Investigation of flux containing GdCl3 on recycling Mg-Gd-Y-Zr scraps
来源期刊:中国有色金属学报(英文版)2008年增刊第1期
论文作者:王玮 吴国华 王渠东 黄玉光 孙明 丁文江
文章页码:292 - 298
Key words:magnesium alloy; gadolinium; mechanical properties; refining, thermodynamics
Abstract: The effects of GdCl3 in flux on the loss of Gd, microstructure, mechanical properties, and corrosion resistance of Mg-Gd-Y-Zr(GW103K) scrapswere investigated by ICP, XRD, SEM and EDS. The results show that the loss of Gd in the alloy decreases with increasing GdCl3 content in the flux, and the mechanism of GdCl3 suppressing the loss of Gd was analyzed by thermodynamic theory. The optimal average mechanical properties could be obtained when the GdCl3 content in the flux was 5% (mass fraction). Compared with the alloy only treated by JDMJ flux, the σb and σs of GW103K alloy were improved from 160.91 MPa and 133.88 MPa to 224.96 MPa and 157.91 MPa by 39.8% and 17.95%, respectively. Immersion test shows the corrosion rate of Mg-Gd-Y-Zr alloy refined by JDMJ+5.0% GdCl3 flux was decreased dramatically from 0.762 mg/(cm2.d) to 0.437 mg/(cm2.d) by 42.7%. GdCl3 has no effect on the fracture pattern of GW103K alloy.
基金信息:the National Basic Research Program of China
the National Key Technology R&D Program of China
Shanghai Subject Chief Scientist Program, China

WANG Wei(王 玮)1, 2, WU Guo-hua(吴国华)1, 2, WANG Qu-dong(王渠东)1, 2, HUANG Yu-guang(黄玉光)1, 2,
SUN Ming(孙 明)1, 2, DING Wen-jiang(丁文江)1, 2
1. National Engineering Research Center of Light Alloy Net Forming, Shanghai Jiao Tong University,
Shanghai 200240, China;
2. State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China
Received 12 June 2008; accepted 5 September 2008
Abstract: The effects of GdCl3 in flux on the loss of Gd, microstructure, mechanical properties, and corrosion resistance of Mg-Gd-Y-Zr(GW103K) scraps were investigated by ICP, XRD, SEM and EDS. The results show that the loss of Gd in the alloy decreases with increasing GdCl3 content in the flux, and the mechanism of GdCl3 suppressing the loss of Gd was analyzed by thermodynamic theory. The optimal average mechanical properties could be obtained when the GdCl3 content in the flux was 5% (mass fraction). Compared with the alloy only treated by JDMJ flux, the σb and σs of GW103K alloy were improved from 160.91 MPa and 133.88 MPa to 224.96 MPa and 157.91 MPa by 39.8% and 17.95%, respectively. Immersion test shows the corrosion rate of Mg-Gd-Y-Zr alloy refined by JDMJ+5.0% GdCl3 flux was decreased dramatically from 0.762 mg/(cm2?d) to 0.437 mg/(cm2?d) by 42.7%. GdCl3 has no effect on the fracture pattern of GW103K alloy.
Key words: magnesium alloy; gadolinium; mechanical properties; refining, thermodynamics
1 Introduction
In recent years, magnesium based alloy have been proved to be highly competitive materials for construction purposes and automotive applications, especially when weight is the critical parameter[1-4]. However, commercially available magnesium alloy can hardly meet the property demands of such parts as automobile engine components because of poor mechanical properties at elevated temperatures, which severely restricts the wider applications of magnesium alloys[5-6]. Adding proper rare earth elements to magnesium alloy is proved to be an effective measure to improve the mechanical properties of magnesium alloys, such as Mg-Gd-Y-Zr alloy, which could not only remove non-metal inclusions, prevent the melt from burr, but also refine the grains, enhance the creep resistance capability, improve corrosion resistance, etc[7-8].
