M, LUO Y, ARMBEUSTER M, PATZKE G R, BEHRENS M. Microwave-hydrothermal synthesis and characterization of nanostructured copper substituted ZnM2O4 (M=Al, Ga) spinels as precursors for thermally stable Cu catalysts [J]. Nanoscale, 2012, 4: 2018-2028.
[16] ZHAO X F, WANG L, XU X, LEI X D, XU S L, ZHANG F Z. Fabrication and photocatalytic properties of novel ZnO/ZnAl2O4 nanocomposite with ZnAl2O4 dispersed inside ZnO network [J]. AIChE J, 2012, 58: 573-582.
[17] LIU B, KHARE A, AYDIL E S. TiO2-B/anatase core-shell heterojunction nanowires for photocatalysis [J]. ACS Appl Mater Interfaces, 2011, 3: 4444-4450.
[18] YAN J H, ZHANG L, YANG H H. CuCr2O4/TiO2 heterojunction for photocatalytic H2 evolution under simulated sunlight irradiation [J]. Solar Energy, 2009, 83: 1534-1539.
[19] ZHANG L, YAN J H, ZHOU M J, YANG Y H, LIU Y N. Fabrication and photocatalytic properties of spheres-in-spheres ZnO/ZnAl2O4 composite hollow microspheres [J]. Appl Surf Sci, 2013, 268: 237-245.
[20] SANG Li-xia, LIU Yu, LI Qun-wei, XU Li-xian, MA Chong-fang, DAI Hong-xing, HE hong. Photocatalytic hydrogen evolution from water over nano perovskite oxides LaFeO3 [J]. Journal of Chinese Rare Earth Society, 2006, 24(s1): 42-45. (in Chinese)
[21] LI D, HANEDA H. Morphologies of zinc oxide particles and their effects on photocatalysis [J]. Chemosphere, 2003, 51: 129-137.
[22] LI F B, LI X Z, HOU M F, CHEAH K W, CHOY W C H. Enhanced photo-catalytic activity of Ce3+-TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control [J]. Appl Catal Gen, 2005, 285: 181-189.
[23] RINCON A G, PULGARIN C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration [J]. Appl Catal B, 2003, 44: 263-284.
[24] CHEN F, YANG X, XU F, WU Q, ZHANG Y. Correlation of photocatalytic bactericidal effect and organic matter degradation of TiO2 Part 1: Observation of phenomena [J]. Environ Sci Technol, 2009, 43: 1180-1184.
[25] ROBERTSON J M C, ROBERTSON P K J, LAWTON L A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms [J]. J Photochem Photobiol A, 2005, 175: 51-56.
[26] GOMES A I, SANTOS J, VILAR V J P, BOAVENTURA R A R. Inactivation of bacteria E-coli and photodegradation of humic acids using natural sunlight [J]. Appl Catal B, 2009, 88: 283-291.
[27] HUANG Z, MANESS P C, BLAKE D M, WOLFRUM E J. Titania and silver-titania composite films on glass-potent antimicrobial coatings [J]. J Photochem Photobiol A, 2000, 130: 163-170.
[28] FU G F, VARY P S, LIN C T. Anatase TiO2 nanocomposites for antimicrobial coatings [J]. J Phys Chem B, 2005, 109: 8889-8898.
[29] PAGE K, PALGRAVE R G, PARKIN I P, WILSON M S, SAVIN L P, CHADWICK A V. Titania and silver-titania composite films on glass-potent antimicrobial coatings [J]. J Mater Chem, 2007, 17: 95-104.
张 丽1,2,3,阎建辉1,2,3,周民杰1, 3,余艳萍1,刘 晔1,刘又年 2
1. 湖南理工学院 化学化工学院,岳阳 414006;
2. 中南大学 化学化工学院,长沙 410083;
3. 湖南理工学院 精细石油化工催化与分离湖南省重点实验室,岳阳 414006
摘 要:通过共沉淀法制备ZnO/ZnAl2O4纳米异质结光催化剂,利用HRTEM、TEM、XRD、BET、TG-DTA和UV-Vis DRS测试方法对样品进行表征。在模拟太阳光照射下,通过测定甲基橙溶液的光催化降解率和对大肠杆菌的杀灭率来评价样品的光催化活性。研究催化剂的组成、焙烧温度、催化剂的用量和不同光源对样品光催化活性的影响。结果表明,当原料中Zn与Al摩尔比为1:1.5时,在600 °C焙烧所得的催化剂具有最佳光催化活性。在模拟太阳光照射下,在50 min内1.0 g/L光催化剂对甲基橙的降解率达98.5%;在60 min内,在相同条件下对大肠杆菌(106 CFU/mL)的杀菌率达到99.8%。
关键词:ZnO/ZnAl2O4;光催化;降解;杀菌;异质结
(Edited by Hua YANG)
Foundation item: Project (21271071) supported by the National Natural Science Foundation of China; Project (21306041) supported by the National Natural Science Young Foundation of China
Corresponding author: You-nian LIU; Tel: +86-731-88836964; E-mail: liuyounian@csu.edu.cn; yanjh58@163.com
DOI: 10.1016/S1003-6326(14)63120-4

