文章编号:1004-0609(2008)06-1135-08
ZnCl2-EMIC离子液体中电沉积锌
马军德,李 冰,颜灵光,陈 彦
(华东理工大学 资源与环境工程学院,上海 200237)
摘 要:采用ZnCl2-EMIC离子液体,用电化学方法对体系在镍电极和钨电极上的反应阴极过程进行研究,分析锌沉积机制,并用直流和脉冲电源进行电沉积实验,研究电流密度、温度和脉冲参数等因素对镀层的影响。结果表明:阴极反应为准可逆反应,锌离子在镍电极和钨电极上形核是三维瞬时形核半球形扩散长大过程,直流电流密度为4mA/cm2,温度为80 ℃时,镀层质量最好;脉冲电沉积得到的镀层要优于直流电沉积得到的镀层,尤其在脉冲宽度为2ms,脉冲间隔为8ms,电流密度为8mA/cm2时,得到的镀层光滑而均匀,颗粒大小接近0.3μm 。
关键词:Zn;ZnCl2;离子液体;电沉积
中图分类号:TQ 153 文献标识码:A
Electrodeposition of zinc from zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt
MA Jun-de, LI Bing, YAN Ling-guang, CHEN Yan
(School of Resource and Environmental Engineering, East China University of Science and Technology,
Shanghai 200237, China)
Abstract: The electrodeposition of zinc from zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt was adopt to study the process of the cathode reaction. The electrodeposition was carried out with direct current and impulse current. The factors influencing the coating were studied. The result indicate that: the electrodeposition of zinc on nickel and tungsten substrates involves instantaneous three-dimensional nucleation. The coating is the best when the current density is 4mA/cm2 and the temperature is 80 ℃. The coating by the method of the impulse electrodeposition is better than the direct current electrodeposition. Especially when f is 100Hz, ton/toff is 2/8 and J is 8mA/cm2, the coating is clean and uniformity. The crystal size is nearly 0.3μ m .
Key words: Zn; ZnCl2; ionic liquids; electrodeposition;
如何在获得高质量金属镀层的同时消除电沉积液对环境的危害已成为绿色电化学和环保工业亟待解决的问题,而离子液体的出现使之成为可能,这种液体具有蒸汽压近似为零,不挥发、无污染、无毒性等特点[1-2]。室温下即可得到在高温熔盐中才能电沉积得到的金属和合金,但没有高温熔盐那样的强腐蚀性,离子液体的特性使之成为电沉积中的崭新液体[3-4]。
PITNER 等[5]利用基于AlCl3 的酸性离子液体研究了锌在金、铂、钨和玻碳电极上的电沉积过程,发现在金属电极上有欠电位现象,而在玻碳电极上只有三元成核过程;得到的是锌和铝的合金。SUN等[6-8]将EMIC与ZnCl2 混合得到能够在空气中稳定存在的离子液体,并用于电沉积锌。循环伏安测试表明,当ZnCl2与EMIC的摩尔比为1?3时,体系呈Lewis碱性,电化学窗口大约为3.0V,与碱性的EMIC-A1C13相同;当ZnCl2与EMIC的摩尔比为0.5?1时,体系呈Lewis酸性,电化学窗口约为2V。在适当的温度和电压下,在酸性离子液体中能够得到锌镀层。
目前,国内文献中关于离子液体的报道也较多
[9-12],但还没有关于离子液体在电沉积方面应用的报道。由于离子液体的特性,可以克服高温熔盐和水溶液在电沉积下所产生的诸多缺点如导致部件产生氢脆、电流效率低、废水处理问题、工作温度高、环境差等
[13]。因此,本文作者对在离子液体ZnCl
2-EMIC中电沉积锌进行了研究,分析其沉积机制,并对影响镀层的各种因素进行研究,为这种新型电解液在电沉积方面的应用提供理论依据。
1 实验
1.1 主要实验仪器和试剂
氯化-1-甲基-3-乙基咪唑(EMIC)(纯度大于97%,日本东京化成株式会社); 氯化锌(分析纯);丙酮(分析纯);脉冲电源(SMD-30数控脉冲电镀电源,邯郸市大舜电镀设备厂);CHI1140电化学分析仪(上海辰华仪器有限公司);JMS-2真空手套箱(南京九门自控技术有限公司);电导仪;铜片(1cm×1cm)(纯度为99%);钨丝(纯度为99.95%);镍丝(纯度为99%);锌片(1cm×1cm)( 纯度为99%); 锌丝(纯度为99%)。
1.2 实验过程
由于离子液体极易吸水,整个过程对水极其敏感,而无水氯化锌具有很强的吸水性,在空气中极易水解。因此,市售分析纯无水氯化锌大多是ZnCl2和Zn(OH)Cl的混合物,所以,在实验之前要对分析纯无水氯化锌进行脱水。
将适量ZnCl2(分析纯)装入直径和长度适当的石英玻璃管中,通入干燥的高纯氮气,加热至150℃并使气流稍大一点,使得能够把水蒸汽完全带出,停止升温,除水完全后再升温,进行阶段性除水。约至550℃时,不再有水汽蒸出。减小气流,控制温度在770~800℃,约750℃时开始有白色ZnCl2固体升华至石英管壁上,升华完毕后,移至手套箱,在氮气保护下,将ZnCl2粉末刮出管外[14]。
离子液体的配制在氮气气氛下的手套箱中进行,并用无水P2O5放在手套箱中做干燥剂,ZnCl2与EMIC以1?1的摩尔比配制,加热到90℃保持2h。
在手套箱中,用电导仪对离子液体在不同温度下进行电导测试。
为了了解室温熔盐中Zn的电沉积过程,需要进行电化学实验,因为普通的参比电极在室温熔盐中不适用,因此,制备Zn/Zn2+参比电极。选取长为90mm,直径为8mm的玻璃管,在玻璃管的一端烧结一块玻璃砂芯。配制2mL左右的ZnCl2-EMIC离子液体(摩尔比1?1),用5mL的医用玻璃针筒将所配离子液体注入玻璃管内,将嵌有高纯锌丝的塑料套管套在玻璃管上,并用密封泥密封固定。
在电镀实验中,阴极采用铜片(2 cm×1 cm×0.01 cm),阳极采用锌片(2 cm×1 cm×0.01 cm)。电极在进行实验之前需要进行从3号砂纸到5号砂纸进行磨光,之后用丙酮去酯,再用H3PO4、H2SO4和HNO3的混合混液(70%?25%?5%)抛光,然后用去离子水洗涤,烘干备用。在锌的电沉积实验中,分别考察直流电流密度、脉冲参数和温度对镀层的影响。在电化学测试中,工作电极分别采用钨丝、镍丝(有效面积0.251 2 cm2),对电极为锌丝(有效面积0.251 2 cm2),参比电极为Zn/Zn2+(有效面积0.251 2 cm2)。
2 结果与讨论
2.1 离子液体的电导率
在手套箱中用电导仪对电解液进行电导测试,加热电解液到80 ℃,然后让其温度自行下降,在下降的过程记录不同温度的电导率。摩尔比为1?1的ZnCl2-EMIC在不同温度下的电导率如表1所列。由表可知,电导率随温度升高而增大。
表1 ZnCl2-EMIC离子液体在不同温度下的电导率
Table 1 Electrical conductivity of ZnCl2-EMIC ionic liquids at various temperatures