The non-metallic inclusions in magnesium alloy are mainly MgO and Mg3N2 which destroy the continuity of the magnesium matrix, and thus impair the mechanical properties of the alloy[9]. MgCl2 has been accepted as
the main composition of the magnesium flux due to its excellent adsorption capability to MgO inclusions[10]. But, rare earth elements tend to burn in air rapidly and usually react with MgCl2 in common flux. This greatly wastes rare earth elements in alloy[11]. Therefore, fluxes containing MgCl2 are not suitable for the refining process of RE magnesium alloy. In practical refining process, no-flux refining or flux without MgCl2 were usually employed[12]. Although this method can avoid the loss of RE partially in alloy, the refining effect is not very satisfactory compared with refining process by fluxes containing MgCl2.
In order to reach the both targets of refining and suppressing the loss of RE in alloy, a new flux containing GdCl3 was developed for Mg-Gd-Y-Zr alloy specially. The effects of GdCl3 additions on the loss of Gd were studied by adding it into melt of Mg-10Gd-3Y-0.5Zr (GW103K) scrap, and the microstructure, mechanical properties and the corrosion resistance of GW103K scrap were investigated as well. Furthermore, the mechanism of GdCl3 suppressing the loss of Gd during the refining process was analyzed thermodynamically.
2 Experimental
2.1 Materials
Industrial GW103K scraps mainly supplied by cuttings of ingots were adopted in the present work. The chemical composition is (mass fraction, %): Gd 10.78, Y 3.15, Zr 0.57, Fe 0.0021, Cu 0.006, Si 0.030, Ni<0.001, Mg balance. Analytically pure GdCl3 was added into a kind of flux named JDMJ, and mixed in QM-ISP pebble mill for 3 h. Then the new flux was added into the melt for refining purpose. The JDMJ flux (MgCl2 45, KCl 25, NaCl 20, others 10) was developed by Shanghai Jiao Tong University, China[13].
2.2 Experimental procedure
GW103K scraps, fluxes and tools used in the experiments were heated to 200 ℃ in a baking oven before experiment in order to eliminate water. 7 kW crucible electric resistance furnace was engaged to smelt the scraps under protection of a shield gas consisting of SF6 (1%, volume fraction), CO2 (bal.). 2.5% (ratio to the whole scraps) new flux was added to refine the melt at 760 ℃. The ingredients of the fluxes are listed in Table 1. After refining, the melt was held for about 30-45 min. And then, at 740 ℃, it was poured into the metallic molds which had been heated up to 400 ℃ previously.
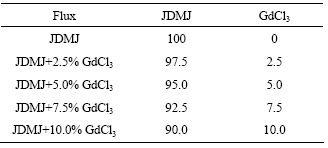
Table 1 Compositions of fluxes (mass fraction, %)
Tensile specimens were cut from the bottom of the castings. The gauge of the specimens is 54.5 mm×15 mm×2 mm (see Fig.1). The ambient temperature tensile tests were carried out on a Zwick/Roell materials testing machine at a tensile speed of 0.5 mm/min.

Fig.1 Sketch of tensile specimens (unit: mm)
The chemical compositions were determined by inductively coupled plasma spectrum machine (ICP, IRIS Advantage 1000). Metallographs and morphologies of the alloy were observed from optical and scanning electron microscope. The compositions of inclusions were analyzed by energy dispersive spectroscope (EDS) attached to the SEM. The phase compositions were investigated by XRD.
The size of specimens for immersion corrosion test was φ35 mm×4 mm. The specimens were polished successively on finer grades of emery papers up to 800 grit. The specimens were immersed in a 5.0% NaCl aqueous solution (pH=8.3) at room temperature ((25±0.5) ℃). Three days later, the specimens were taken out and cleaned in a solution of 15% Cr2O3+1% AgNO3 in 500 mL water in boiling condition for about 5 min. The mass of the corroded specimen was measured and then the corrosion rate (CR, mg/(cm2?d)) could be calculated.
3 Results
3.1 Gd concentration
Fig.2 shows the relationship between the Gd

Fig.2 Content (a) and loss (b) of Gd in GW103K scraps refined with different fluxes
concentration and GdCl3 addition during the refining process. The results indicate that, with increasing GdCl3 content in flux, the Gd concentration in alloy increases correspondingly (Fig.2(a)). Fig.2(b) shows the relationship between the loss of Gd in alloy and GdCl3 addition directly. It can be seen that the loss of Gd could be limited to 0.28% when the GdCl3 addition was 10.0%. This illustrates that this refining process by the new flux can suppress the loss of Gd in alloy to a certain degree. The study also shows that this process has not obvious effects on the loss of rare earth element Y in alloy. The loss of Y kept at about 10.0%, as shown in Fig.3.
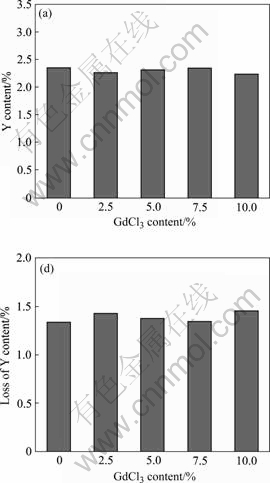
Fig.3 Content (a) and loss (b) of Y in GW103K scraps refined with different fluxes
3.2 Mechanical properties and corrosion resistance
The relationship between the ambient temperature mechanical properties and the GdCl3 addition is shown in Fig.4. Initially, the ultimate tensile strength (σb), yield strength at 0.2% offset (σs) were improved with increasing GdCl3 content in the flux. Compared with pure JDMJ flux refining process, when the GdCl3 addition in flux increases to 5.0%, σb and σs improve from 160.91 MPa and 133.88 MPa to peak value 224.96

Fig.4 Effect of GdCl3 contents on mechanical properties of GW103 scraps
MPa and 157.91 MPa by 39.8% and 17.95%, respectively. Keeping on increasing GdCl3 content in the flux to 10%, σb and σs decrease contrarily to 191.35 MPa and 136.49 MPa. The elongation (δ) of the alloy appears the same behavior as σb and σs. δ reaches the lowest value of 0.91% when the melt is only refined by pure JDMJ flux. Similarly, δ enhanced to the highest 2.6% after being refined by JDMJ+5.0%GdCl3 flux, and decreased to 1.55% when GdCl3 continuously increased to 10.0%. Therefore, the best synthetical mechanical properties of the alloy could be obtained while the melt was refined with JDMJ+5.0%GdCl3 flux.
The corrosion rates of the alloy treated with different refining processes are shown in Fig.5. These refining processes can be identified as no refining, pure JDMJ flux refining and refining process with JDMJ flux containing various GdCl3 contents. The decreasing corrosion rates of GW103K scrap is in the following order: no refining>pure JDMJ flux refining>JDMJ+5.0% GdCl3 flux refining. Compared with no

Fig.5 Effect of different treatments on corrosion rate of GW103 scraps
refining process which is set as a reference experiment here, the corrosion rate under the condition of JDMJ+5.0% GdCl3 flux refining descends dramatically from 0.762 mg/(cm2?d) to 0.437 mg/(cm2?d) by 42.7%. The morphological characteristics of the corroded surfaces of the specimens after being immersed in 5.0% NaCl aqueous solution for 3 d are shown in Fig.6, where (a), (b) and (c) represent the samples refined by JDMJ, JDMJ+5.0% GdCl3, JDMJ+10.0% GdCl3, respectively. It can be seen that corrosion pits homogeneously distribute on the specimen surfaces. The difference lies in the fact that the pits become denser and deeper in the order as follows: JDMJ+5.0% GdCl3>JDMJ+10.0% GdCl3>JDMJ. It is known that the deleterious effects of non-

Fig.6 SEM images of corrosion surface morphology under different flux refining conditions: (a) JDMJ refining; (b) JDMJ+5.0% GdCl3 refining; (c) JDMJ+10.0% GdCl3 refining
metallic inclusions and flux inclusions on corrosion resistance of magnesium alloys can be attributed to the galvanic coupling between the inclusions and magnesium matrix[14]. The specimens refined with 5.0% GdCl3 exhibits excellent corrosion resistance because of its remarkable ability of removing non-metallic inclusions from the melts. The reduction of non-metallic inclusions in the melt decreases the cathode areas and thus improves the corrosion resistance of the alloy. But with increasing GdCl3 content, more flux inclusions are introduced into the melt meanwhile, which brings about the negative effect on the corrosion resistance.