2.2 锌的电沉积机制
2.2.1 循环伏安法
实验中采用三电极体系对1?1 ZnCl2-EMIC离子液体进行电化学测试。图1所示为80℃时以镍丝(有效面积0.251 2cm2)、铂丝(有效面积0.251 2cm2)为工作电极,锌丝为对电极(有效面积0.251 2cm2),参比电极为Zn/Zn2+(有效面积0.251 2cm2)的循环伏安曲线。在镍电极上从开始向负方向扫描到-0.5V为止,再向正方向扫描到2V。由图可知,负方向扫描上有2个还原峰(a、b),对应的有3个氧化峰(c、d、e)。经分析可知,由于参比电极为Zn/Zn2+,所以锌还原电位应该在0V附近,主还原峰(b)和主氧化峰(c)为大量锌的沉积和溶解,但在0.06V时出现锌的还原,1V附近出现明显的2个小氧化峰(d、e), 根据Kolb-Gerischer的欠电位关系式[15-16]:

由图1可知,1V附近出现氧化峰(d)可能是对应欠电位沉积的锌的溶解,而峰(e)可能与镍电极本身的氧化有关,在0.4V附近的氧化峰为大量锌的氧化。在0.06V时出现锌的还原可能是欠电位沉积,峰不是很明显。
本实验中用铂电极来估定电解液中的水分,在铂电极上只有一对还原峰和氧化峰(见图1(b)),是锌的还原与氧化,在铂电极上锌形核长大需要一定的过电位,在-0.025V处锌开始还原,图中并没有出现氢离子的还原峰,由此可以知道,电解液中水分极低。
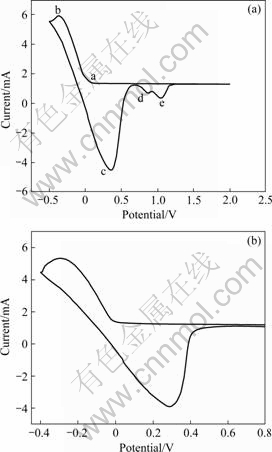
图1 80 ℃时摩尔比为1?1的ZnCl2–EMIC离子液体的循环伏安曲线
Fig.1 Cyclic voltage current characteristic curves of ZnCl2-EMIC at 80℃ and 100mV/s: (a) Nickel electrodes; (b) Platinum electrodes
图2(a) 所示为工作电极为镍丝(有效面积0.251 2cm2),摩尔比为1?1的ZnCl2-EMIC室温熔盐在80℃下以不同扫描速率的循环伏安曲线。由图可知,随着扫描速率的增加,峰电流 增大,
增大, 向左移动,
向左移动, 向右移动,初步确定反应为准可逆的。图2(b)所示为阴极峰电流和扫描速率平方根的关系曲线。由图可知,峰电流
向右移动,初步确定反应为准可逆的。图2(b)所示为阴极峰电流和扫描速率平方根的关系曲线。由图可知,峰电流 和扫描速率平方根v1/2不成线形关系,在高速扫描情况下偏离直线,由此可以确定反应为准可逆反应。
和扫描速率平方根v1/2不成线形关系,在高速扫描情况下偏离直线,由此可以确定反应为准可逆反应。

图2 80 ℃不同扫描速率时摩尔比为1?1 ZnCl2-EMIC熔盐的循环伏安曲线及阴极峰电流和扫描速率平方根的关系
Fig.2 Cyclic voltage current curves of ZnCl2–EMIC at 80℃ and different scanning rates (a) and dependence of Ipc on v1/2(b)
2.2.2 计时电流法
电位阶跃法是研究成核和生长的常用方法。采用三电极体系对离子液体进行电流—时间暂态分析。图3所示为分别以钨丝(有效面积0.251 2 cm2)、镍丝(有效面积0.251 2 cm2)为工作电极,锌丝(有效面积0.251 2 cm2)为对电极,Zn/Zn2+(有效面积0.251 2cm2)为参比电极,80℃时不同电位下摩尔比为1?1的ZnCl2-EMIC室温熔盐的计时电流曲线。

图3 80℃时熔盐在不同电极上的I—t曲线
Fig.3 I—t curves of melt on different electrodes at 80℃: (a) Tungsten electrodes; (b) Nickel electrodes
由图3可以看出,实验开始时电流很大,这是因为电极双电层的充电过程及晶核的孕育阶段所致。双电层电流阶跃后逐渐减弱,然后电流继续增大,这是因为锌被还原,成核并长大。电结晶初期由于晶核的形成和新相的生成,电流迅速上升,在电流达到最大值后出现电流衰减,此时,锌电结晶过程经历了生长中心的交叠并向外生长,同时伴随生长中心的消失和新生长中心的形成,最后电流缓慢下降,受线性扩散控制,两者双电层充电结束后,电流下降快慢不一样。这是因为钨电极上锌的沉积需要更高的形核过电位,故双电层充电后电流负移更明显。由于锌可在镍电极上发生一定的欠电位沉积,形核的过电位较小,故相同极化电位下负移不明显。生长中心为半球状而微晶的生长速度受溶液中电活性离子的扩散控制时,不同成核机理的暂态电流公式为[17]

通过对式(2)和(3)进行微分变换,可分别得到tm,Im和I2/ 的表达式,结果列于表2。
的表达式,结果列于表2。
表2 由式(2)和(3)导出的关系
Table 2 Expressions derived from Eqns. (2) and (3)