3.3 Microstructure
Fig.7 shows the microstructures of GW103K alloy refined with JDMJ and JDMJ+5.0% GdCl3 flux. For the specimens refined with JDMJ flux, many non-metallic inclusions, even including some big inclusion clusters, can be observed (see Fig.7(a)). However, these non-metallic inclusions in specimens which were refined with JDMJ+5% GdCl3 are much less than that of the former and the grain boundaries seems much cleaner. Table 2 shows the statistical volume fraction of the inclusions in alloy by using Leco image software. Apparently, the removal of non-metallic inclusions from melts is useful for improving the mechanical properties of the alloy.

Fig.7 Optical microstructures of GW103K scraps refined by different fluxes: (a) JDMJ refining; (b) JDMJ+5.0%GdCl3 refining
Table 2 Statistical volume fraction of inclusions in GW103K alloy refined by various fluxes
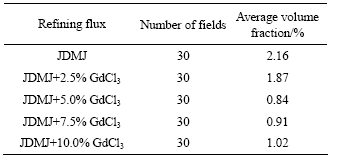
Fig.8 indicates the results of X-ray diffraction analysis of GW103K alloy refined with JDMJ (Fig.8(a)) and JDMJ+5.0% GdCl3 (Fig.8(b)) respectively. The phase composition of the alloy almost has not been changed, and still consisted of matrix phase α-Mg and eutectic phase Mg5(GdY).
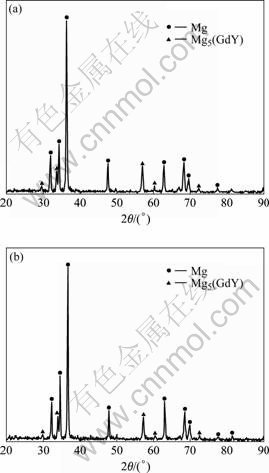
Fig.8 X-ray diffraction (XRD) patterns of GW103K scraps refined by different fluxes: (a) JDMJ refining; (b) JDMJ+5.0% GdCl3 refining
The fracture surface morphologies of the specimens after ambient temperature tensile tests are shown in Fig.9. The specimens were refined with JDMJ and JDMJ+5.0% GdCl3 respectively. It is obvious that the fracture pattern

Fig.9 Fractographs of tensile samples of GW103K scraps: (a) JDMJ refining; (b) JDMJ+5.0% GdCl3 refining
has not been changed substantially by the flux refining process. The fracture mechanism is still quasi-cleavage crack. During the tensile test, it is not easy for cracks to spread strictly according to the given crystallography plain. The micro-morphology feature of the fracture belongs to river pattern of cleavage crack, but not genuine cleavage one. Accurately, it belongs to transcrystalline fracture, which is mixed with small cleavage plane, steps, or tears arrises and rivers.
4 Discussion
The advantage of the new flux containing GdCl3 focuses on two points: fluxes maintaining MgCl2 can remove non-metallic inclusions such as MgO effectively; GdCl3 suppresses the loss of rare earth elements during the melting process. However, too much GdCl3 addition in the flux results in flux-inclusion, which harms the mechanical properties of the alloy. Flux-inclusions could be confirmed by whether there is Cl in it. Actually, Cl is found by EDS analysis as shown in Fig.10.