由表2可知,对于不同的成核方式,其无因次曲线I2/ —t/tm的形状不同。将图3的数据进行处理,时间和电流分别减去双电层充电过程的t0和I0得出:t′=t-t0、
—t/tm的形状不同。将图3的数据进行处理,时间和电流分别减去双电层充电过程的t0和I0得出:t′=t-t0、 =tm-t0,I′=I-I0、
=tm-t0,I′=I-I0、 =Im-I0,然后将图3及式(2)和(3)转换
=Im-I0,然后将图3及式(2)和(3)转换 —
— 无因次关系,结果如图4所示。
无因次关系,结果如图4所示。
比较瞬时成核和连续成核的无因次曲线,发现,连续成核的无因次曲线呈现一个较尖锐的峰,而瞬时成核是一个较宽的峰。从图4可以看出,在实验的电位范围内,测得的数据点基本与瞬时成核曲线相吻合,镍电极上双电层的充电过程较钨电极时间要长,所以形核起始电流稍较大些,但基本和瞬时成核曲线相吻合,因此,认为锌在镍、钨电极上电结晶是按三维瞬时形核半球形的扩散长大过程。

图4 对应图3的无因次曲线
Fig.4 Non-dimensional plots of (I/Im)2 vs t/tm data corresponding to potential steps in Fig.3: (a) Tungsten electrodes; (b) Nickel electrodes
2.3 锌的电沉积
2.3.1 锌的直流电沉积
采用铜片(1cm×1cm)作阴极材料,锌片(1cm×1cm)为阳极材料,在ZnCl2-EMIC体系中进行电沉积实验,研究不同温度下、不同电流密度对镀层的影响。在电镀的过程中,温度在较低时,如50℃时,镀层很容易发黑,表面堆积了一层灰色的物质,经XRD分析得出,灰色堆积层仍然为锌。图5所示为含灰色成分的镀层的XRD谱。

图5 含灰色成分镀层的XRD谱
Fig.5 XRD pattern of coating containing gray composition
图6所示为不同温度下电镀15 min的镀层表面的SEM像。由图可知,在同一电流密度下,50℃时得出的镀层晶粒要比80℃时得出的镀层晶粒细小,且不均匀,镀层很薄。因为在同一电流密度下,50℃时离子液体电导率小,需要更大的过电位,那么形核速度较80℃时大,所以晶粒较小,而且电流密度增大就易导致镀层发黑。

图6 不同温度下制备镀层的SEM像
Fig.6 SEM images of coating prepared at different tempera- tures (Molar ratio of EMIC to ZnCl2 of 1?1, J=1.39 mA/cm2): (a) 50℃; (b) 80℃
在同一温度下,在电流密度为1mA/cm2时,晶粒较小,大小1.5μm左右,不均匀致密,如图7(a)所示。由图可以看出:镀层表面呈乳白色,光滑,且适当增大电流密度到1.39mA/cm2时,晶粒增大到2.5μm左右,如图7(b) 所示。由图可知,当电流密度继续增大时,随着电流密度继续增大,晶粒越来越细致,当增大到4mA/cm2时,晶粒大小为1μm左右,如图7(c)所示。由图可知,镀层表面乳白色,光滑。电流密度在0~1.39mA/cm2时,随着电流密度增大沉积层的晶粒变粗,这是由于电流密度低,过电位则小,导致晶电流密度继续增大时,过电位增加,晶核形成的速度显著增加,沉积层结晶细致。在允许电流密度范围内,沉积层结晶均较细。若电流密度超过允许上限5mA/cm2时,由于阴极附近缺少放电金属离子,一般在棱角和凸出部位放电,出现结瘤或枝状结晶,如图7(d)所示。由图可知,镀层表面呈灰色,与50℃时制备的镀层一样,灰色部分仍然为锌。

图 7 80℃不同电流密度下镀层的SEM像
Fig.7 SEM images of coating at 80 ℃ and different current densities: (a) J = 1 mA/cm2; (b) J = 1.39mA/cm2; (c) J = 4mA/cm2; (d) J = 6mA/cm2
2.3.2 锌的脉冲电沉积
脉冲电沉积的结果如表3所列。用单向脉冲电源对离子液体进行电沉积,可控的电流密度更加的宽,当电流密度增大到10mA/cm2时,很难得到好的镀层,表面堆积了灰色的物质。镀层表面的SEM像如图8所示。由图可知,当电流密度在8mA/cm2,脉冲宽度9ms,脉冲间隔1ms时得到的镀层表面呈白色、光滑、颗粒细小均一,边缘有晶枝。

图8 80℃时下镀层表面的SEM像
Fig.8 SEM image of coating at 80℃ (f=100Hz, ton/toff =9/1, J=10mA/cm2 )
使用脉冲电镀能够获得较直流更加细小的颗粒,这是因为脉冲电镀在电流导通时,接近阴极的金属离子在高电流密度下沉积成细小的颗粒;当电流关断时,扩散层消失,阴极周围的放电离子又重新恢复到初始浓度[18]。这样阴极表面扩散层内的金属离子浓度就得到了及时补充,扩散层周期间隙式形成,减薄了扩散层的实际厚度,从而改善了镀层的性能。
在80℃下电流密度为8mA/cm2、不同频率和通断比时得到的镀层SEM像如图9所示。由图可以看出,在f = 100Hz、ton/toff = 2/8条件下镀层质量最好,得到的镀层表面光滑且均匀,晶粒大小接近0.3μm 。f = 50 Hz、ton/toff = 2/8和f = 100Hz、ton/toff = 5/5条件下也能获得比较好的镀层。而其他出现结瘤或枝状结晶,宏观上略显灰色,因此,可以认为该灰色部分也为锌。
表3 80 ℃下锌的脉冲电沉积结果
Table 3 Experiment results of Zn electrodeposition by PCP at 80 ℃