In order to illustrate that GdCl3 could suppress the loss of Gd during the refining process, we tried to analyze from the aspect of thermodynamics. Generally, rare earth elements in Mg melt could react with MgCl2 in flux and then results in this loss. For the present study, the following chemical reaction should be taken into account:
3(MgCl2) + 2[Gd] = 2(GdCl3) + 3[Mg] (1)
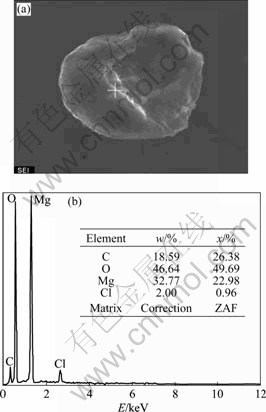
Fig.10 SEM photographs of flux inclusion in GW103K scraps (a) and EDS analysis (b)
where the substances in round parenthesis are in molten flux, and the ones in square parenthesis are in Mg melt. Under standard conditions, the standard Gibbs free energy of the reaction (1) could be obtained in terms of relative thermodynamic data[15]:
![]() =-79810 J/mol
=-79810 J/mol
![]() <0 indicates that the reaction can occur spontaneously under standard conditions.
<0 indicates that the reaction can occur spontaneously under standard conditions.
Under practical conditions, the change of Gibbs free energy of reaction (1) should be calculated by Eqn.(2).
?G1=![]() +RTln
+RTln![]() (2)
(2)
where aMg, ![]() , aGd, and
, aGd, and ![]() are activities of Mg, MgCl2, Gd and GdCl3 in the melt, respectively. The activities here are replaced by corresponding mole concentration. For this experiment, n[Mg], n[GdCl3], n[MgCl2] and n[Gd] can be calculated as
are activities of Mg, MgCl2, Gd and GdCl3 in the melt, respectively. The activities here are replaced by corresponding mole concentration. For this experiment, n[Mg], n[GdCl3], n[MgCl2] and n[Gd] can be calculated as
n[Mg]=
![]() = 0.966
= 0.966
n[MgCl2]=
![]() = 0.003 23
= 0.003 23
n[Gd]=
![]() = 0.019 9
= 0.019 9
n[GdCl3]=![]() = 0.000 13
= 0.000 13
Substituting T with 1 013 K, R with 8.314 J/(mol·K), then
?G1=![]() +RTln
+RTln![]() =
=
![]() +RTln
+RTln![]() =-20 798.22 J/mol
=-20 798.22 J/mol
Although ?G1 is still a negative value, it is not too negative compared with ![]() . This means the addition of GdCl3 slows down the reaction velocity between Gd and MgCl2 significantly, i.e. the loss of Gd can be suppressed in a certain degree.
. This means the addition of GdCl3 slows down the reaction velocity between Gd and MgCl2 significantly, i.e. the loss of Gd can be suppressed in a certain degree.
The negative value of ?G1 describes that the refining process with JDMJ+5.0% GdCl3 could not hold up the loss of Gd in alloy according to the reaction (1). But, compared with ![]() , the much higher value of ?G1 illustrates that this process slows down the reaction velocity between Gd and MgCl2 significantly, i.e. the loss of Gd in alloy is suppressed in a certain degree by adding GdCl3 into Mg melt.
, the much higher value of ?G1 illustrates that this process slows down the reaction velocity between Gd and MgCl2 significantly, i.e. the loss of Gd in alloy is suppressed in a certain degree by adding GdCl3 into Mg melt.
5 Conclusions
1) Introducing GdCl3 into JDMJ flux is an effective means to refine GW103K alloy melt. GdCl3 addition can not only remove non-metallic inclusions effectively but also suppress the loss of Gd in alloy as well. The mechanism of this suppression effect is interpreted by thermodynamic calculation.
2) The refining process with JDMJ flux containing GdCl3 can greatly improve the mechanical properties of GW103K alloy. The optimal addition of GdCl3 appears at 5.0%, where σb, σs and δ reach the maximum of 224.96 MPa, 157.91 MPa and 2.60%, respectively.
3) Corrosion resistance of the GW103K scrap is greatly improved by GdCl3 treatment. The corrosion rate of the alloy refined by JDMJ+5.0% GdCl3 flux is declined to 0.437 mg/(cm2?d).
4) The microstructure and fracture pattern of GW103K scrap are not changed by refining process with JDMJ flux containing GdCl3. The phase compositions of the alloy still consist of matrix phase α-Mg and eutectic phase Mg5(GdY), and the fracture mechanism remains as quasi-cleavage crack.
Acknowledgements
This work was funded by the National Basic Research Program of China (No.2007CB613701), National Key Technology R&D Program of China (No. 2006BAE04B07-2) and Program of Shanghai Subject Chief Scientist (No.08XD14020).
References
[1] MORDIKE B L, EBERT T. Magnesium properties—applications— potential [J]. Mater Sci Eng A, 2001, 302(1): 37-45.
[2] WU Y F, DU W B, NIE Z R, CAO L F, ZUO T Y. Thermodynamic calculation of intermetallic compounds in AZ91 alloy containing calcium [J]. Trans Nonferrous Met Soc China, 2006, 16(2): 392-396.
[3] FLETERN R J. Magnesium alloys shine bright for north American die casters [J]. Die Casting Engineering, 1998, 42(2): 82-83.
[4] BAGHNI I M, WU Y S, LI J Q, ZHANG W. Corrosion behavior of magnesium and magnesium alloys [J]. Trans Nonferrous Met Soc China, 2004, 14(1): 1-10.
[5] DU W B, WU Y F, NIE Z R, SU X K, ZUO T Y. Effects of rare earth and alkaline earth on magnesium alloys and their applications status [J]. Rare Metal Materials and Engineering 2006, 35(9): 1345-1348. (in Chinese)
[6] GAO X, HE S M, ZENG X Q, PENG L M, DING W J, NIE J F. Microstructure evolution in a Mg-15Gd-0.5Zr(wt.%) alloy during isothermal aging at 250 ℃ [J]. Mater Sci Eng A, 2006, 431(1/2): 322-327.
[7] NIE J F, GAO X, ZHU S M. Enhanced age hardening response and creep resistance of Mg-Gd alloys containing Zn [J]. Scripta Materialia, 2005, 53(9): 1049-1053.
[8] HE S M, ZENG X Q, PENG L M, GAO X, NIE J F, DING W J. Microstructure and strengthening mechanism of high strength Mg-10Gd-2Y-0.5Zr alloy [J]. Journal of Alloys and Compounds, 2007, 427(1): 316-323.
[9] GAO H T, WU G H, DING W J, ZHU Y P. Purifying effect of new flux on magnesium alloy [J]. Trans Nonferrous Met Soc China, 2004, 14(3): 530-536.
[10] ZHANG S C, KUAN H Q, CAI Q Z, WEI B K. The inclusions in magnesium alloy and assessment methods [J]. Foundry Technology, 2001(4): 3-6. (in Chinese)
[11] WANG Q D, LV Y Z, ZENG X Q, DING W J, ZHU Y P. Application of rare earth metals in cast magnesium alloy [J]. Special Casting and Nonferrous Alloys, 2002, S1: 293-295. (in Chinese)
[12] WU G H, KANG S H, YOU B S, YIM C D SU J R. Effects of non-flux purification on the microstructure and mechanical properties of AZ31+xCa Mg alloy [J]. Materials Science Forum, 2007, 546/547: 217-220.
[13] ZHAI C Q, DING W J, XU X P, DENG Z W, YU Z Z. Development of new type hazardless fluxes used in the melting of Mg-alloys [J]. Special Casting and Nonferrous Alloys, 2002(S1): 292-295. (in Chinese)
[14] GAO H T, WU G H, DING W J, LIU L F. Study on Fe reduction in AZ91 melt by B2O3 [J]. Mater Sci Eng A, 2004, 368(1/2): 311-317.
[15] LIANG Y J, CHE Y C, LIU X X. Handbook of inorganic thermodynamic data [M]. Shenyang: North-East University Press, 1993. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project(2007CB613701) supported by the National Basic Research Program of China; Project(2006BAE04B07-2) supported by the National Key Technology R&D Program of China; Project(08XD14020) supported by the Program of Shanghai Subject Chief Scientist
Corresponding author: WU Guo-hua, Professor; Tel: +86-13671800698; Fax: +86-21-34202794; E-mail: ghwu@sjtu.edu.cn