图9 80℃时不同频率和通断比下电镀15min镀层的SEM像
Fig.9 SEM images of coating at different impulse frequencies and different ratios of ton to toff at 80℃ (J =8mA/cm2): (a) f = 100Hz, ton/toff =2/8; (b) f =50Hz, ton/toff = 2/8; (c) f =10Hz, ton/toff = 2/8; (d) f =500Hz, ton/toff = 2/8; (e) f =100Hz , ton/toff = 5/5; (f) f = 100Hz, ton/toff = 9/1
3 结论
1) ZnCl2-EMIC离子液体中电沉积锌的反应为准可逆反应,锌在镍和钨电极上形核是三维瞬时形核半球形扩散长大过程。在同一电流密度下,50 ℃时得出的镀层晶粒要比80 ℃时得出的镀层晶粒细小,但不均匀。
2) 在80℃时,当电流密度在0~1.39mA/cm2变化时,随着电流密度增大,沉积层的晶粒变粗;电流密度继续增大时,过电位增加,晶核形成的速度显著增加,沉积层结晶细致。若电流密度超过允许上限5.0 mA/cm2时,得到的镀层表面呈灰色无金属光泽,出现结瘤或枝状结晶。
3) 脉冲电沉积较直流电沉积镀层表面质量更好,采用脉冲电镀时,电流密度达到8mA/cm2,脉冲宽度2ms,脉冲间隔8ms, 电流密度为8mA/cm2时,得到的镀层表面光滑且均匀,晶粒大小接近0.3μm。
REFERENCES
[1] 陈双平, 王寿武, 汪守建, 戴卫东, 冯 莉. 离子液体的性质和制备方法[J]. 精细化工中间体, 2004, 34(5): 10-12.
CHEN Shuang-ping, WANG Shou-wu, WANG Shou-jian, DAI Wei-dong, FENG Li. The character and preparation method of ionic liquids[J]. Fine Chemical Intermediates, 2004, 34(5): 10-12.
[2] 王均凤, 张锁江, 陈慧萍, 李 闲, 张密林. 离子液体的性质及其在催化反应中的应用[J]. 过程工程学报, 2003, 3(2): 177-185.
WANG Jun-feng, ZHANG Shuo-jiang, CHEN Hui-ping, LI Xian, ZHANG Mi-lin. The character of the ionic liquids and the appliance on catalysis reaction[J]. Chinese Journal of Process Engineering, 2003, 3(2): 177-185.
[3] 张英锋, 李长江, 包富山, 张永安. 离子液体的分类、合成与应用[J]. 化学教育, 2005(2): 7-12.
ZHANG Ying-feng, LI Chang-jiang, BAO Fu-shan, ZHANG Yong-an. The classify synthesize and application of the ionic liquids[J]. Chemistry Education, 2005(2): 7-12.
[4] 刘艳升, 严 骏, 徐春明, 胡玉峰. 离子液体在电沉积金属和半导体材料中的应用[J]. 化学通报, 2004, 67: 470-475.
LIU Yan-sheng, YAN Jun, XU Chun-ming, HU Yu-feng. The application of the ionic liquids electrodeposition of metal and semiconductor material[J]. Chemistry, 2004, 67: 470-475.
[5] PITNER W R, HUEESY C L. Electrodeposition of zinc from ionic liquids[J]. J Electrochem Soc, 1997, 144: 3095-3104.
[6] HSIU S I, HUANG J F, SUN I W. Lewis acidity dependency of the electrochemical window of zinc chloride-1-ethy1-3- methylimidazolium chloride ionic liquids[J]. Electrochimica Acta, 2002, 47: 4367-4372.
[7] HUANG J F, SUN I W. Electmdeposition of Pt-Zn in a Lewis acidic ZnC12-1-ethyl-3-methylmidazolium chloride ionic liquid[J]. Electrochimica Acta, 2004, 49: 3251-3258.
[8] CHEN P Y, SUN I W. Electrodeposition of cobalt and Zinc-cobalt alloys from a lewis acidic zinc chloride-1-ethyl-3- methylimidazolium chloride molten salt[J]. Electrochimica Acta, 2001, 46: 1169-1177.
[9] 刘 卉, 陶国宏, 寇 元. 功能化的离子液体在电化学中的应用[J]. 化学通报, 2004(11): 795-801.
LIU Hui, TAO Guo-hong, KOU Yuan. The application of the ionic liquids on electrochemistry[J]. Chemistry, 2004(11): 795-801.
[10] 张景涛, 朴香兰, 朱慎林. 离子液体及其在萃取中的应用研究进展[J]. 化工进展, 2001, 20(12): 16-19.
ZHANG Jing-tao, PU Xiang-lan, ZHU Shen-lin. The research of application on extraction of ionic liquids[J]. Chemical Industry and Engineering Progress, 2001, 20(12): 16-19.
[11] 张普玉, 娄 帅, 金邻豫, 李文斌. 离子液体应用研究进展[J]. 精细化工, 2005, 22(5): 324-327.
ZHANG Pu-yu, LOU Shuai, JIN Lin-yu, LI Wen-bin. The research of the application of the ionic liquids[J]. Fine Chemical Intermediates, 2005, 22(5): 324-327.
[12] 赵大成, 徐海涛, 高 歌, 徐 鹏, 刘凤岐. 室温离子液体中的聚合反应[J]. 化学进展, 2005, 17(4): 700-705.
ZHAO Da-cheng, XU Hai-tao, GAO Ge, XU Peng, LIU Feng-qi. Polymerization reaction of the ionic liquids[J]. Chemical Industry and Engineering Progress, 2005, 17(4): 700-705.
[13] 安茂忠. 电镀锌及锌合金发展现状[J]. 电镀与涂饰, 2003, 22(6): 35-40.
AN Mao-zong. The status of electroplate zinc and alloy[J]. Electroplating and Coating, 2003, 22(6): 35-40.
[14] 贾会珍, 张 萍, 李 媛, 韩明会. 高纯度无水氯化锌的一种实验室制备方法[J]. 石家庄学院学报, 2005(5): 22-25.
JIA Hui-zhen, ZHANG Ping, LI Yuan, HAN Ming-hui. A sort of laboratory preparation of high pure zinc chloride[J]. Journal of Shijiazhuang University, 2005(5): 22-25.
[15] GERISCHER H, KOLB D M, PRZASNYSKI M. Tunneling processes at highly doped zinc oxide electrodes in contact with aqueous electrolytes (Ⅰ): Electron exchange with the conduction band[J]. Surf Sci, 1974, 45: 662-666.
[16] LI L F, TOTIR G G, TOTIR D O, CHOTTINER G S. Underpotential deposition of lithium on aluminum in ultrahigh- vacuum environments[J]. Phys Chem, 1999, 103: 164-168.
[17] SCHARIFLKER B, HILLS G. Theoretical and experimental studies of multiple nucleation[J]. Electrochemical Acta, 1983, 28(7): 45-48.
[18] 刘 勇, 罗义辉, 魏子栋. 脉冲电镀的研究现状[J]. 电镀与精饰, 2005, 27(5): 25-29.
LIU Yong, LUO Yi-hui, WEI Zi-dong. The study status of impulse electroplate[J]. Electroplating and Coating, 2005, 27(5): 25-29.
基金项目:国家自然科学基金资助项目(50474027)
收稿日期:2007-09-30;修订日期:2008-01-16
通讯作者:李 冰,教授,博士;电话:021-64252170;E-mail: drlibing@163.com
(编辑 龙怀中)